An Investigation on the Effects of Varying Temperatures on Gelatin Denaturation in Response to Enzymatic Reactions from Fruit Extracts
Introduction
During holiday celebrations, my family would hold gatherings in our home, and I would always help my mother prepare some traditionally known desserts in the Filipino culture, such as buko pandan (coconut salad with jelly) and fruit salad. I was advised to use canned pineapples instead of fresh ones because the latter contained more active enzymes, which "melted" the gelatin in the salad. When we discussed biomolecules in class, I became particularly interested with the topic, as it is relatively associated with biological processes in the human body such as digestion and metabolism. Thus, upon learning that gelatin - a major component in fruit salads - was made up of the biomolecule protein, I have decided to investigate the different factors that affected fruit enzymes and how these catalysts subsequently affected the breakdown of proteins. This would be demonstrated through an experiment involving the use of materials that I was already familiar with - fruits and gelatin.
Research question
To what extent do varying temperatures (5℃, 55℃, 75℃, 100℃) affect the pH and the denaturation of gelatin in response to exposure to fruit enzymes, such as bromelain, papain, and amylases found in pineapple (Ananas comosus), orange and green papayas (Carica papaya), and banana (Musa acuminata), respectively, by measuring the change in pH (±0.01) and volume (±0.5mL)?
Hypotheses
For the change in volume
H1: Gelatin denaturation occurs the most in fruit samples exposed to room temperature condition, as this temperature provides an internal environment where enzymes function most effectively.
H0: There is no difference observed in gelatin denaturation when exposed to fruit samples from all temperature conditions.
For the change in pH
H2: The pH of all fruit samples decreases as the temperature increases.
H0: Varying temperature conditions do not have a significant effect on the pH of all fruit samples.
Background Information
Gelatin is made up of an animal protein called collagen, the most abundant protein in the human body found in bones, muscles, and skin. It is a hard, insoluble, and fibrous protein that are packed together to form long, thin fibrils, which act as supporting structures to give the skin elasticity and strength [1].
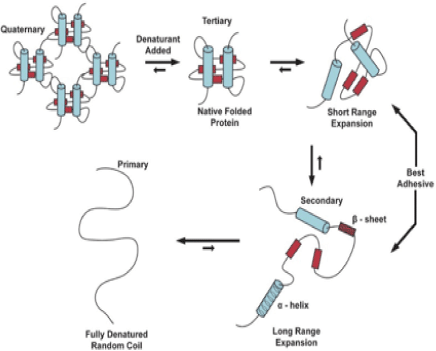
With the presence of heat, the long chains of amino acids coiled by the weak bonds in gelatin begin to unwind. In this process, the hydrogen bonds and non-polar hydrophobic interactions are disrupted by heat due to kinetic energy. With an increase in heat, the molecules move rapidly and violently, disrupting the bonds in the process. The process of denaturation in secondary and tertiary proteins is illustrated in Figure 1, depicting how the structures are destroyed into random coils under heated conditions [2].
After some time, the gelatin cools down and solidifies; in this process, the collagen reforms, and the initial protein structure is no longer achieved due to the disruption of the normal alpha-helix and beta sheets from denaturation [2]. Ergo, the protein now reforms in a random, tangled structure. Water is trapped in the middle of the long chain proteins in the process, turning the liquid state of protein into a semi-solid mass. The protein structure of a semi-solid mass remains the same even under room temperature.
Enzymes in Fruits
Catalytic enzymes are known to accelerate the breakdown of peptide bonds found in the link of long chain proteins by lowering the activation energy. With this, the links of long chain proteins break loose into smaller proteins. The trapped water from the bonds is then released, and the solution returns into liquid. This explained why the gelatin in the experiment became liquefied as it came in contact with the fruit samples.
Fruits are an abundant source of enzymes that aid in the digestion of ingested protein and carbohydrates. According to Kaur, et al. (2014) [3], bromelain is a sulfhydryl enzyme found in the fruit and stem of pineapple, renowned for its role in the promotion of remedying digestive disorders and as a natural meat tenderizer. It is an essential component in the proteolysis and digestion of protein in the human body. The proteolytic enzyme functions by attacking the internal peptide bonds of the protein chain [4]. To effectively function, the optimum pH of 7.1 must be reached. It is most stable from the pH ranges of 3.9 - 4.2, and its optimum temperature is 55℃ [5].
Aside from the diversity of functions bromelain offers, its proteolytic activity is known to be 10 times higher than that of papain [6]. Papain is another proteolytic enzyme obtained from the latex of raw papaya fruit (Carica papaya) [7]. Similar to the function of bromelain as a meat tenderizer and a digestive agent, papain also boasts the same abilities, as it breaks protein down into smaller fragments of amino acids and peptides [8]. Based on the research of Babu (2019) [9], papain is optimally active between a pH of 5 to 7.5 at 70 - 90℃.The proteolytic enzymes bromelain and papain were compared side-by-side based on their use as a meat tenderizer for squid (Loligo vulgaris) in Gokoglu, et al. (2017) [10], and results showed that better results were obtained from papain compared to bromelain.
Lastly, another fruit that contains an abundant number of enzymes is banana (Musa acuminata). The fruit is composed of amylases, which aid in the breakdown of complex carbohydrates into easier to absorb simple sugars in the body [11]. The maximum activity of amylase was found to be within the pH range of 6 - 7, and it is most active at 62℃ according to Kinsella & Mao (2006) [12].
Factors Affecting Enzyme Activity
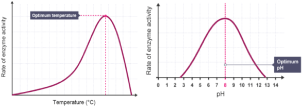
While it is true that enzymes work best in an internal environment that mimics the human body temperature (37℃), there still exist factors that influence enzyme activity. Enzymes have an optimum temperature at which they can work best. It is observed in the illustration in Figure 2 that as the temperature gradually increases, the rate of enzyme activity also increases. This is true, but only until the peak of optimum temperature is reached. At optimum temperature, the enzyme works at its fullest potential; however, as presented in the curvilinearity of the graph, the rate of enzyme activity continuously declines as the temperature increases and exceeds the optimum point. At this part, the initial shape of the active site of the enzyme becomes disrupted when exposed to extreme temperatures, disabling the attachment of a substrate to the enzyme’s active site.
Aside from temperature, enzymes also respond to changes in pH. An enzyme can undergo conformational changes when a change in pH is induced in its environment. This is explained through the charge in amino acids. Within the enzyme are positively- and negatively- charged amino acids that are attracted to one another. The shape of the enzyme is attributed to the attractive forces between the amino acids. When the pH is changed, the charges in the amino acids are affected; thus, the amino acids are no longer attracted to one another. As a result, the enzyme undergoes a conformational change - most often permanently - which produces a denatured enzyme [13]. Similar to temperature, the effect of pH depicts the same trends in the rate of enzyme activity. However, there is also an optimum pH in the graph at which the enzyme is likewise able to function most effectively, yet this is only true to a certain extent because the rate of enzyme activity encounters a decline once the enzyme exceeds its optimum pH measurement.
Methodology
Variables
Independent variable: Varying temperatures (5℃, 55℃, 75℃, 100℃)
Dependent variables: Change in volume (gelatin denaturation) (mL) (±0.5mL), change in pH (±0.01)
Controlled variables
1. The volume of the gelatin (mL) was evenly distributed to five empty beakers with a measurement of 60 mL in each.
2. The mass of the fruits (g) was measured on a digital weighing scale to be exactly 40g. This was done to prevent unequal measurements in gelatin denaturation. It was more reasonable to measure the fruits in mass (g) than in volume (mL) since the fruits had different consistencies after being blended - some were in chunks and some were more liquefied.
3. The room temperature condition was the control group in this experiment. It did not undergo any extreme temperature conditions and remained at room temperature at all times. It was placed far away from an external heat source. Hence, no changes were observed in the dependent variables under this condition.
4. To prevent unequal measurements in gelatin denaturation, the duration of fruit exposure in gelatin (hrs) was controlled. Once the fruit sample was poured into the gelatin, a timer was set at 1.5 hours.
5. The time of temperature exposure of fruit samples (mins) remained consistent throughout the experiment; all timers lasted for twenty (20) minutes.
6. To ensure that the gelatin’s response to fruit enzymes was not varied in all trials, the gelatin mix used in all samples - Knox unflavored gelatin - was standardized.
7. The fruit samples for all trials under each condition were prepared using the same fruit.
Materials
• 4 fruits (pineapple, orange papaya, green papaya, banana)
• 6 boxes of Knox unflavored gelatin
• 5 pieces of 100 mL beakers (±0.5mL)
• Oven mitts
• Mixing spatula
• Chopping board
• Kitchen knife
• Mobile phone as a timer (±1s)
• 4 in 1 pH meter (±0.01)
*This brand was chosen due to its automatic calibration feature.
• Tanita digital weighing scale (±0.1g)
*This brand was chosen due to its availability in the kitchen.
• Philips blender
• Oven
• Refrigerator
Preliminary Set-up
1. The initial mass of the beakers containing gelatin was pre-weighed to measure the mass of the fruit juice when it was later placed.
2. The gelatin mix was prepared separately in a large bowl, then poured into five (5) empty beakers at 60 mL. These were placed inside the refrigerator to solidify for three (3) hours.
3. While waiting for the gelatin to solidify, the fruit was sliced into smaller pieces then placed in a blender until it reached a liquid consistency. Following this procedure, the initial pH of the fruit juice was measured.
Procedure
1. Once taken out of the refrigerator, the gelatin samples were placed on the table at room temperature to cool down for thirty (30) minutes. This was to ensure that the cold temperature, which the samples were previously exposed to, did not become a confounding variable in directly affecting the gelatin denaturation.
2. While waiting for the gelatin to cool, the selected fruit was exposed to its respective condition for twenty (20) minutes. In the 5℃ condition, the sample was placed inside the freezer; for the following conditions, the samples were placed inside the pre-heated oven.
3. The fruit was left to cool down for thirty (30) minutes to ensure that its temperature did not have a direct effect on the gelatin denaturation. The pH of the fruit sample was then measured.
*The purpose of exposing the fruit to varying temperatures was solely for fruit enzyme denaturation; thus, there was no need to keep the temperatures constant throughout the experiment.
4. Next, the fruit juice was poured into the surface of the gelatin in five (5) beakers. Each beaker was representative of the five (5) trials under the temperature condition the fruit was exposed to.
5. A mobile phone was used to set a timer for 1.5 hours.
6. After the allotted time, the change in volume (mL) was measured.
7. After completing the experiment for the first five (5) trials, the same procedure was repeated for the following trials. Each fruit consisted of twenty-five (25) trials and there were five (5) conditions in total.
Figure 3: The set-up of gelatin with banana under 75℃
Figure 4: The set-up of gelatin with banana under 75℃ after 1.5 hours
Risk assessment
Safety Concerns: Since most of the samples needed to be exposed to high temperatures, oven mitts were worn when the oven was accessed to prevent getting burns. The assistance of a family member at home was needed while using the oven and using the knife to cut the fruits to ensure that all safety precautions were followed.
Environmental concerns: The samples were disposed of in the trash bin to lessen the chances of food contamination.
Ethical concerns: There were none surrounding this experiment.
Presentation of Raw Data
Table 1: Change in pH in all fruit samples under varying temperature conditions after 1.5 hours
Table 2: Change in gelatin volume (mL) in all fruit samples under varying temperature conditions after 1.5 hours
Observations
From temperatures 5℃ to 75℃ in all fruit samples, it was seen that the fruits penetrated through the gelatin surface and turned the affected part into a liquid-like consistency after the exposure. Figure 5 illustrated the complete disintegration of gelatin in response to the effect of pineapple, as seen in the color transition of gelatin from a translucent appearance to an opaque, bright yellow. In Figure 6, there was a production of effervescence in green papaya after a prolonged time, showing an indication of the release of oxygen from water due to the breaking of the hydrogen bonds in gelatin caused by the catalytic enzyme bromelain.
Processed data
To solve for the average changes in volumes and pH, this equation was used:
Table 3: Average change in gelatin volume (mL) in all fruit samples under varying temperature conditions after 1.5 hours.
Table 4: Average change in pH inall fruit samples under varying temperature conditions after 1.5 hours.
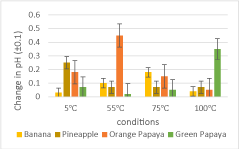
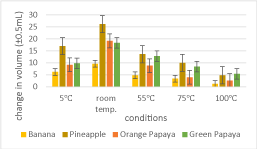
In Figure 7, it was demonstrated that at higher temperatures, enzymatic activity became weaker in denaturing gelatin because the rate of enzymatic activity declined after the optimum temperature, for each temperature has been surpassed. Based on the results of the experiment, the condition with the most active enzymes was at room temperature, and the lowest was at 5℃. Among all the fruits, pineapple had the most noticeable effects on gelatin denaturation; on the contrary, the fruit with the lowest enzymatic activity was in banana. In Figure 8, the trends seemed inconsistent in all fruit samples, as some increased then decreased and vice versa, whereas one continuously decreased across the increasing temperatures. This clearly illustrated that pH did not decrease as temperature increased.
Optimally active at 55℃, papain from papaya at 70℃ to 90℃, and amylases from banana at 62℃, all the fruit samples worked most effectively at room temperature. Bromelain was said to be most stable between 3.9 to 4.1 pH, and this was proven true in the study, as the pH of pineapple in all conditions approximately fell within the range. Between orange and green papayas, the latter resulted in a more denatured gelatin than orange papaya. Papain was expected to be optimally active between 5 to 7.5 pH, and this was confirmed in the study, as the pH measurements of the samples in all conditions fit within the supposed range. In banana, its pH ranged from 4.5 to 4.9, which did not coincide with the literature values wherein amylases were said to be optimally active between 6 to 7 pH.
Statistical Test
The error bars in Figures 7 and Figure 8 suggested that some data were more spread around the mean than others. Hence, a Shapiro-Wilk test was used to examine whether the samples (including outliers) were normally distributed to determine the appropriate statistical test to be used later. The tests on the average change in volume and the average change in pH were ran on Microsoft Excel.
Table 5 showed that all samples were normally distributed, where the values of W were found to be closer to 1, a predictor of the data being normally distributed. Aside from this, it was also observed that the p-value of all conditions was greater than the chosen σ level 0.05, hence the data being normally distributed. The same results were observed in Table 6 where the data was found to be normally distributed in all groups:
Due to the data being normally distributed, a one-way ANOVA test was conducted using StatPlus to determine its significance. This statistical test was chosen because there were numerous samples present, and the study only had one independent variable. Tables 7 and Table 8 below showed the results from the test on the change in volume (mL) and the change in pH. Due to the equal number of samples present in each group, the homogeneity assumption for the variances was ignored.
With an obtained p-value of 0.00338, which was less than the significance level of 0.05, Table 7 demonstrated that the data was significant. Based from Table 7, the 100℃ condition had the lowest standard deviation of 1.95 among the five groups, indicating that the differences in the condition were closer to one another. The room temperature condition resulted in a standard deviation of 6.80, which meant that the differences were larger in this condition. The same was observed in the mean - the 100℃ condition had the lowest mean (3.50) and the room temperature condition had the highest mean (18.4), indicating that the enzymes were able to work best under room temperature. With the data being significant at p < 0.05, and pineapple having the highest mean in the change in volume, the first alternate hypothesis for the change in volume was accepted. This result supported the notion that enzymes work best in an internal environment because it shares a close resemblance to the human body temperature (37℃), wherein they play a vital role in maintaining biological processes. It was therefore understood that pineapple, at room temperature, resulted to the most prominent disruption of protein chains in gelatin, which led to releasing trapped water from the bonds. This explained why its outcome produced the most liquid-like consistency as it came in contact with the fruit (See Figure 5).
The obtained p-value of 0.42462 provided in Table 8 proved that the data was insignificant, as it was greater than the significance level of 0.05. Among the five conditions, the 75℃ condition had the lowest standard deviation of 0.0624, indicating that the differences in the condition were closer to one another. The 55℃ condition had the highest standard deviation of 0.196, which meant that the differences in the condition were larger compared to the former. The same was observed in the mean - the 75℃ condition had the lowest mean (0.113) and the 55℃ condition had the highest mean (0.16), which meant that the largest decrease in pH values was observed in the latter. There were no observed changes in pH at room temperature condition because it was not exposed to any extreme temperature conditions. For the reason that the data was not significant at p < 0.05, the null hypothesis for the change in pH failed to be rejected. Thus, it was inferred that varying temperature conditions did not have a significant effect on the pH of all fruit samples.
Following this, the change in pH across the temperature conditions was graphed to better understand the trend present in the variables:
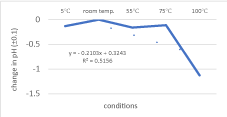
An inverse relationship between pH and temperature with a moderate correlation of determination (R2 = 0.5156) is presented in Figure 9. From 5℃ to room temperature, there was an increase in pH; as the temperature reached 55℃, the change in pH gradually decreased, and fairly increased towards 75℃. At 100℃, there was a drastic decline as the pH continued to decrease to -1.1275. Due to this, it was deduced that temperature did not have a significant effect on pH, but they shared an inverse relationship and a moderate correlation.
Evaluation
Conclusion
Based on the results of this study, the enzymes in all the fruit samples worked most effectively under room temperature, as this mimicked the normal body temperature at which enzymes become optimally active. Among all the fruits involved, pineapple demonstrated the most activity in denaturing gelatin, and banana with the least activity. A reason for the occurrence that bromelain resulted to a higher denaturing capability than amylases was that bromelain was a proteolytic enzyme known to digest proteins, whereas amylases were instead involved in the breakdown of carbohydrates into simple sugars. Moreover, at room temperature, it was implied that the enzymes remained unaffected as they did not denature or undergo any conformational changes at this condition due to the absence of extreme temperatures, hence the gelatin became more resistant to the action of enzymes. Due to this and with the data being significant, the first alternative hypothesis for the change in volume was confirmed by the data, insinuating that gelatin denaturation occurred the most in fruit samples exposed to room temperature condition, as this temperature provided an internal environment where enzymes function most effectively.
In measuring the change in pH, temperature did not have a significant effect on pH, so a strong relationship between the variables could not be established. Among the temperature conditions, the highest change in pH was found to be at 55℃ and the lowest at 75℃. Among all the fruit samples, the highest change in pH occurred in orange papaya; the lowest change, in banana. Hence, the second null hypothesis for the change in pH was refuted by the data. This suggested that varying temperature conditions did not have a significant effect on the pH of all fruit samples. However, it was still worth noting that temperature and pH shared a moderate inverse relationship (R2 = 0.5156), as demonstrated in Figure 9. To explain this, as the temperature increased, the subsequent molecular vibrations caused ionization to occur, hence more hydrogen ions were formed. As a result, the pH dropped ("How Does Temperature Affect pH?"), leading to an inverse relationship. Ultimately, the results showed that temperature still played a part in the measurement of pH.
Strengths
The experiment certainly had strengths due to it having several controlled variables in the procedure. There were 5 trials used in 4 fruit samples under each temperature condition, which ensured a more consistent representation of results. Although the measuring devices used in the experiment could not guarantee a high level of accuracy due to the presence of measurement errors, the uncertainty measurements for all apparatuses used in the experiment and the standard error in Figures 7 and Figures 8 still helped improve accuracy. Another strength would be the use of standardized samples from Knox unflavored gelatin, which stayed consistent throughout all the trials. This ensured that the gelatin in all samples reacted the same way to the fruit enzymes.
Limitations
A limitation to the procedure included the lack of appropriate measuring apparatuses due to it being a home-based experiment. A kitchen scale was used to weigh the fruit samples instead of a laboratory weighing scale, which could only measure the mass of the samples until the second decimal place. Using the latter would have lessened random and measurement errors due to its precision in detecting the mass of the sample until the smallest decimal. Also, it was worth noting that although the fruit samples came from the same batch, their ripeness was not assessed or measured before the experiment. Ethylene production in fruits causes ripening, and in this process, new enzymes are produced [13]. This poses a hindrance in acquiring more accurate data because the fruits may have been at different levels of ripeness, affecting the proteolytic activity of the enzymes in protein denaturation in the gelatin. This may have consequently resulted to the presence of outliers in the data, ergo affecting its significance.
Extensions
For future studies, experimenting in a laboratory setting is recommended due to access to appropriate apparatus. This would allow a more controlled environment for the variables, and using measurement tools with smaller uncertainties would lessen the percentage error in data. Because the temperature conditions used in the experiment were pre-set temperatures in the oven, the enzymatic activity under conditions closer to body temperatures (for example, 35℃ to 40℃) was not tested. This would have been an interesting take on understanding better the chemical processes occurring inside our bodies. Additionally, the gelatin denaturation should occurat constant temperature, unlike in this study, where only a 20-minute exposure was performed in denaturing fruit enzymes. Additionally, the selection of fruit samples may be expanded to a wider variety of fruits whose enzymes may also aid in digestion. Better yet, it would be a worthwhile opportunity to test the proteolytic properties that uncommon fruits may possibly contain. Most importantly, it would be advisable to quantitatively measure the ripeness level of the fruit samples beforehand to reduce possible confounding variables in the experiment. Perhaps this could be done by using a spectrophotometer to inspect the colored pigment molecules or light absorption intensity of the fruit’s skin as a means of determining its age.
References
- McIntosh J (2017) What is collagen, and why do people use it? Medical News Today.
- Denaturation of proteins.
- Kaur T, Kaur A, Grewal R (2015) Kinetics studies with fruit bromelain (Ananas comosus) in the presence of cysteine and divalent ions. J Food Sci Technol 52: 5954-5960.
- Wijeratnam S (2016) Pineapple. Encyclopedia of Food and Health.
- Bromelain. Creative Enzymes.
- Bromelain. National Center for Complementary and Integrative Health.
- Arvanitoyannis I, Varzakas T (2008) Fruit/Fruit juice waste management: Treatment methods and potential uses of treated waste. Waste management for the food industries.
- McDermott A (2018) 6 ways to use papain. Health line.
- Babu K (2019) The dyeing of silk. In. Papain. (2nd Edn). Science Direct.
- Gokoglu N, Ucak I, Yatmaz H, et al. (2017) Effect of bromelain and papain enzymes addition on physicochemical and textural properties of squid (Loligo vulgaris). Journal of food measurement and characterization 11: 347-353.
- Oswald C (2018) Digestive Enzymes: Amylase, protease, and lipase. Integrative Therapeutics.
- Kinsella JE, Mao WW (1981) Amylase activity in banana fruit: Properties and changes in activity with ripening. Journal of Food Science 46: 1400-1403.
- What happens in cells and what do cells need? Factors affecting enzyme denaturation.
- Koning R (1994) Fruit growth and ripening.
Corresponding Author
Dave Arthur R Robledo, International Baccalaureate Diploma Program (IBDP), Saint Jude Catholic School, Manila, Philippines.
Copyright
© 2022 Mae Chu M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.