A Review of Pyrethroid and Mitin Analysis in Environmental Matrices by Sample Extraction & Preparation, ELISAs with a Unique Focus on GC/NICI-MS
Abstract
Since the 1980s, pyrethroids, such as permethrin and cyfluthrin, have replaced other more intransigent and unreliable pesticides/insecticides as proofing agents for a range of applications, for example, to protect fabrics against moth invasion and bed net materials for employment in African countries in prevention of invasive mosquito attack.
However, these pyrethroids do present a small health hazard and care has to be taken during the process of application and handling, particularly, where the quantities applied are considerably above the levels allowed in environments occupied by or adjacent to human habitation, water, flora and fauna.
A number of analytical techniques have been used to monitor these pesticides, including chromatography mass spectrometry and ELISAs. Correlation between these techniques has been successfully investigated by researchers in the 1990s in relation to determining the levels of these pyrethroids in water courses, flora, fauna and in bed net materials. Parallel techniques have been used to analyse for mitins in environmental samples but employing LC/ESI-MS-MS.
This concise review outlines the earlier work and provides a brief summary of some of the more recent studies in the application of techniques since to highlight the research work undertaken with a unique focus on the utilization of GC/NICI-MS in conjunction with ELISA for pyrethroid analysis in environmental samples and for LC/ESI-MS-MS for mitins in water environments. Selected and representative figures and tables are featured in the papers described to highlight the results obtained.
Keywords
Pyrethroids, Mitins, GC/NICI-MS, LC/ESI-MS-MS, ELISA, Environmental matrix, Sample preparation, Quantification, Correlation
Introduction
Pyrethroids
The pyrethroids are recognized as a major class of synthetic organic insecticides. Since commercial production in 1976, this group of compounds has achieved world-wide use with widespread agricultural and health applications. Of the major classes, pyrethroids as a group are among the most potent as insecticides but have been considered to exhibit comparatively low toxicity to mammals.
The pyrethroids reviewed here are broad spectrum synthetic insecticides with a greater photostability, enhanced insecticidal activity and low mammalian toxicity. Their effectiveness against a wide range of insects at extremely low dosage and limited persistence in the environment stimulated interest for use in agriculture around the world. At the same time, many countries have conducted research on the residual effects of these compounds on the environment after use. Contamination of fresh water ecosystems by pyrethroids occurs either due to the direct discharge of industrial and agricultural effluents or through the effluents of sewage works where it is accumulated by the surrounding biosphere. The concentrations that have been measured in the soil suggest that this may be a serious problem in the areas to which such effluent is discharged. In the UK, recommendations have been issued for the safe use of some pyrethroids. The Food and Agricultural Organisation and WHO have prescribed residue limits for some pyrethroids in the area of agricultural and livestock products [1-3]. Other references provide data on the environmental quality standards (EQS) for pyrethroids [4].
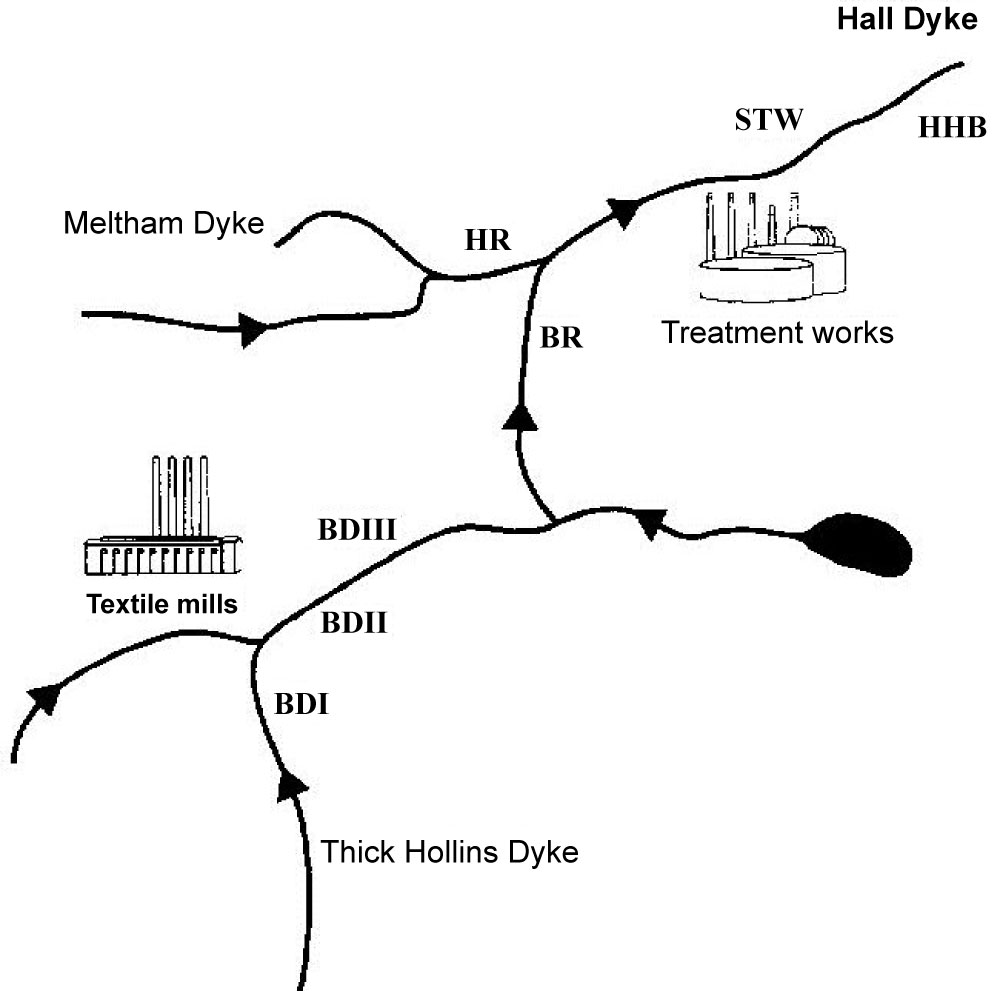
Pyrethroids were introduced to replace Eulan WA New (a complex mixture of polyamino diphenyls, PADs, and polychloro sulphonamides, PCSDs) as mothproofing agents, which was detected and monitored in water courses by the National Rivers Authority, NRA (now Environment Agency, EA) resulting from effluent streams. A prime example, of the application of environmental monitoring was in the carpet industry, for example, in the vicinity of the Meltham catchment, West Yorkshire, UK. As a result there was a need to monitor the pollution of streams and rivers in the vicinity as considerable migration occurred from the factory because of leaching from the fabric into wooden flooring into which pyrethroids had been adsorbed. The relative persistence of pyrethroids because of lower biodegradability also presented some difficulties. The monitoring required involved sampling stream water, sediment, flora and fauna.
Methods for analysis of pyrethroid insecticides in a variety of matrices have previously been presented and reviewed. Most of these analytical procedures in the early work used GC-ECD and employed a variety of clean-up stage, see for example Bolygo and Zakar [5]. Jenkins, et al. [6] have reported the development and validation of a universal HPLC method with an update of references.
The methods have been applied to analyse these insecticides in water, crops and vegetables. The current review provides an extension of this early work to emphasize how the methodology had advanced in the 1990s to 2000s, and since, to demonstrate the validity of the more recent research work, in the light of current circumstances.
Mitins
The pattern of mothproofing agents has changed since the 1970s. Dieldrin was shown to be highly toxic to mammals and very persistent in the environment and replaced by formulations based on substituted ureas, urons.
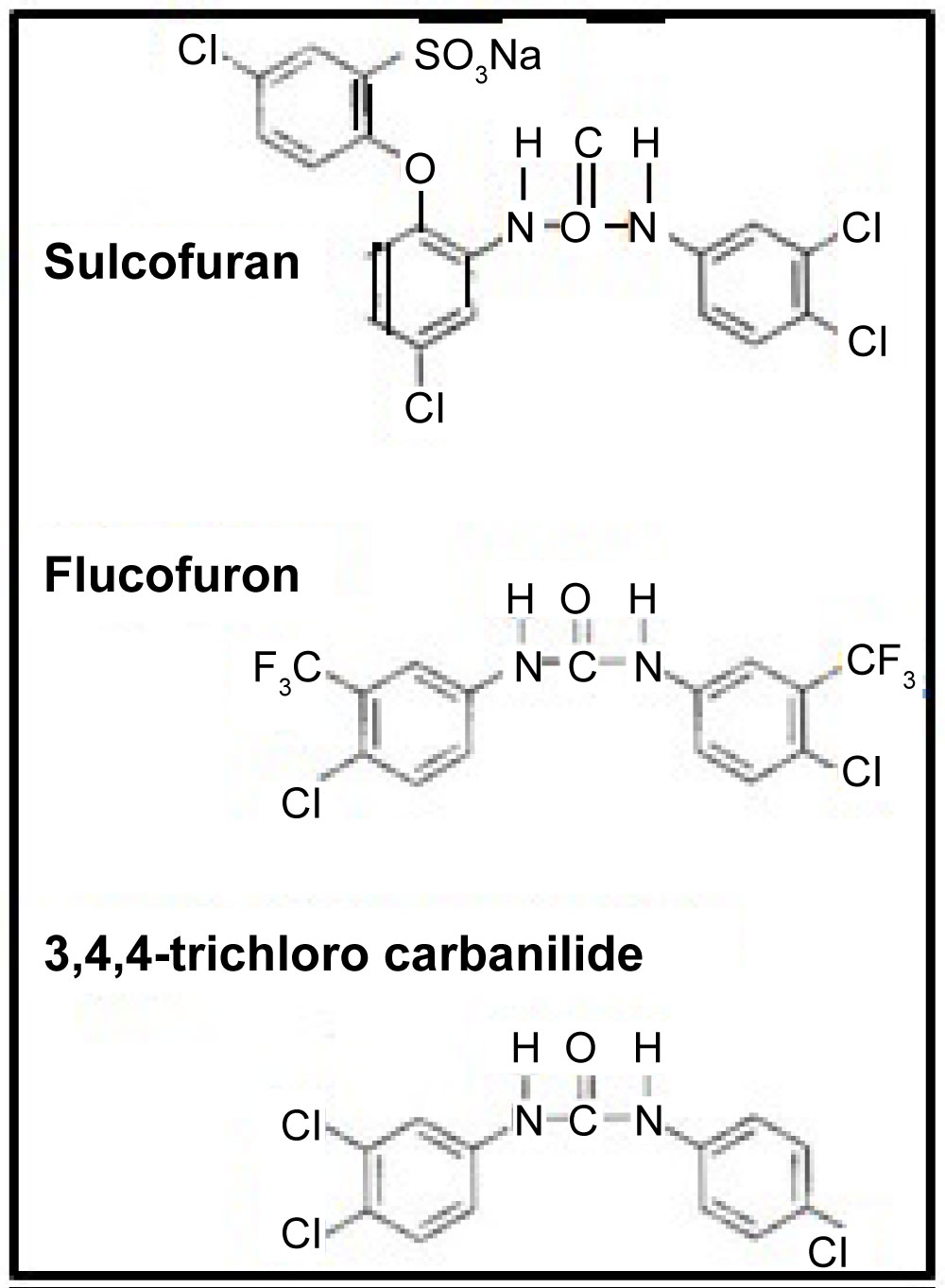
The term mothproofing described the treatment of wool or wool-based fabrics to prevent damage by larvae of a number of insect pests that are capable of digesting keratin. Sulcofuron and flucofuron, as examples, exert their toxic effect on the target organism by inhibiting the synthesis of the enzyme required to break down keratin. These agents bond to wool fibres in the same way as dyes and are added in the process of dyeing. Restrictions apply to the formulations and in addition to being toxic, these must be stable to the application conditions, resistant to washing and light and effective for prolonged periods of the textile. Mitin is the trade name for the agent, which was produced by Ciba Geigy (Basle, Switzerland) and was marketed in the UK under Mitin FF (80% sulcofuron) and Mitin LP (7.6% flucofuron).
Regulations designed to limit the pollution of surface waters were introduced in the UK in 1993. Contamination of freshwater eco-systems occurs directly owing to the discharge of industrial effluents or indirectly through the discharge from sewage treatment works. In tests on activated sludge it was found that mitins were strongly absorbed akin to their persistence in the environment. The environmental quality standards (EQS) for sulcofuron and flucofuron in fresh water required to support fish are 25.0 and 1.0 µg l-1, respectively [7,8].
The work in this review also focuses on the use of liquid chromatography with negative ion electro spray ionization tandem mass spectrometry (LC/ESI-MS-MS) for the determination of sulcofuron and flucofuron and the development of LLE, and SPE techniques for the determination of the analytes in surface waters, employing trichlorocarbanilide as an internal standard. The methods were successfully applied to samples obtained from a contaminated ecosystem.
Tadeo in 2019 has reviewed the analysis of pesticides in food and environmental samples [9].
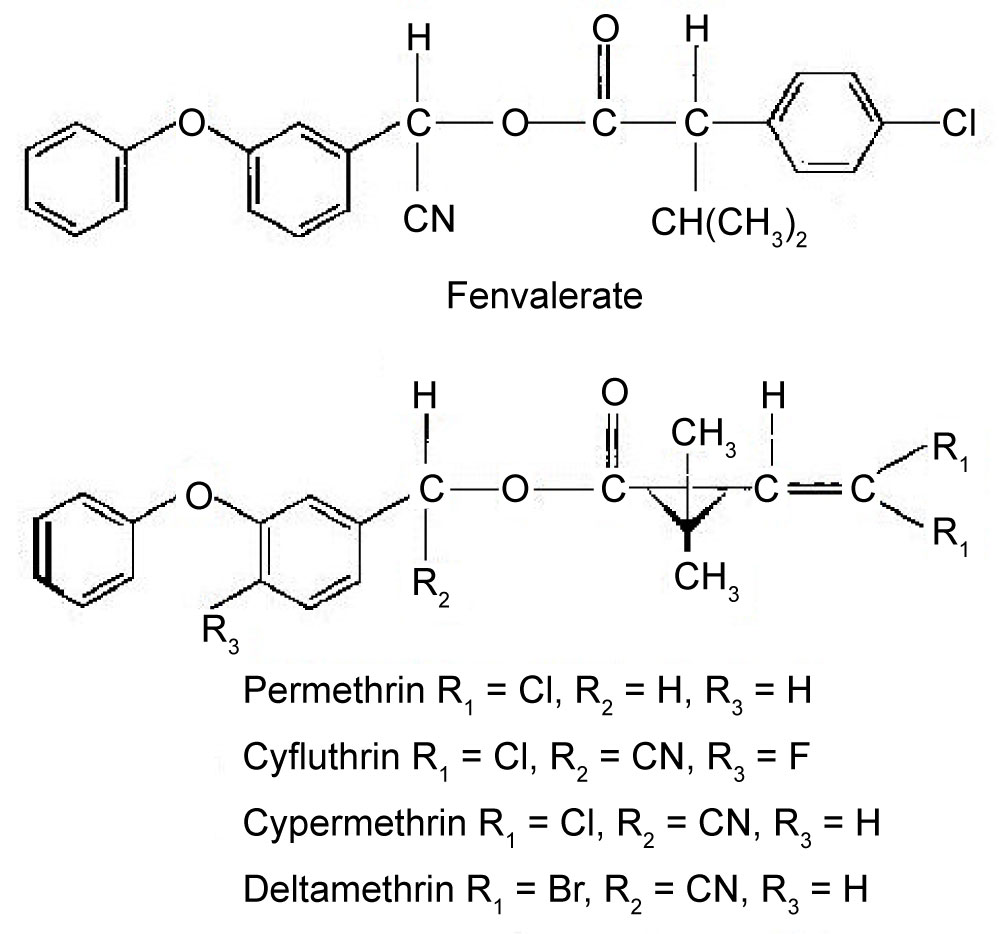
The structures of permethrin, cyfluthrin, cypermethrin, deltamethrin, and fenvalerate, are illustrated in Figure 1.
The following section describes the methodology used and the published results of studies undertaken in the period 1990 to 2003 by different researchers in the Departments of Chemistry and Biological Sciences at the University of Salford. In the last section, a summary of the advances in the application of these methodologies in the period 2003 to date is made. The full report of the project is contained in Mothproofing Agents and Water Course Management (1996) R & D Project Record [10].
Research Work at the University of Salford, UK
ELISA development
Elisas were developed specifically for a selected group of pyrethroids (viz., permethrin, cyfluthrin) and mitins like sulcofuron and flucofuron.
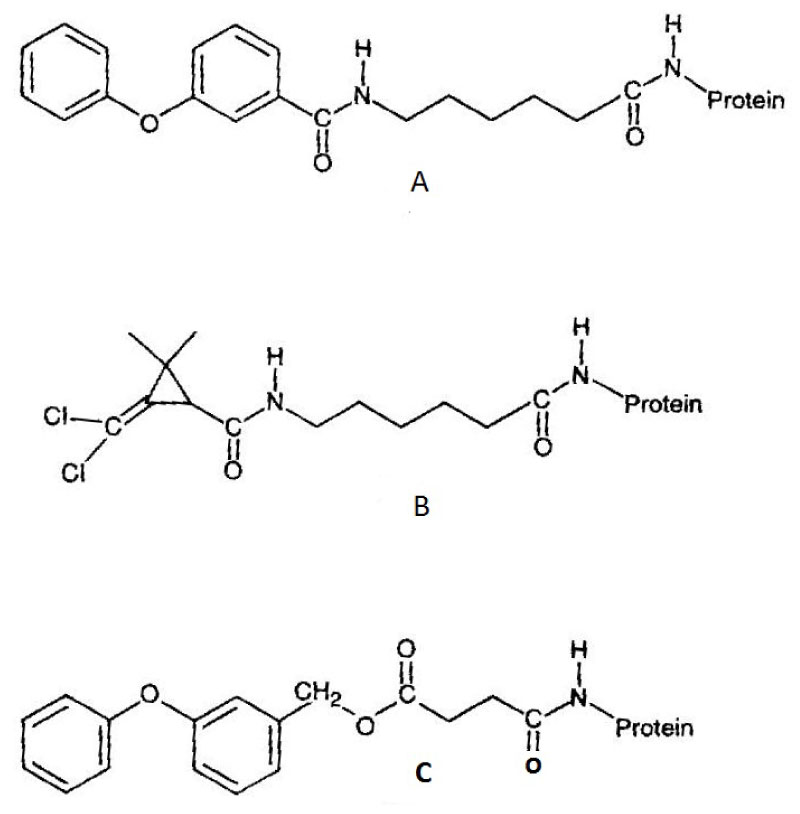
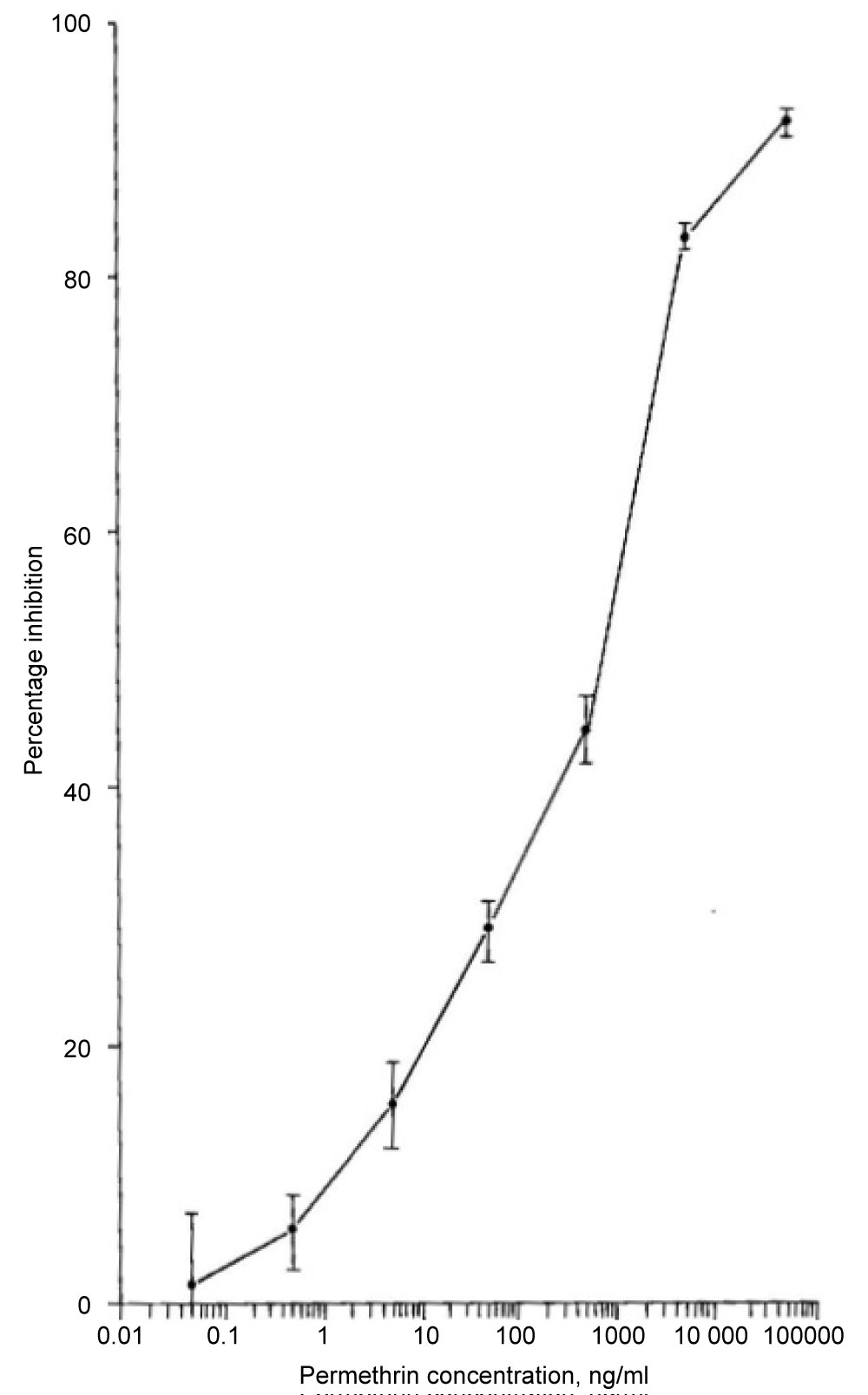
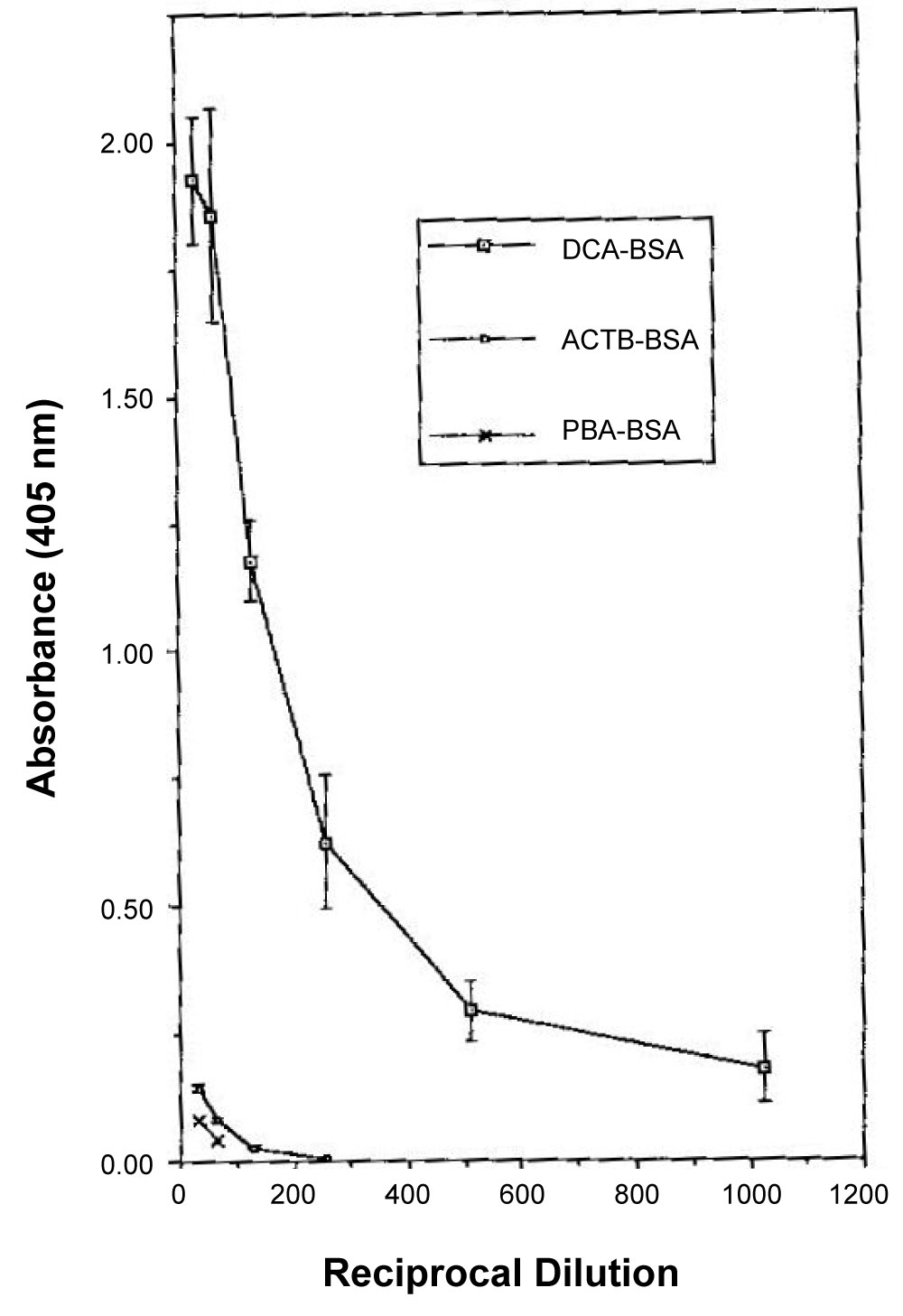
pAbs: Polyclonal antisera were produced in rabbits against two different synthetic immunogens, one of which incorporated 3‐phenoxybenzoic acid (PBA) while the other contained dichlorovinyl cyclopropane carboxylate (CPA) [11]. The immunogens were constructed such that the hapten was coupled to the carrier protein through a peptide bond to a six carbon spacing group (6‐amino hexanoic acid, 6‐AHA). Both the anti‐PBA and anti‐CPA antisera obtained were able to detect permethrin when used in an indirect competitive enzyme‐linked immunosorbent assay (IC‐ELISA) format (see Figure 2). The detection limits typically obtained with both antisera were 10 mg l‐1 with 50% inhibition of antibody binding (ho) at 100 mg l‐1. Cross‐reactivity with the pyrethroids cyfluthrin, phenothrin and deltamethrin was observed for both antisera, the degree of which was related to the structural similarity of the compound to the immunizing hapten. Further development of the immunoassay for permethrin was examined through use of the anti‐PBA antiserum. Assay performance was improved by negative immunoaffinity chromatography of the anti‐PBA antiserum, in which antibodies directed against the six carbon spacing group were removed. In conjunction with an avidin‐biotin amplification step, typical detection limits were 1 mg l‐1 with an I50 value of 15 mg l‐1. Assay performance was considerably enhanced by use of a microtiter plate coating antigen which possessed a four carbon spacing group between the hapten and carrier protein. The hapten was also coupled to the spacing group through an ester bond. Typical detection limits for permethrin were 0.5 µg l‐1, with an I50 value of 1 mg l‐1 (Figure 3). This assay was also unaffected by the inclusion of methanol at concentrations of up to 10% by volume. The study indicated the potential usefulness of antibodies raised against compounds which mimic moieties present within larger hapten molecules (anti‐hapten mimic antibodies), particularly where the target analyte is not amenable to direct conjugation to a carrier protein.
mAbs: Monoclonal murine anti-pesticide antibodies were produced by in vitro immunisation (IVI) of cultured splenocytes with the pesticides sulcofuron and flucofuron [12]. The majority of both anti-flucofuron and anti-sulcofuron antibodies obtained were of the IgM isotype, rather than IgG. When used in an indirect enzyme-linked immunosorbent assay (ELISA), the antibodies bound to plate coating antigens which incorporated haptens that mimicked moieties present within the immunising pesticide. The antibodies exhibited a high degree of specificity, with the degree of cross-reactivity related to the structural similarity between the hapten present in the plate coating antigen and the moieties present within the immunising pesticide. These results indicated that antibodies specific to both sulcofuron and flucofuron had been produced by IVI. Figures 4 and Figure 5 illustrate the IC elisa response vs concentration for flucofuron and sulcofuron, respectively Synthesis of both hapten analogues and immunogens as required for methods based on in vivo immunisation was avoided, whilst antibody production was also comparatively more rapid than traditional methods and minimised animal discomfort.
Gas chromatography/Mass spectrometry analysis in negative ion chemical ionization mode (GC/NICI-MS): Formation of negative ions as diagnostic fragments
GC/MS analysis in NICI mode was used for the analysis of pyrethroids because the negative ion observed is specific to the target analyte of interest and more so than for electron Ionisation (EI), which produces the considerably less diagnostic phenoxybenzene carboxylate positive ion fragment. Also cis and trans isomer negative ion fragments and isotope ratio profiles assist in positive identification of the target analytes. Quantisation of the ions is conducted using these isomer and isotopic ratio profiles. Furthermore, the sensitivity is enhanced over EI-MS, which assists in complying with detection limits as required by legislation. The general reactions for the formation of negative ions are as follows:
Reagent gas reactions CH4 + e- → CH4+ 2e- ; CH4+ + CH4 --> CH3 + CH5+ PICI mode
Pyrethroid target ROR' + e- → [ROR']-- → R + [OR']-- NICI mode, dissociative electron capture
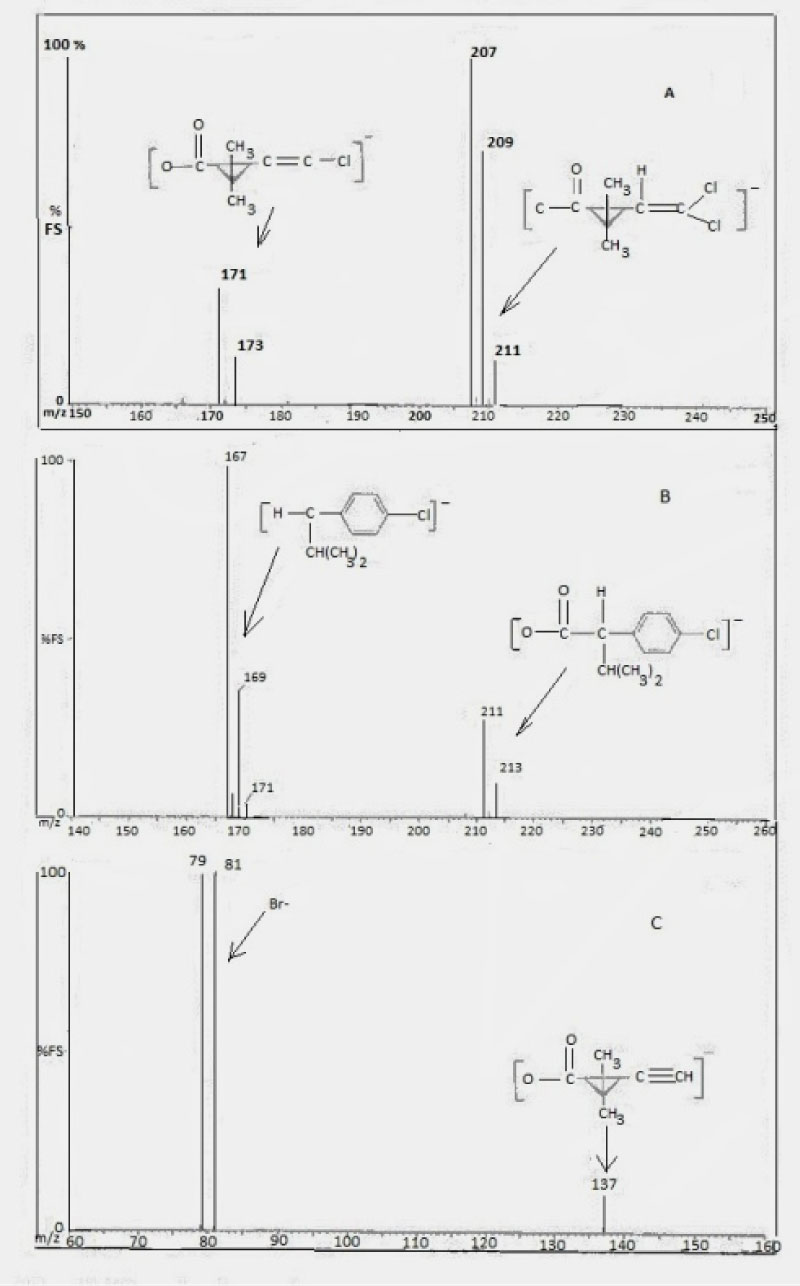
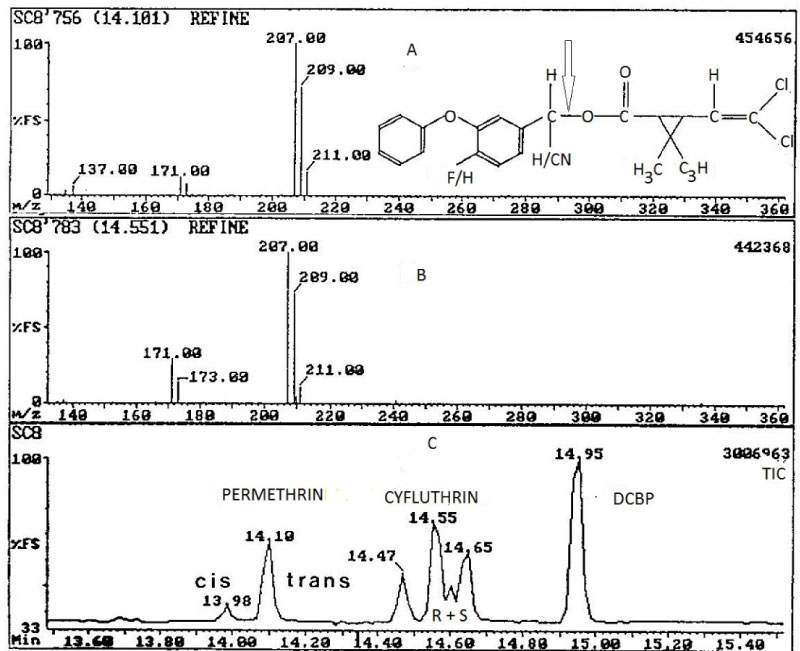
Where [OR']-- is the diagnostic negative ion fragment for the target pyrethroid for which the structures are of the form, R''dimethyl cyclopropane carboxylate anion, m/z 207, 209, where R'' is dichloro vinyl end group for permethrin, cypermethrin and cyfluthrin pyrethroids being considered. Deltamethrin, undergoes dissociative electron capture to produce the isotopic profile for Br- [13,14] although m/z 137 [15] observed can also be utilized in analysis (dissociation energy dependent). λ-Cyhalothrin yields m/z 241, 243 [15] and fenvalerate, m/z 211, 213 [13-15]. Figures 6, Figure 7 and Figure 8 show the mass spectral and chromatographic profiles for the pyrethroids investigated (Table 1).
ELISA and GC/MS correlation
Comparative studies were conducted to correlate Elisa and GC/MS data to determine the effectiveness of Elisa for permethrin analysis at concentrations above the maximum residue level (MRL). The following paragraphs describe the development of comparative analytical for permethrin [16] and an application to fish sampled from headwater stream [17].
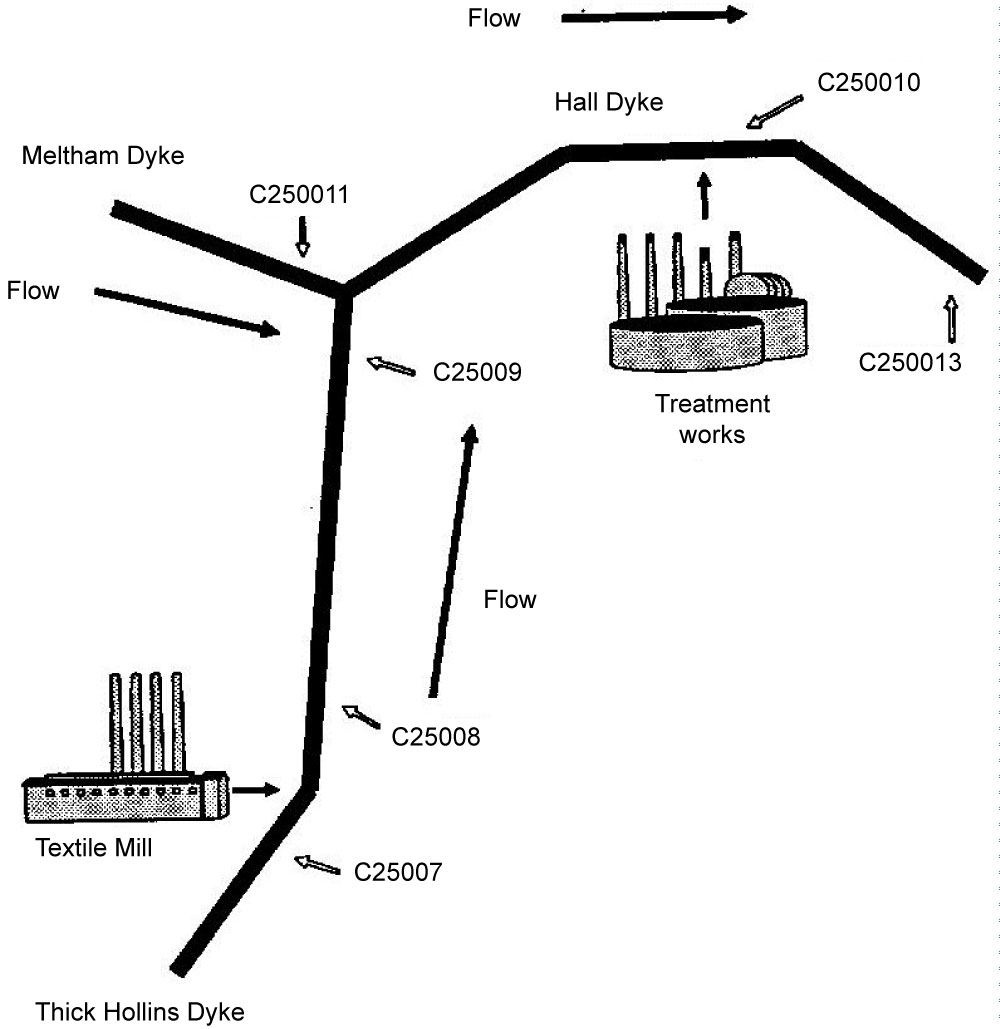
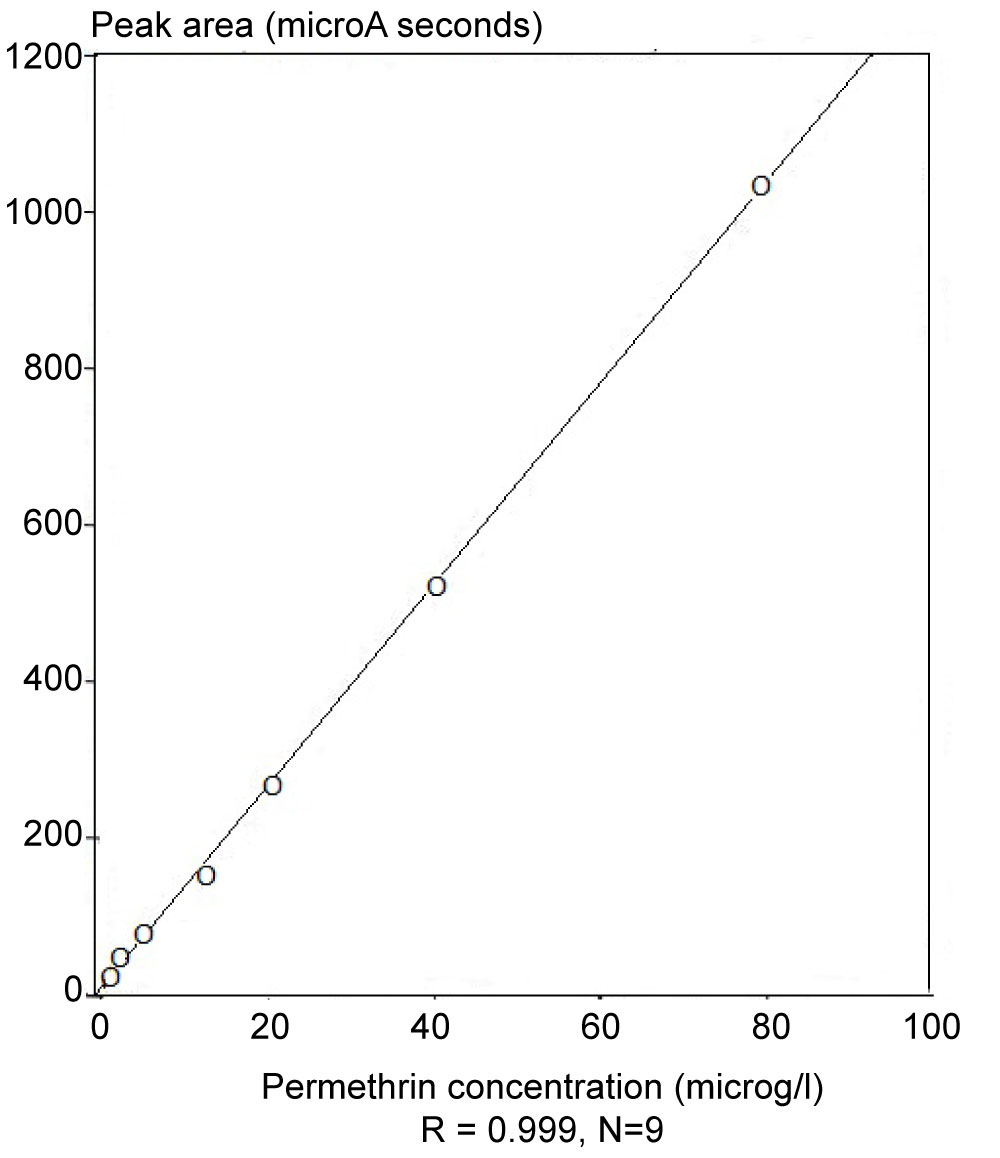
The synthetic pyrethroid permethrin has widespread use in agriculture and, as a result, is found in a variety of matrices. Chemical methods based on GC/MS operated in NICI mode (GC‐NICI/MS) have been developed [16] Sample extraction techniques, such as, ultrasonication and steam distillation have also been examined. Significant improvements have been made, compared with traditional methods, in both sample extraction time and detection level for a range of matrices. Enzyme‐linked immunosorbent assay (ELISA) methods have also been developed for permethrin (refer to Figure 3, section 2.1.1, for the IC-ELISA calibration curve for permethrin). This study addressed the comparison of ELISA and chemical assays for permethrin in a variety of both environmental and laboratory‐spiked samples. The sensitivities, specificities and potential applications of these methods were discussed. Figure 9 illustrates the high quality data for the calibration of permethrin in the concentration range, 0 to 80 µg l-1, using GC/NICI- MS. Figure 9 illustrates the location of the sampling sites and Tables 2, Table 3 and Table 4 summarise the data correlation between GC/NICI-MS and ELISA for permethrin in water, sediment and white muscle from fish.
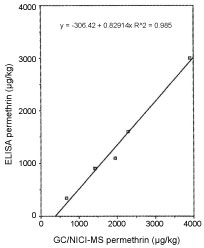
Brown trout (Salmo trutta) were captured from a headwater stream contaminated with moth‐proofing agents [17] (Figure 10 and Figure 11). Synthetic pyrethroid residues within the tissues were obtained by ultrasonic extraction and examined by GC/NICI‐MS. Both permethrin and cyfluthrin had been bioaccumulated by the fish and the mean concentrations ± standard deviation recorded were 2046 ± 203 µg kg‐1 and 25.4 ± 46.9 µg kg‐1 respectively. The concentrations of these pyrethroids are the highest recorded for freshwater fish captured from a natural ecosystem. At the time of sampling, permethrin could be detected within the stream water (0.034 µg l‐1) while cyfluthrin could not. A mean relative bioconcentration factor of approximately 60,000 was derived for the brown trout which was also greater than any previously determined for freshwater fish. The fish tissue extracts were also analyzed through use of an ELISA for permethrin. The ELISA reported consistently lower concentrations than those obtained by GC/NCI‐MS. This was attributed to analyte losses during preparation of the sample extracts in a form suitable for incorporation into the ELISA. The concentrations obtained by ELISA were significantly correlated with those obtained by GC/NICI‐MS (R2 = 0.985, n = 5). Overall, the ELISA appeared to be suitable for the detection of permethrin in fish when present at concentrations in excess of the MRL suggested for many foods by the Commission of the European Economic Community (Table 5).
Unpublished results presented as posters at meetings [18,19] focused on the correlation between Elisa and GC/NICI-MS for determining permethrin residues in mosquito net materials [20]. Approximate linearity in the correlation was observed for the concentrations determined.
GC NICI-MS development and Applications
Methods were developed for pyrethroids in environmental matrices. GC-NICI-MS with selected-ion monitoring (SIM) showed both high sensitivity and excellent specificity for permethrin and cyfluthrin [21].
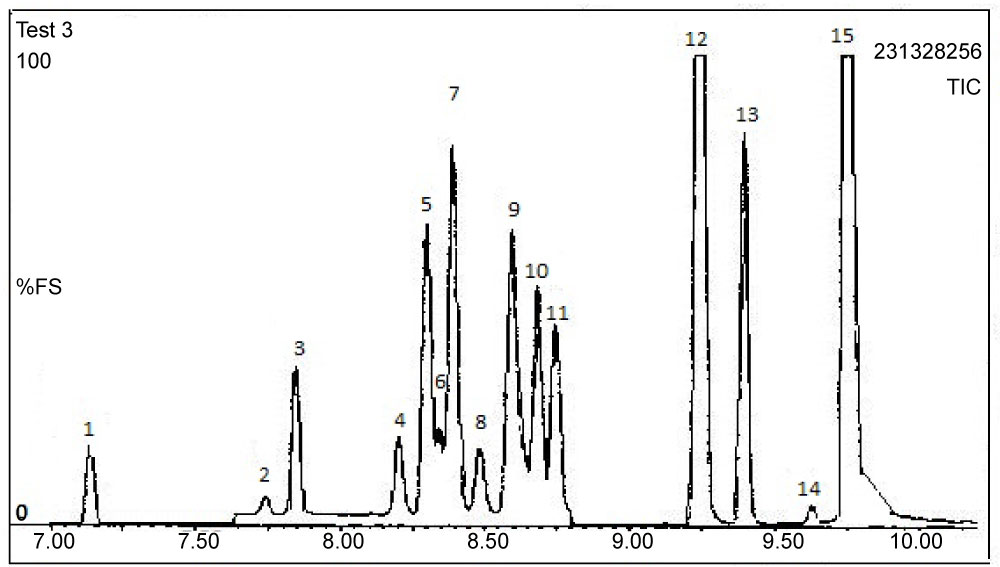
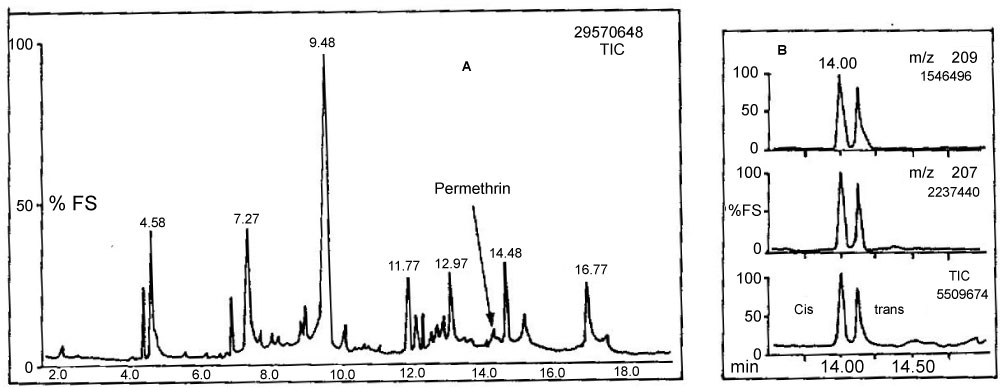
The detection limit for both permethrin and cyfluthrin was 50 fg, whilst a linear response was observed from 50 fg to 80 pg. A sample extraction method using an ultrasonic bath was developed enabling simultaneous processing of multiple samples. Good percentage recoveries of both permethrin and cyfluthrin from spiked sediments were obtained (97.3 ± 4.8% and 93.9 ± 5.3%, respectively) and sample clean-up was avoided. These methods were also successfully applied to samples obtained from a contaminated ecosystem, the highest concentrations recorded in water and sediment samples were 0.048 µg l−1 and 335 µg kg‐1, respectively. Figure 12 illustrates the NICI mass spectra and the TIC for cis/trans permethrin and R & S isomers for cyfluthrin. Tables 6 and Table 7 summarise the recoveries for the two analytes from spiked water and sediment samples, respectively. Table 8 shows the data for permethrin and cyfluthrin levels in water and sediment samples from sites A B and C in the River Calder cartchment, West Yorkshire, UK.
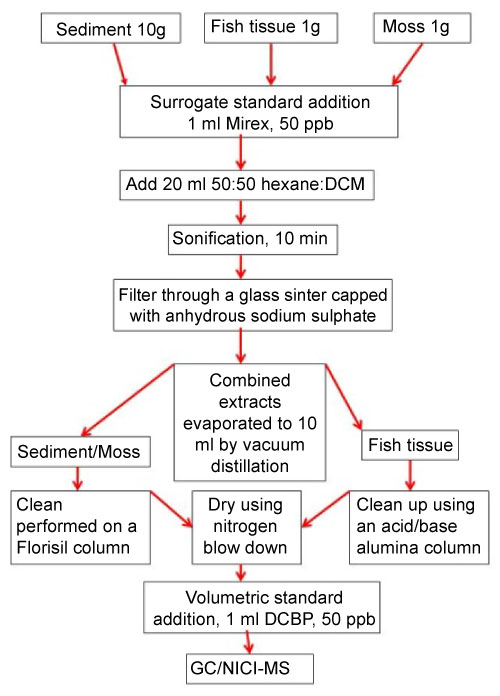
A GC/MS method was developed for the simultaneous determination of five synthetic pyrethroid insecticides, namel, permethrin, cyfluthrin, cypermethrin, fenvalerate and deltamethrin, in soil, moss and fish tissue [13]. These pyrethroids were extracted with hexane‐dichloromethane by ultrasonication and cleaned up on Florisil (soil and moss) and mixed acid/base alumina (fish) columns prior to determination by GC/NICI-MS in SIM mode. All the pyrethroids were analyzed simultaneously in a single run on a DB5‐MS 15 m capillary column. Recoveries of the pyrethroids from the three matrices at fortification levels of 10, 50 and 100 µg/kg ranged from 80 to 117%. Four determinations were made at each concentration level for each matrix. The practical determination limit of the method was in the range 0.5 to 5 µg/kg depending on the compound. This method was also applied to samples obtained from a contaminated ecosystem.
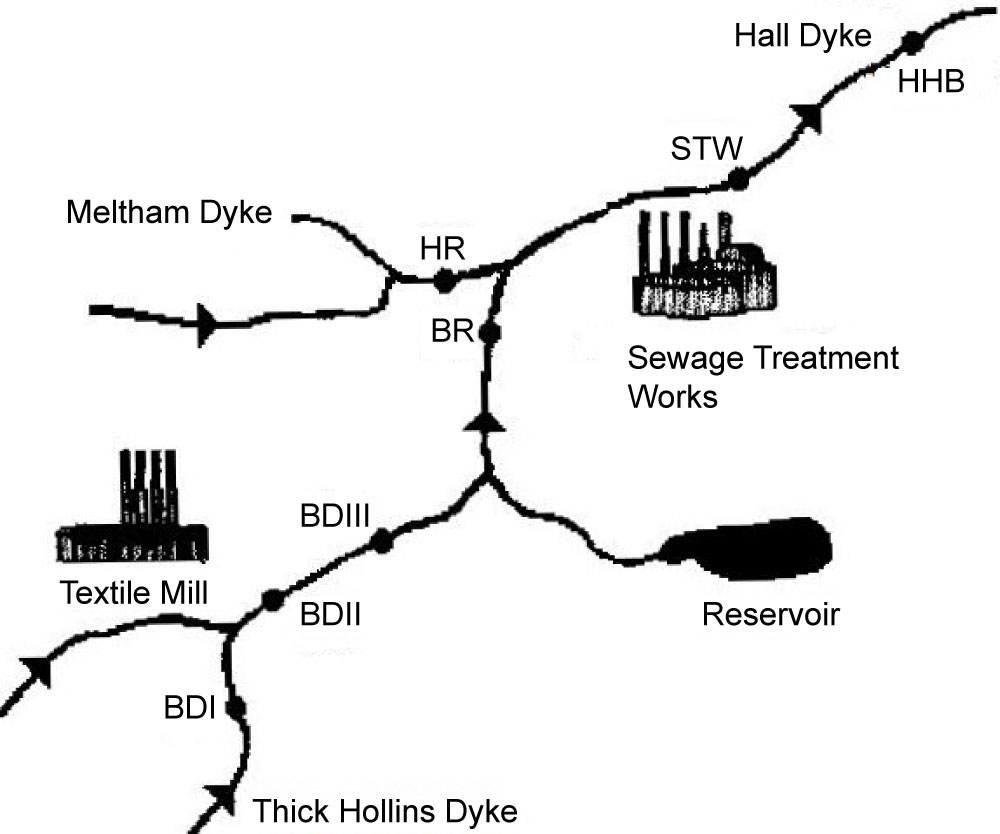
Figure 13 shows the location of the sampling sites in the Meltham Catchment, West Yorkshire, UK. Figure 14 illustrates the scheme for sample preparation for analysis by GC/NICI-MS. Tables 9 and Table 10 summarise the recoveries of pyrethroids for different solvent system used and fortification levels. Tables 11A and Table 11B summarise the levels of permethrin and cyfluthrin, A in sediment and moss from the catchment sites and B in fish for a selected site HHB. Table 12 summarises the levels of the five pyrethroids (µg/kg) in fish samples from the River Calder at selected sites.
An effective analytical method for the simultaneous determination of five synthetic pyrethroid insecticides in soil was developed and method performance data presented [14]. The pyrethroid residues were extracted with hexane-dichloromethane in an ultrasonic bath. The extract was cleaned up on a Florisil column prior to determination by GC-NICI-MS in SIM mode. The highest detection sensitivities were achieved in the SIM mode where the instrument was adjusted to collect only a few ions which were indicative for the compound to be searched for, instead of scanning the entire spectrum over the whole mass range. The gain in sensitivity was the result of longer specific sampling times for each of the ions selected. Recovery studies were performed at 10, 50 and 100 ppb fortification levels of each pyrethroid and of the internal standard, mirex, and the percentage recoveries ranged from 81.7 ± 4.2 to 108.2 ± 2.6%. Four determinations were made at each concentration level along with a procedural blank. The quantification limit of the method was in the range of 0.012 to 4.4 ppb, depending on the compound. This method was also applied to sediment samples collected from the environment of a River Catchment which was monitored for the presence of target pyrethroids. Figure 15 illustrates the quality of the linearity for the calibration of the concentrations of permethrin, which provides a prime example of the reliability and sensitivity of GC/NICI-MS employed in these studies. Table 13 summarises the performance data for calibration of the pyrethroids and limits of detection (LOD). Table 14 summarises the levels of permethrin (cis and trans) and cyfluthrin in sediments from sites 1 to 7 in the Meltham catchment, West Yorkshire, UK.
An analytical method based on GC combined with NICI-MS in SIM mode has been optimized for the simultaneous determination of five synthetic pyrethroid insecticides in water, sediment, moss and fish tissue [22]. The pyrethroids were originally extracted from the environmental matrices by steam distillation extraction (SDE) but this has since been superseded by liquid-liquid extraction (LLE) for water samples and ultrasonic extraction (USE) for solid matrices with particular attention for solvent selectivity. Recovery studies were performed on all the environmental matrices for each pyrethroid and the internal standard, mirex. The percentage recoveries ranged from 80.6 ± 5.6 to 116.2 ± 4.3, where n = 4. The limit of quantitation for the method was determined statistically from the linear calibration curve, giving values in the range 0.01 to 4.4 pg of material introduced into the system depending on the pyrethroid in question. The method was applied to environmental samples collected from a contaminated river ecosystem currently being monitored for the target analytes. The limit of detection (LOD) formulation for calculating the LODs for pyrethroids reviewed and Table 15 summarises the instrumental detection limits for the respective pyrethroids. Table 16 shows the recoveries of two selected pyrethroids, permethrin and cyfluthrin, employing three different extraction techniques. Table 17 summarises the recoveries of the pyrethroids reviewed from water, sediment, moss and fish tissue at realistic fortification levels using LLE for water and the preferred technique, USE, for solid matrices.
Applications to other targets: Mitins using liquid chromatography combined with negative-ion electrospray ionization tandem mass spectrometry (LC/ESI-MS-MS)
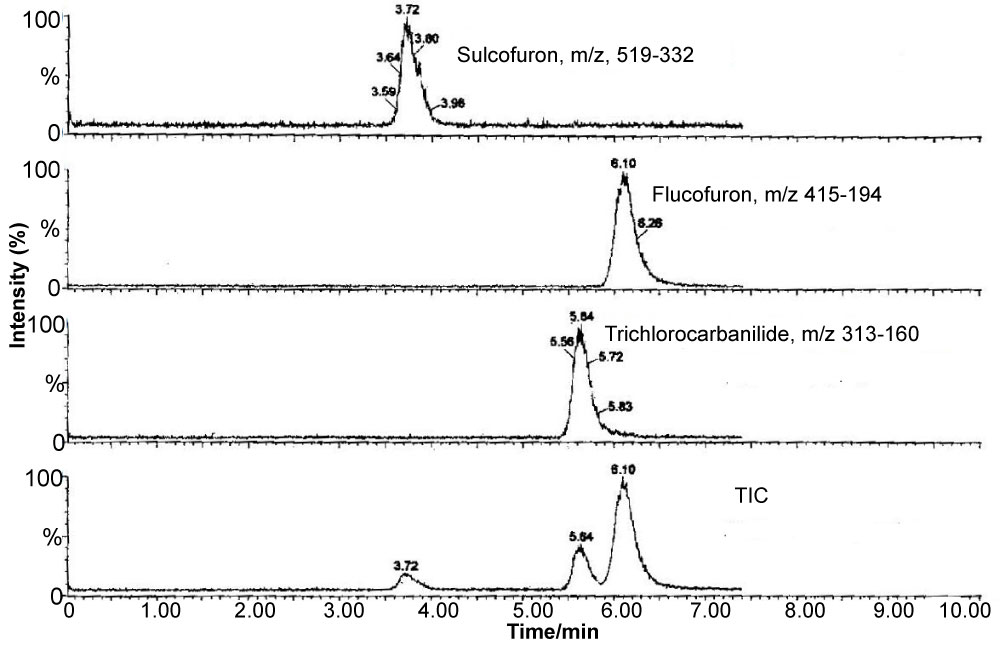
Flucofuron and sulcofuron, both examples of mitins, were employed as the active ingredients in mothproofing formulations for the protection of textile fabrics by the dyeing industry. Monitoring of their presence in components of the river ecosystem is a regulatory requirement so precise extraction techniques, combined with sensitive detection systems, are required to obtain valid data concerning the levels of target pollutants present. This study [23,24] continued the development LC/ESI-MS-MS operated in the multiple reaction monitoring mode for the determination of these analytes in complex matrices. The paper describes the development of liquid-liquid extraction (LLE) and solid-phase extraction (SPE) techniques for the determination of the analytes in environmental river water. The methods employed an internal standard, trichlorocarbanilide (TCC), to check the extraction efficiencies but not to correct environmental data. The extraction efficiencies obtained with LLE were 73.2 ± 6.7, 112.4 ± 8.6 and 96.4 ± 14.3% (n = 5) compared with 74.3 ± 8.4, 115.9 ± 3.1 and 112.7 ± 4.5% (n = 4) employing SPE for sulcofuron, flucofuron and TCC (100 ng l–1 matrix fortification level), respectively. The SPE results are consistent with those obtained for LLE, although the precision of the SPE method was better than that of the LLE method. These methods were then successfully applied to samples obtained from a contaminated ecosystem. Figure 16 illustrates the structures of the mitins and the standard. Figure 17 shows typical LC/ESI-MS-MS multiple reaction monitoring (MRM) reconstructed ion traces and TIC for sulcofuron, flucofuron and trichlorocarbanilide standard. Figure 18 illustrates the sampling sites in the Meltham catchment of the River Calder, West Yorkshire, UK. Table 18, Table 19 and Table 20 summarise the data obtained for the two mitins investigated.
Sorption - desorption equilibria for pyrethroids in soils
Several related studies were conducted to establish sorption desorption equilibria for pyrethroids in soils, the first investigating the suitability of binary solvent systems for establishing the recoveries of pyrethroids from soils and the second a study of the sorption - desorption equilibria in soils.
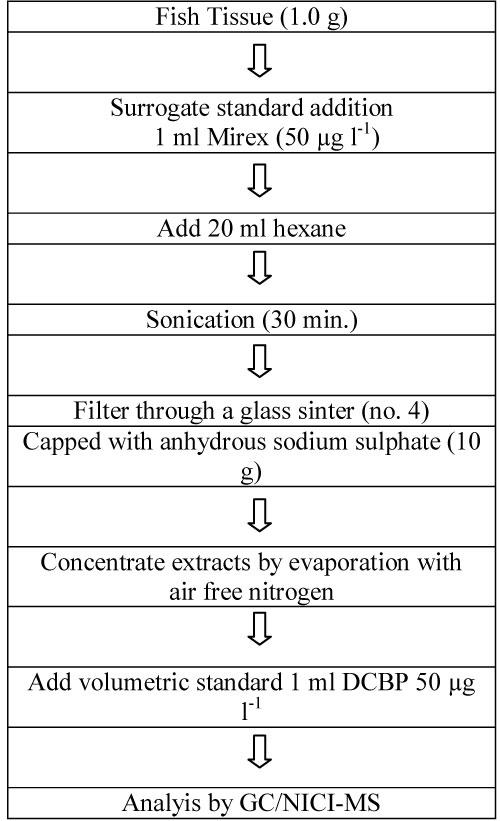
Development of a binary solvent system for analysis of pyrethroids on soil: Ultrasonic extraction was used to develop a suitable binary solvent system for the analysis of synthetic pyrethroid pesticides and mirex on soil [25]. The analysis was carried out by GC/NICI-MS). In the initial experiments, accurately weighed soil samples were spiked with a mixture of standard solution pyrethroids and mirex and shaken for 24 h to ensure homogeneity, then extracted with solvent. The extracts were evaporated to dryness before the volumetric internal standard was added.
The binary solvents used in this study were various mixtures of hexane: acetone, hexane: dichloromethane (DCM), isooctane: acetone and isooctane: dichloromethane, representing different classes of polarity. The recoveries of all pyrethroids and mirex were satisfactory over three solvent systems, but results of isooctane: DCM produced low recoveries. The average recovery increased with the extraction time, but the increase was not statistically significant. A 30-min optimum extraction was deemed sufficient for recovering pyrethroids from soil. After 30 min, extraction decreased owing to the re-distribution of the analyte on the soil matrix.
Table 21 summarises the percentage recoveries of six pyrethroids using different solvent systems and compositions at 1 mg l-1 concentration level.
Sorption-desorption equilibria for pyrethroids on soils: Sorption-desorption equilibria of six pyrethroids (permethrin, cyfluthrin, cypermethrin, λ-cyhalothrin, deltamethrin and fenvalerate) and mirex were determined in soils possessing a range of organic content (1.15-2.46%) [26]. Solutions (in deionized water, pH 6.5-7.4) of the samples were shaken using a mechanical shaker for 24 h. The suspensions were centrifuged and aliquots of clear supernatant were passed through a C-18 column (SPE extraction). The eluates were concentrated to dryness before a volumetric standard was added. The analytes were determined by GC/NICI-MS either in SIM or scan (SCN) mode. Sorption isotherm parameters (n and k) were calculated according to the Freundlich equation. The values of n are around unity. Permethrin and cyfluthrin were the least sorbed pyrethroids, k < 2, mirex and fenvalerate the most. The effect of the pH on sorption was examined also (at pH values 2, 4, 6 and 9). Sorption behaviour on different soils and silica was also examined. Desorption studies were conducted on the same pyrethroid solutions. After sorption, the supernatant was replaced with a similar volume of deionized water. Desorption was achieved by removing all the supernatant from the centrifuged samples and then replacing it with deionized water. This equilibration process was repeated five times. Each time the suspension was centrifuged, concentrated and analyzed using GC/NICI-MS. The residual amount of pyrethroid on the soil was calculated as the difference between the initial amount and the desorbed amount (mass balance) (Table 22).
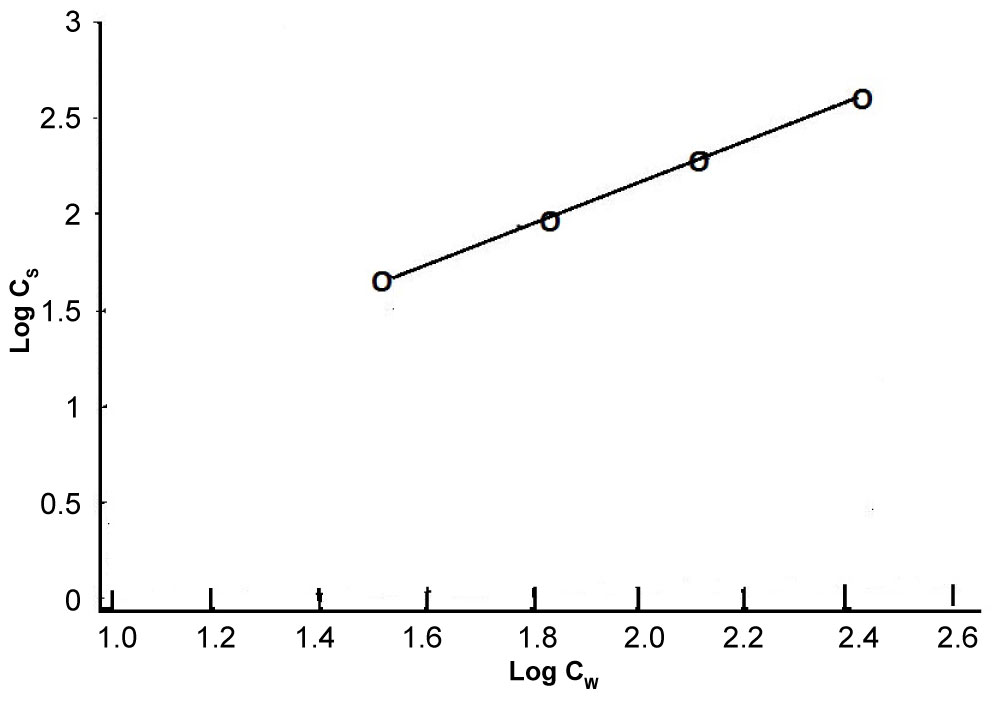
Figure 19 shows a typical sorption isotherm curve for cyfluthrin as an example and Table 22 summarises the Freundlich parameters for the six pyrethroids investigated using the formula:
where Cs (mg kg‐1) is the total chemical concentration associated with the sorbent, Cw (mg l-1) is the total chemical concentration in solution and k is adsorption coefficient The log form is used to plot the sorption isotherm curve, as illustrated in Figure 19.
GC/NICI-MS using stable isotope analogue standards as reference
Unreported studies at Salford in the 1990s focused on the synthesis of d6 permethrin substituted at the cyclopropane exocyclic methyl groups, i.e., CD3.CD3. The shift of 6 mass units from the parent facilitates the monitoring of the analogue and target analyte at low mass resolution using SIM. Preliminary results on environmental samples from water course sources provided information about the enhancement in the determination and quantisation of permethrin [27].
The following more recent paper has relevance to the above unreported work and is included here for this purpose.
Koch, Clark and Tessier [28] have utilized stable isotope analogues for analysing pyrethroids in environmental samples from effluent waters from wastewater treatment facilities to increase the accuracy of determination of the target pyrethroids. Stable isotope internal standards are useful in correcting for matrix effects and instrumental variability when environmental samples such as wastewaters and biosolids are analyzed by mass spectral methods. This paper reports the use of deuterium-labeled analogues of eight pyrethroid insecticides to improve accuracy for the analysis of environmental samples by negative chemical ionization gas chromatography with mass spectrometric detection (NCI-GC-MS). Data for the analysis of effluent water from wastewater treatment facilities are presented which demonstrate that the method is rugged and capable of achieving limits of quantification (LOQs) as low as 0.5 ng/L (ppt), with individual recoveries within the range of 81-94% for those compounds with minimal control background concentrations. In addition, an alternate use of the deuterium-labeled standards is proposed for the determination of method recoveries at low levels that would normally have been precluded due to background pyrethroid levels present in environmental samples being used for control fortifications.
Summary of Recent Advances in the Applications of GC/NICI-MS to Environmental Systems
In period 2003 to date a number of studies have reported on the applications of GC/NICI-MS to the determination of pyrethroids in environmental and biological matrices including plasma.
For example, Ramesh and Ravi [15] have analysed pyrethroid insecticides in whole blood and serum. A new rapid and sensitive analytical method using NICI-GC-MS in SIM mode has been developed for the determination of residues of different synthetic pyrethroid insecticides, allethrin, bifenthrin, cypermethrin, cyphonothrin, cyfluthrin, λ-cyhalothrin, deltamethrin, fenvalerate, fenpropathrin, permethrin, prallethrin, and trans-fluthrin, in whole blood. The residues of pyrethroid molecules were extracted from the whole blood using a hexane and acetone (8:2, v/v) solvent mixture without separating the serum. The method was found sensitive to detect the residues of pyrethroids up to the level 0.2 pg/mL. Experiments conducted with the whole blood samples at the fortification level 1-100 pg/mL showed 9-103% recovery, whereas blood serum samples collected after the fortification of pyrethroids in whole blood showed 36-54% recovery. Recovery experiments conducted by direct fortification of pyrethroids in blood serum samples showed 96-108%. The applications of the analytical method were tested by analyzing 73 human blood samples collected from the population exposed continuously to different pyrethroid-based formulations. None of the blood samples showed residues of pyrethroids. The results were also confirmed by the detection of the appropriate amounts in a number of these samples, which had subsequently been spiked with known quantity of pyrethroids.
Koch and Tessier [28], as already described in section 2.7, have employed stable isotope analogues to increase the confidence in the determination of pyrethroids in effluent waters.
Barcelo, et al. [29] have used GC/MS-MS to analyse twelve pyrethroid insecticides in environmental and food samples. First, a comparison of two different ionization modes, EI and NICI, was carried out using MS and MS-MS. NICI-MS-MS provided the best results in terms of selectivity and sensitivity giving very low detection limits of 0.11 to 450 fg injected. The reliability of the method was confirmed through the evaluation of quality parameters such as accuracy (70-100%), and repeatability and reproducibility, with coefficients of variation below 15% and 10%, respectively. The applicability of the GC/MS-MS method to real samples and influence of matrix effects were evaluated through the analysis of spiked water, sediment and milk at 0.25 ng L-1, 5 ng g-1 dry weight (dw) and 25 ng g-1 (dw), respectively, of each pyrethroid insecticide considered. Using GC/NICI-MS-MS, matrix spectral interferences were minimized providing method limits of detection (MLODs) of 0.05-2.59 ng L-1, 0.10-87.7 pg g-1 dw, 2.29-1071 pg g-1 lipid weight (lw) for water, sediment and milk, respectively. To the best of knowledge considered, the MLOD values found in this study were better than those reported in previous studies; in particular for sediment and food samples, the values were one order of magnitude lower.
Recent Results on the Application of ELISA to Determine Pyrethroids in Bednets
A very recent report [30] has appeared on the assessment of analytical methods for the determination of pyrethroids in bednets of which an outline is described below.
The objective was to present and evaluate simple, cost-effective tests to determine the amount of insecticide on treated materials. Methods were developed and evaluated a competitive immunoassay on two different platforms: a label-free impedimetric biosensor (EIS biosensor) and a lateral flow. Both approaches were validated by gas chromatography (GC) and ELISA, gold standards for analytical methods and immune assays, respectively. Finally, commercially available pyrethroid-treated ITN samples were analysed. Different extraction methods were evaluated. results Insecticide extraction by direct infusion of the ITN samples with dichloromethane and dioxane showed recovery efficiencies around 100% for insecticide-coated bednets, and > 70% for insecticide-incorporated bednets. These results were comparable to those obtained with standard sonication methods. The competitive immunoassay characterisation with ELISA presented a dynamic range between 12 nM and 1.5 lM (coefficient of variation (CV) below 5%), with an IC50 at 138 nM, and a limit of detection (LOD) of 3.2 nM. EIS biosensor had a linear range between 1.7 nM and61 nM (CV around 14%), with an IC50 at 10.4 nM, and a LOD of 0.6 nM. Finally, the lateral flow approach showed a dynamic range between 150 nM and 1.5 lM, an IC50 at 505 nM and a LOD of67 nM. It was ELISA can replace chromatography as an accurate laboratory technique to determine insecticide concentration in bednets. The lateral flow approach developed can be used to estimate ITN insecticide concentration in the field. This new technology, coupled to the new extraction methods, should provide reliable guidelines for ITN use and replacement in the field.
General Reference of Relevance to the Analysis of Pyrethroids
A very recent reference of general relevance to the trace analysis of pyrethroids has appeared on line as a review by Albaseer [31] of comments on the current state of the art in sampling and chromatographic methods. This review is worthy of inclusion because of where the author is located, in a university institution, in war torn Yemen.
Conclusions
Although not widely employed, GC/NICI-MS, in correlation studies with ELISAs, has been uniquely applied to determine pyrethroid residues in environmental systems from the 1990s to early 2000s. In addition, the employment of LC/ESI-MS-MS in the quantification and monitoring of selected mitins in water courses is an instrumental technique, which is shown to be of high quality in this review. The update of papers reported indicates that this premise remains and it can be concluded that the earlier research is still of current relevance.
Acknowledgements
The author wishes to acknowledge the contributions of the research students, research assistants and members of staff in the Departments of Biological Sciences and Chemistry at the time the research work was undertaken, whose names appear in the resulting publications, cited in the references following, including Dr. Dai Davies (retired, The Dept. of Biological Sciences), Dr. Graham Bonwick (Dept of Biological Sciences/University of Chester/Fera Science), Professor Chris Smith (North East Wales Institute/Cortecs/MMU), Dr. Peter Hancock (Dept. of Chemistry/Waters Corporation), Dr. Puziah Abdul Latif (Dept. of Chemistry/Malaysia), Dr. Mohammed Yasin (Dept. of Chemistry/Astrazeneca/Iraq)), Dr. Mohammed Adam (Dept. of Chemistry/Sudan), Dr. C Sun (Dept. of Chemistry/Nanjing-China), Dr. Simon White (Dept. of Chemistry/Waters Corporation), David Catlow (Astrazeneca).
References
- Guide to Codex recommendations concerning pesticide residues. Pt. 5 (1984) Recommended method of sampling for the determination of pesticide residues.
- (2014) Joint FAO/WHO meeting on pesticide residues - World health organization.
- http://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/pesticides/en/.
- (2015) Environment quality standards.
- Bolygo E, Zakar F (1983) Gas-liquid chromatographic screening method for six synthetic pyrethroid insecticides. J Assoc Off Anal Chem 66: 1013-1017.
- Jenkins DW, Hensens A, Lloyd J, et al. (2013) Development and validation of a 'universal' HPLC method for pyrethroid quantification in long-lasting insecticidal mosquito nets for malaria control and prevention. Trop Med Int Health 18: 2-11.
- Standing Committee of Analysts (1992) Chlorophenylid, flucofuron and sulcofuron in water (tentative methods), in methods for the examination of waters and associated materials, HM Stationary Office, London.
- (2000) Selected water quality standards
- Tadeo J L (2019) Analysis of pesticides in food and environmental samples. (2nd edn), CRC Press, pp 423.
- Mothproofing Agents and Water Course Management (1996) R&D Project Record, ©Environment Publication code: NE-12/96-25-A-AXAP.
- Bonwick GA, Putman M, Baugh PJ, et al. (1994) Immunoassay development for permethrin residues. Food & Agricultural Immunology 6: 341-356.
- Bonwick GA, Cresswell JE, Tyreman AL, et al. (1996) Production of murine monoclonal antibodies against sulcofuron and flucofuron by in vitro immunization. J Immunological Methods 196: 163-173.
- Yasin M, Baugh PJ, Hancock P, et al. (1995) Synthetic pyrethroid insecticides analysis by gas chromatography operated in negative-ion chemical ionization mode in soil, moss and fish tissue. Rapid Commun Mass Spectrom 9: 1411-1417.
- Yasin M, Baugh PJ, Bonwick GA, et al. (1996) Analytical method development for the determination of synthetic pyrethroid insecticides in soil by gas chromatography - mass spectrometry operated in negative-ion chemical ionisation mode. J Chromatogr A 754: 235-243.
- Ramesh A, Elumalai Ravi PE (2004) Negative ion chemical ionization-gas chromatographic-mass spectrometric determination of residues of different pyrethroid insecticides in whole blood. J Anal Toxicol 28: 660-666.
- Bonwick GA, Abdul-Latif P, Sun C, et al. (1994) Comparison of chemical methods and immunoassay for the detection of pesticide residues in various matrices. Food & Agricultural Immunology 6: 267-276.
- Bonwick GA, Yasin M, Hancock P, et al. (1996) Synthetic pyrethroid insecticides in fish: Analysis by gas chromatography - mass spectrometry operated in negative ion chemical ionization mode and ELISA. Food & Agricultural Immunology 8: 185-194.
- Ali MA, P Baugh PJ, Davies DH, et al. (2004) Correlation Studies between Elisa and GC-MS for the Analysis of pesticide residues in mosquito net materials. Poster presented at the 2nd National Meeting on Environmental Mass Spectrometry, April 01, University college chester.
- Ali MA, Baugh PJ, Davies DH, et al. (2004) Correlation studies between Elisa and GC-MS for the analysis of pesticide residues in mosquito net materials. Poster presented at the 28th Annual BMSS meeting, University of Derby, September 5-8.
- Ali M A (2003) PhD Thesis, University of Salford, UK.
- Bonwick GA, Sun C, Abdul-Latif P, et al. (1995) Determination of permethrin and cyfluthrin in water and sediment by gas chromatography - mass spectrometry operated in the negative ion chemical ionisation mode. J Chromatogr 707: 293-302.
- Hancock PM, M Yasin M, Baugh PJG, et al. (1997) Optimization of high quality analytical methods for the monitoring of pyrethroid mothproofing agents by gas chromatography - mass spectrometry operated in negative-ion chemical ionization mode. Intern J Environ Anal Chem 67: 81-95.
- Hancock PM, White SJG, Catlow DA, et al. (1997) Determination of the Mitins, Sulcofuron and Flucofuron, using liquid chromatography combined with negative‐ion electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom 38: 195-200.
- Hancock PM, Walsh M, White SJG, et al. (1998) Extraction and determination of the Mitins sulcofuron and flucofuron from environment river water. Analyst 123: 1669-1974.
- Ali MA, Baugh P J (2003) Pyrethroid soil extraction, properties of mixed solvents and time profiles using GC/MS-NICI. Int J Environ Anal Chem 83: 909-922.
- MA Ali MA, Baugh PJ (2003) Sorption-desorption studies of six pyrethroids and Mirex on soils using GC/MS-NICI. Int J Environ Anal Chem 83: 923-933.
- Hancock PM (1998) PhD Thesis, Undergraduate Dissertation, University of Salford, McGill C, UK.
- Koch del A, Clark K, Tessier DM (2013) Quantification of pyrethroids in environmental samples using NCI-GC-MS with stable isotope analogue standards 61: 2330-2339.
- Feo M L, Eljarrat E, Barcelo D (2011) Performance of gas chromatography/tandem mass spectrometry in the analysis of pyrethroid insecticides in environmental and food samples. Rapid Communications in Mass Spectrometry 25: 869-876.
- Castellarnau M, Ramon-Azcon J, Gonzalez-Quinteiro Y, et al. (2017) Assessment of analytical methods to determine pyrethroids content of bednets. Tropical Medicine and International Health 22: 41-51.
- Albaseer S (2018) Trace analysis of synthetic pyrethroids: Comments on the current state of the art in sampling and chromatographic methods. Research Journal of Chemical Sciences 8: 13-22.
Corresponding Author
Peter J Baugh, Leader of Environmental and Food Analysis Special Interest Group, EFASIG, The British Mass Spectrometry Society, c/o 23, Priory Road Sale M33 2BU, UK.
Copyright
© 2019 Baugh PJ. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.