Similarities among Alzheimer's Disease, Parkinson's Disease and Dementia may Call for a Similar Treatment
Abstract
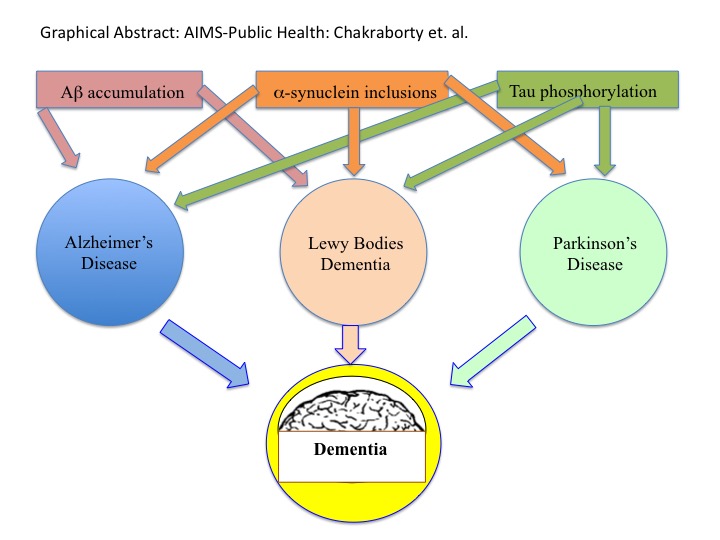
Alzheimer's Disease (AD), Parkinson's Disease (PD), and Dementia are all age-related neurodegenerative diseases. In the US, AD victims are increasing every year from 5.5 million in 2018 to 13.8 million by 2050. PD patient are calculated to increase from 7K to 1 million by 2030. Results from accumulation of extracellular amyloid-β (Aβ) peptide and deposition of intracellular tau aggregated tangles. The prognosis of AD and PD both is the onset of Dementia, which causes memory impairment irreversibly, thinking capabilities, orientation, comprehension, learning any new things, and taking the judgment. Dementia is currently the seventh leading cause of death among all diseases worldwide. Here we will discuss the similarities in the disease nature and cause of AD, PD and Dementia at their cellular and molecular level to find a common therapy for them (Graphical Abstract).
Keywords
Alzheimer's disease (AD), Parkinson's Disease (PD), Dementia, Tangles, Plaques, Cognitive function
Abbreviations
AD: Alzheimer's Disease; LOAD: Late-Onset AD, SPs: Senile Plaques; NMDA: N-methyl-D-aspartate; Aβ: Amyloid-Beta Peptide; EphB2 receptors: Ephrin type-B receptor 2; NFTs: Neurofibrillary Tangles; ApoE: Apolipoprotein E; pTau: Hyperphosphorylated tau; BACE1: β-site APP Cleaving Enzyme type-I; APP: Amyloid Precursor Protein; LTD: Long-term Depression; EOAD: Early-Onset AD; MTs: Microtubules; PSEN1 and PSEN2: Presenilins 1 and 2; ptau: Phosphorylated 'tau'; AICD: APP Intracellular Domain; Cdk-5: Cyclin-Dependent Kinase 5; sAPPβ: Soluble Ectodomain-β; cdc-2: Cell-Cycle Kinase; sAPPα: soluble Ectodomain-α; ROS: Reactive Oxygen Species
Introduction
The typical neurodegenerative diseases are Parkinson's disease (PD) and Alzheimer's disease (AD) and Dementia [1]. Both PD and AD display mitochondrial dysfunction and oxidative stress [2-4], while Dementia is a broad term that stands for an irreversible loss of thinking ability, memory, and other mental capabilities [5,6]. In fact, Dementiais considered as the end results of AD and PD. All these diseases are believed to be due to aging. This article will discuss the similarities and differences among these neural diseases and find for some appropriate treatment possibilities [7-75] (Table 1, Table 2, Table 3, Table 4, Table 5, Table 6 and Table 7).
Conclusions
The greatest risk factor for AD, PD and Dementia is age. It appears thattau and Aβ are the hallmarks for both AD and PD in the brain and eventually for Dementia [76,77].
This review indicates large similarities in genetic risk factors between diseases, AD, PD and Dementia. The genes that were discussed above have the possibility as a potential biomarkers.
Using KEGG pathway analysis [78], Wang, et al. discovered that PD and AD were both dysfunctional in synaptic vesicle and mitochondrial oxidative metabolism pathways [79]. The enriched genes in AD cases was greater than PD. Although PD and AD have common characteristics [80]; cognition and patients with learning or memory damage in AD was more severe than in PD [81].
Epigenetic regulatory mechanisms, such as chromatin remodeling, DNA methylation, histone variant and histone post-translational modification have been suggested to regulate numerous aspects of axonal development and neuronal survival [82]. One study presented evidence that changes in H3K27ac or H3K4me3 occurred in connection with genetic variants in AD. This is an important function for immune-associated enhancers and promoter proteins in determining AD susceptibility [83]. Another study demonstrated that H4K16ac, a histone associated with DNA repair and neurodegenerative disorders, is significantly reduced in the cortex of AD patients. This suggests that the aged brains of these individuals are incapable of up-regulating H4K16ac [84]. In addition, multiple reports have associated loss of H3K4me3, a protein related to gene activation, with the deterioration found in PD. Overexpression of H3K4me3 can accelerate A-T mutation that mitigates behavioral impairments and neurodegeneration [85-87].
HDAC inhibitors can prevent neuro-degeneration in models of AD [88-91]. This paper demonstrate that epigenetic profiles are regulated in neurodegenerative diseases and gives a better understanding of these mechanisms that can provide the foundation for developing more precisely targeted epigenome therapies. For example, recent work suggesting that epigenetic editing can improve cognition in AD highlights the potential of epigenetic regulation-based gene therapy for neurodegenerative disorders [92].
Gene modification of stem cells prior to transplantation can be useful for increasing cell survival and making them more effective [93]. Due to the loss of cholinergic neurotransmitters in AD, gene-modified cells transplantation can produce acetylcholine (Ach) and could be beneficial to the patients. Primary fibroblast cell line genetically engineered to express choline acetyltransferase showed the capacity to produce Ach after transplantation into the hippocampus of rats [94]. Another example of the use of the facilitation of genetherapy for AD is the over expression of neprilysine (NEP), an Aβ degrading protease that has been shown to ameliorate extracellular amyloids [95].
Transgenic mice (APP/PS1) injected with lentiviral vector expressing NEP showed a reduction in Ab deposits [96], and MSCs overexpressing the NEP gene demonstrated the ability to degrade Aβ peptides in vitro [97]. Similar results were obtained in vivo with transgenic mice that were transplanted with fibroblasts engineered with lentivirus carrying NEP [98].
Recently we have created modified neural stem cells which can differentiate, produce dopamine, BDNF/GDNF [99-101]. Our notion, therefore, is that the cell-replacement therapy of AD/PD/Dementia patients with modified neural cells could be relevant [102-106].
Acknowledgments
We acknowledge all our colleagues for their help during the preparation of the manuscript by providing all the relevant information. Thanks to Ms. Bethany Pond for her Editorial assistants.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authors' Contribution
Both the authors have contributed equally to preparing this article, reading, and approving the final manuscript.
Conflict of Interests
The authors declare no conflict of interests.
Consent for Publications
Both the authors have agreed to submit this paper for publication.
Ethical Approval
Not applicable.
References
- Nussbaum RL, Ellis CE (2003) Alzheimer's disease and Parkinson's disease. N Engl J Med 348: 1356-1364.
- Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443: 787-795.
- Khan TA, Hassan I, Ahmad A, et. al. (2016) Recent updates on the dynamic association between oxidative stress and neurodegenerative disorders. CNS Neurol Disord Drug Targets 15: 310-320.
- Denzer I, Munch G, Friedland K (2016) Modulation of mitochondrial dysfunction in neurodegenerative diseases via activation of nuclear factor erythroid-2-related factor 2 by food-derived compounds. Pharmacol Res 103: 80-94.
- Mayeux R, Chen J, Mirabello E, et al. (1990) An estimate of the incidence of dementia in idiopathic Parkinson's disease. Neurology 40: 1513-1517.
- Ebmeier KP, Calder SA, Crawford JR, et al. (1991) Dementia in idiopathic Parkinson's disease: Prevalence and relationship with symptoms and signs of Parkinsonism. Psychological Medicine 21: 69-76.
- Dickson DW (1997) Neuropathological diagnosis of Alzheimer's disease: A perspective from longitudinal clinicopathological studies. Neurobiol Aging 18: S21-S26.
- Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science 297: 353-356.
- German DC, Manaye K, Smith WK, et al. (1989) Midbrain dopaminergic cell loss in Parkinson's disease: Computer visualization. Ann Neurol 26: 507-514.
- Obeso JA, Rodriguez Oroz MC, Goetz CG, et al. (2010) Missing pieces in the Parkinson's disease puzzle. Nat Med 16: 653-661.
- Jankovic J (2008) Parkinson's disease: Clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79: 368-376.
- https://www.nia.nih.gov/health/alzheimers/symptoms.
- https://parkinsonsnewstoday.com/2017/08/03/11-facts-parkinsons-may-not-know/.
- https://www.healthline.com/health/dementia/early-warning-signs.
- Dewey ME, Saz P (2001) Dementia, cognitive impairment and mortality in persons aged 65 and over living in the community: A systematic review of the literature. Int J Geriatr Psychiatry 16: 751-761.
- James BD, Leurgans SE, Hebert LE, et al. (2014) Contribution of Alzheimer disease to mortality in the united states. Neurology 82: 1045-1050.
- Weuve J, Hebert LE, Scherr PA, et al. (2014) Deaths in the united states among persons with Alzheimer's disease (2010-2050). Alzheimers Dement 10: e40-e46.
- Harlin MCC, Mullan M, Brown J, et al. (1991) Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature 349: 704-706.
- Hamilton RL (2000) Lewy bodies in Alzheimer's disease: A neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol 10: 378-384.
- Clinton LK, Blurton Jones M, Myczek K, et al. (2010) Synergistic interactions between Abeta, tau, and alpha-synuclein: Acceleration of neuropathology and cognitive decline. J Neurosci 30: 7281-7289.
- Kalinderi K, Bostantjopoulou S, Fidani L (2016) The genetic background of Parkinson's disease: current progress and future prospects. Acta Neurol Scand 134: 314-326.
- McKeith IG, Galasko D, Kosaka K, et al. (1996) Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurolog 47: 1113-1124.
- Zawia NH, Lahiri DK, Cardozo Pelaez F (2009) Epigenetics, oxidative stress, and Alzheimer disease. Free Radic Biol Med 46: 1241-1249.
- Bertram L, Tanzi RE (2005) The genetic epidemiology of neurodegenerative disease. J Clin Invest 115: 1449-1457.
- Kehoe P, De Vrieze FW, Crook R, et al. (1999) A full genome scan for late onset Alzheimer's disease. Hum Mol Genet 8: 237-245.
- Curtis D, North BV, Sham PC (2001) A novel method of two-locus linkage analysis applied to a genome scan for late onset Alzheimer's disease. Ann Hum Genet 65: 473-481.
- Healy DG, Abou Sleiman PM, Lees AJ, et al. (2004) Tau gene and Parkinson's disease: a case-control study and meta-analysis. J Neurol Neurosurg Psychiatr 75: 962-965.
- Sherrington R, Rogaev EI, Liang Y, et al. (1995) Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature 375: 754-760.
- Rogaev EI, Sherrington R, Rogaeva EA, et al. (1995) Familial Alzheimer's disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer's disease type 3 gene. Nature 376: 775-778.
- Levy Lahad E, Wasco W, Poorkaj P, et al. (1995) Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science 269: 973-977.
- Polymeropoulos MH, Lavedan C, Leroy E, et al. (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science 276: 2045-2047.
- Kitada T, Asakawa S, Hattori N, et al. (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392: 605-608.
- Bonifati V, Rizzu P, van Baren MJ, et al. (2003) Mutations in the DJ-1 gene associated with autosomal recessive early-onset Parkinsonism. Science 299: 256-259.
- Valente EM, Abou Sleiman PM, Caputo V, et al. (2004) Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science 304: 1158-1160.
- Zimprich A, Biskup S, Leitner P, et al. (2004) Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44: 601-607.
- Paisan Ruiz C, Jain S, Evans EW, et al. (2004) Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron 44: 595-600.
- Kelly J, Moyeed R, Carroll C, et al. (2019) Gene expression meta-analysis of Parkinson's disease and its relationship with Alzheimer's disease. Mol Brain 12: 16.
- Kazmierczak A, Czapski GA, Adamczyk A, et al. (2011) A novel mechanism of non-Aß component of Alzheimer's disease amyloid (NAC) neurotoxicity. Interplay between p53 protein and cyclin-dependent kinase 5 (Cdk5). Neurochem Int 58: 206-214.
- Zhang X, Gao F, Wang D, et al. (2018) Tau pathology in Parkinson's disease. Front Neurol 9: 809.
- Brun A (1987) Frontal lobe degeneration of non-Alzheimer type. I. Neuropathology. Arch Gerontol Geriatr 6: 193-208.
- Hutton M, Lendon C L, Rizzu P, et al. (1998) Association of missense and 5'-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393: 702-705.
- Kwon OD (2017) Is there any relationship between Apolipoprotein e polymorphism and idiopathic parkinson's disease? J Alzheimers Dis Parkinsonism 7: 292.
- Verpillat P, Camuzat A, Hannequin D, et al. (2002) Apolipoprotein E gene in front temporal dementia: An association study and meta-analysis. Eur J Hum Genet 10: 399-405.
- Strittmatter WJ, Saunders AM, Schmechel D, et al. (1993) Apolipoprotein E: High-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial alzheimer disease. Proc Natl Acad Sci USA 90: 1977-1981.
- Schmechel DE, Saunders AM, Strittmatter WJ, et al. (1993) Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset alzheimer disease. Proc Natl Acad Sci USA 90: 9649-9653.
- Belin AC, Bjork BF, Westerlund M, et al. (2007) Association study of two genetic variants in mitochondrial transcription factor a (TFAM) in alzheimer's and parkinson's disease. Neurosci Lett 420: 257-262.
- Zhu XC, Cao L, Tan M S, et. al. (2017) Association of Parkinson's disease GWAS-linked loci with Alzheimer's disease in Han Chinese. Mol Neurobiol 54: 308-318.
- Goate A, Chartier-Harlin M C, Mullan M, et al. (1991) Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature 349: 704-706.
- Dermaut B, Singh SK, Engelborghs S, et al. (2004) A novel presenilin 1 mutation associated with Pick's disease but not beta-amyloid plaques. Ann Neurol 55: 617-626.
- Cooper CA, Jain N, Gallagher MD, et al. (2017) Common variant rs356182 near SNCA defines a Parkinson's disease endophenotype. Ann Clin Transl Neurol 4: 15-25.
- Karimi-Moghadam A, Charsouei S, Bell B, et al. (2018) Parkinson disease from mendelian forms to genetic susceptibility: New molecular insights into the neurodegeneration process. Cell Mol Neurobiol 38: 1153-1178.
- Campelo C, Silva RH (2017) Genetic variants in SNCA and the risk of sporadic Parkinson's disease and clinical outcomes: A review. Parkinson's Dis 2017: 4318416.
- Maple-Grodem J, Chung J, Lunde KA, et al. (2018) Alzheimer disease associated variants in SORL1 accelerate dementia development in Parkinson disease. Neurosci Lett 674: 123-126.
- Diner I, Hales CM, Bishof I, et al. (2014) Aggregation properties of the small nuclear ribonucleoprotein U1-70K in Alzheimer disease. J Biol Chem 289: 35296-35313.
- Santiago JA, Potashkin JA (2017) Blood transcriptomic meta-analysis identifies dysregulation of hemoglobin and iron metabolism in parkinson' disease. Front Aging Neurosci 9: 73.
- Bell RD, Sagare AP, Friedman AE, et al. (2007) Transport pathways for clearance of human Alzheimer's amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab 27: 909-918.
- Deane R, Sagare A, Hamm K, et al. (2008) ApoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest 118: 4002-4013.
- Miners JS, Baig S, Palmer J, et al. (2008) Abeta-degrading enzymes in Alzheimer's disease. Brain Pathol 18: 240-252.
- Macours N, Poels J, Hens K, et al. (2004) Structure, evolutionary conservation, and functions of angiotensin- and endothelinconverting enzymes. Int Rev Cytol 239: 47-97.
- Axelsena TM, David PD, Woldbyeb DPD (2018) Gene Therapy for Parkinson's Disease, An Update. J Parkinson Dis 8: 195-215.
- Jiang J, Zhu Q, Gendron Tania F, et al. (2016) Gain of toxicity from ALS/FTD-linked repeat expansions in C9ORF72 is alleviated by antisense oligonucleotides targeting GGG GCC containing RNAs. Neuron 90: 535-550.
- DeVos SL, Miller RL, Schoch KM, et al. (2017) Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy. Sci Transl Med 9: eaag0481.
- Mueller-Steiner S, Zhou Y, Arai H, et al. (2006) Antiamyloidogenic and neuroprotective functions of cathepsin B: implications for Alzheimer's disease. Neuron 51: 703-714.
- Miller BC, Eckman EA, Sambamurti K, et al. (2003) Amyloid-ß peptide levels in brain are inversely correlated with insulysin activity levels in vivo. Proc Natl Acad Sci USA 100: 6221-6226.
- Farris W, Mansourian S, Chang Y, et al. (2003) Insulin-degrading enzyme regulates the levels of insulin, amyloid ß-protein, and the ß-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci USA 100: 4162-4167.
- Boado R (2007) Blood-brain barrier transport of non-viral gene and RNAi therapeutics. Pharm Res 24: 1772-1787.
- Zheng H, Jiang M, Trumbauer ME, et al. (1995) ß-amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell 81: 525-531.
- Arrant AE, Onyilo VC, Unger DE, et al. (2018) Progranulin gene therapy improves lysosomal dysfunction and microglial pathology associated with frontotemporal dementia and neuronal ceroid lipofuscinosis. J Neurosci 38: 2341-2358.
- Amado DA, Rieders JM, Diatta F, et al. (2019) AAV-mediated progranulin delivery to a mouse model of progranulin deficiency causes T cell-mediated toxicity. Mol Ther 27: 465-478.
- Thompson LM (2008) Neurodegeneration: a question of balance. Nature 452: 707-708.
- Mucke L (2009) Neuroscience: Alzheimer's disease. Nature 461: 895-897.
- Colette Daubner S, Le T, Wang S (2011) Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys 508: 1-12.
- German CL, Baladi MG, McFadden LM, et al. (2015) Regulation of the Dopamine and Vesicular Monoamine Transporters: Pharmacological Targets and Implications for Disease. Pharmacol Rev 67: 1005-1024.
- Tang J, Xu H, Fan X, et al. (2008) Embryonic stem cell derived neural precursor cells improve memory dysfunction in Aß (1-40) injured rats. Neurosci Res 62: 86-96.
- Li Z, Gao C, Huang H, et al. (2010) Neurotransmitter phenotype differentiation and synapse formation of neural precursors engrafting in amyloid-ß (1-40) injured rat hippocampus. J Alzheimers Dis 21: 1233-1247.
- Steinbeck JA, Koch P, Derouiche A, et al. (2012) Human embryonic stem cell-derived neurons establish region-specific, long-range projections in the adult brain. Cell Mol Life Sci 69: 461-470.
- Blurton-Jones M, Kitazawa M, Martinez-Coria H, et al. (2009) Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci USA 106: 13594-13599.
- Lue LF, Guerra A, Walker DG (2017) Amyloid Beta and Tau as Alzheimer's Disease Blood Biomarkers: Promise From New Technologies. Neurol Ther 6: 25-36.
- Chojdak-Lukasiewicz J, Malodobra-Mazur M, Zimny A, et al. (2020) Plasma tau protein and Aß42 level as markers of cognitive impairment in patients with Parkinson's disease. Adv Clin Exp Med 29: 115-121.
- Kanehisa M, Goto S (2000) KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28: 27-30.
- Wang Q, Lia W-X, Dai S-X, et al. (2017) Meta-Analysis of Parkinson's Disease and Alzheimer's disease revealed commonly impaired pathways and dysregulation of NRF2-Dependent genes. J Alzheimer's Dis 56: 1525-1539.
- Boller F, Mizutani T, Roessmann U, et al. (1980) Parkinson disease, dementia, and Alzheimer disease: Clinicopathological correlations. Ann Neurol 7: 329-335.
- Ross CA, Poirier MA (2004) Protein aggregation and neurodegenerative disease. Nat Med 10: S10-S17.
- Steffan JS, Bodai L, Pallos J, et al. (2001) Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature 413: 739-743.
- Ferrante RJ, Kubilus JK, Lee J, et al. (2003) Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington's disease mice. J Neurosci 23: 9418-9427.
- Chen W, Dong G, Wu Y, et al. (2017) Dual NAMPT/HDAC inhibitors as a new strategy for multitargeting antitumor drug discovery. ACS Med Chem Lett 9: 34-38.
- Bustos FJ, Ampuero E, Jury N, et al. (2017) Epigenetic editing of the Dlg4/PSD95 gene improves cognition in aged and Alzheimer's disease mice. Brain 140: 3252-3268.
- Kacher R, Lamazière A, Heck N, et al. (2019) CYP46A1 gene therapy deciphers the role of brain cholesterol metabolism in Huntington's disease. Brain 142: 2432-2450.
- Scrivo A, Bourdenx M, Pampliega O, et al. (2018) Selective autophagy as a potential therapeutic target for neurodegenerative disorders. Lancet Neurol 17: 802-815.
- Xiang HG, Zhang JF, Lin CC, et al. (2020) Targeting autophagy-related protein kinases for potential therapeutic purpose. Acta Pharm Sin B 10: 569-581.
- Du F, Yu Q, Yan S, et al. (2017) PINK1 signaling rescues amyloid pathology and mitochondrial dysfunction in Alzheimer's disease. Brain 140: 3233-3251.
- Xilouri M, Brekk OR, Landeck N, et al. (2013) Boosting chaperone-mediated autophagy in vivo mitigates a-synuclein-induced neurodegeneration. Brain 136: 2130-2146.
- Decressac M, Mattsson B, Weikop P, et al. (2013) TFEB-mediated autophagy rescues midbrain dopamine neurons from a-synuclein toxicity. Proc Natl Acad Sci USA 110: E1817-E1826.
- Filézac de L'Etang A, Maharjan N, Cordeiro Braña M, et al. (2015) Marinesco-Sjögren syndrome protein SIL1 regulates motor neuron subtype-selective ER stress in ALS. Nat Neurosci 18: 227-238.
- Ian PM, Liang Tang Y (2008) Genetic modification of stem cells for transplantation. Adv Drug Deliv Rev 60: 160-172.
- Fisher LJ, Raymon HK, Gage FH (1993) Cells engineered to produce acetylcholine: Therapeutic potential for Alzheimer's disease. Ann N Y Acad Sci 695: 278-284.
- Iijima-Ando K, Hearn SA, Granger L, et al. (2008) Overexpression of neprilysin reduces Alzheimer amyloid-beta42 (Abeta42)-induced neuron loss and intraneuronal Abeta42 deposits but causes a reduction in cAMP-responsive element binding protein-mediated transcription, age-dependent axon pathology, and premature death in Drosophila. J Biol Chem 283: 19066-19076.
- Marr RA, Rockenstein E, Mukherjee A, et al. (2003) Neprilysin gene transfer reduces human amyloid pathology in transgenic mice. J Neurosci 23: 1992-1996.
- Habisch HJ, Schmid B, von Arnim CA, et al. (2009) Efficient processing of Alzheimer's disease amyloid-beta peptides by neuroectodermally converted mesenchymal stem cells. Stem Cells Dev 19: 629-633.
- Hemming ML, Patterson M, Reske-Nielsen C, et al. (2007) Reducing amyloid plaque burden via ex vivo gene delivery of an Abeta-degrading protease: A novel therapeutic approach to Alzheimer disease. PLoS Med 4: e262.
- Chakraborty A, Diwan A (2021) Modified neural stem cells: A new regimen for cell therapy of Alzheimer's and Parkinson's disease. Current Trends in Neurology.
- Chakraborty A, Diwan A (2020) Cell-Cell interaction: A method to upgrade the Neural Cells Function. Journal of Neurology & Neurophysiology 11: 001-003.
- Chakraborty A, Diwan A (2021) Coculturing NSCs with melanocyte increased its dopamine and neural factor secretion. Acta Scientific Neurology 4: 70-78.
- Chakraborty A, Diwan A (2020) Alzheimer and it's possible Therapy: A Review. Journal of Experimental Neurology 1: 115-122.
- Chakraborty A, Diwan A (2019) Selection of cells for Parkinson's disease cell-therapy. Int J Stem Cell Res Ther 6: 063.
- Chakraborty A, Diwan A (2021) Dementia in Parkinson's Disease: It's therapeutics. Innovative Journal of Neurology and Neuroscience 1: 1-4.
Corresponding Author
Ashok Chakraborty, PhD, Sr. Research Scientist, Allexcel, Inc., 1 Controls Drive, Shelton, CT 06484, USA.
Copyright
© 2022 Chakraborty A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.