A Defunct Lead-Acid Battery Recycling May Lead Strong Soil Pollution: A Case of Study in Mexico
Abstract
Inadequate waste management from the lead-acid battery recycling (LABR) industry can severely pollute the soil. This work aimed to assess heavy metal contamination of a defunct LABR site in Mexico. Total, DTPA-extractable, water-soluble metal concentrations and soil fractionation were analyzed. Speciation of Na and Pb was calculated by Geochemist's workbench 10 Software package based on soil solution analysis. Spatial distribution of soil features are presented in maps by the Kriging method. Total Pb and Cu concentrations reached up to 474,105 mg kg-1 and 3,911 mg kg-1, respectively, representing the greatest risk at the site. The highest DTPA-extractable Pb concentration was 12,000 mg kg-1, and the water-soluble Pb was 4.5 mg kg-1. High concentrations of available PO43- (1,354 mg kg-1), SO42- (34,086 mg kg-1) and Na (33,101 mg kg-1) were found. Lead fraction decreased as follow: Exchangeable (284,580 mg kg-1) > bound on to sulfides > weakly bound to OM >bound onto Fe and Mn oxides > residual phase > strongly bound to OM. The predominant chemical species in soil solution were Pb2+ (up to 86%) and Na+ (99%).
Keywords
Lead distribution, Copper, Salinity, Lead speciation
Introduction
Lead-acid batteries (LAB) are widely used for many industries and are the source of electric energy in every single vehicle. LAB are built with metal grids (25% to 30% of their weight), electrode paste (35% to 45%), sulfuric acid solution (10% to 15%), connectors and poles of Pb alloy, grid separators made up of PVC (5% to 8%), ebonite (1% to 3%) and a plastic case. LAB cells are composed of a Pb electrode (anode) and a Pb oxide electrode (cathode) immersed in a solution of sulfuric acid, metallic grids and connections [1], for this reason, used-batteries are considered as hazardous waste.
Lead-acid battery recycling (LABR) attempts to recover Pb and plastic from old batteries. In the USA, 99% of the LAB is recycled, because the efficiency of Pb recovery in 1990 was up to 95% [2]. Globally, 80% of the Pb produced is destined for LAB manufacturing and 95% of Pb used in batteries comes from the recycling process. Lead recycling has economic and ecological advantages but some disadvantages, one disadvantage is the release of Pb to the environment. A fraction of the Pb from batteries remains in the produced sludge as PbSO4, PbO2, and PbO.PbSO4 [3], which can be disposed in the soil. Therefore, inadequate LABR process and residue disposal may seriously pollute soil and groundwater [4] as occur in some developing countries. Hence, workers engaged in LABR are frequently exposed to contaminants because they often manipulate the material by hand without safety equipment [5]. Lead is considered as the second priority risk substance at sites in the USA National Priorities List [6]. The world most seriously polluted sites (in 2012 and 2015) are commonly related to the LABR industry and Pb deposition [5,7].
The present research involves the soil of a LABR factory which was closed by the Mexican Environmental Agency (MEA), due to complaints from residents of the neighborhood. This agency suspected high Pb concentrations in soil. Nevertheless, there were no reports, files or other available information about the procedure used for recycling neither the batteries nor the protocol for waste management. As a result, the MEA needs an environmental assessment of the defunct LABR site.
Precise information about the distribution and dynamics of the pollutants in the soil is required to define to define a remediation strategy. This study aimed to assess the concentrations of metals and salts and their spatial variability in the soil at a defunct LABR factory site located in Tepetlaoxtoc, Mexico.
Materials and Methods
Soil sampling
The study area is located at central Mexico (19.550831, -98.794714) in a former recycling factory of lead-acid batteries; the factory was near to the Hondo creek. The soil survey was carried out using a systematical sampling procedure [8]. A grid of 5 × 5 m was laid out and at each intercept composite soil samples (5-20 cm depth) were taken (a total of 54 samples). Six additional composite non-polluted soil samples were taken near to the LABR site to determine natural soil heavy metal concentrations.
Fertility and soil solution analyses
Immediately after soil collection, oxide-reduction potential (ORP) was measured in soil samples in a 1: 2.5 soil: Water ratio slurry with a pH meter (Thermo Orion 420 A) using an ORP electrode. The equipment was calibrated using Light's solution [9]. After ORP determination, the soil was air-dried and sieved (≤ 2 mm) before other analyses.
Soil samples were air dried and sieved for the analysis of soil features, Pb and Cu concentrations. Plant available nutrients: Phosphorus [10], sulfate-[11] and N (KCl extraction and Kjeldahl distillation) [12] were measured. Besides, total carbonates was determined by the calcimeter method [13] and organic matter (OM) by the loss on ignition procedure [14].
Electrical conductivity (EC), pH and oxide reduction potential (ORP) were measured as mentioned above in liquid extracts from saturation pastes (soil solution) [15]. The water-soluble ions were determined: SO42- (modified 9038 method) [16], PO43- [10], HCO3-, CO32-, Ca and Mg [17], Cl, Na and K (argentometric and flame photometrically methods) [18]. Analysis of PO43- and SO42- in this section differs from those determined in Section 2.2.1, as the soil solution contains soluble compounds which react in the liquid phase of the soil.
Heavy metal concentrations were also determined in the soil solution before chemical speciation simulation, mimicking the soil solution reactions. Sodium adsorption (SAR) and potassium adsorption (PAR) ratios were calculated to determine the relationship between Na+, K+, Ca2+, and Mg2+ as follows:
Where: Na+, K+, Ca2+ and Mg2+ are the concentrations of these cations (milliequivalents L-1).
Heavy metal soil concentrations
Total metal concentrations were determined after acid digestion in HNO3-HClO4-H2O2 (3:1:1) mixture (modified 3050B) [19]. The extractable metals were determined with DTPA-TEA-CaCl2 solution (DTPA-extractable) in a 1:5 soil: Solution ratio [20]. Bioavailability index [21] of the metals was calculated as follows: [(DTPA-TEA-CaCl2 extractable concentration/total metal concentration)*100]. Saturated paste extract was prepared to measure the soluble salts and metals in soil.
Fractionation of metals was analyzed by sequential extraction following the Pagnanelli modified method [22]. The fractions obtained were: Exchangeable (EX); bound onto Fe and Mn oxides (FeMn); weakly bound to OM (WOM); firmly bound to OM (SOM) and linkedto sulfide phase (S). The last stage of the fractionation was the residual fraction (R): Acid digestion of the samples with 5 mL of HNO3-HClO4-H2O2 (3:1:1) mixture. Metals were quantified by flame atomic absorption spectrometry (Perkin Elmer model 3110).
Geostatistical analysis
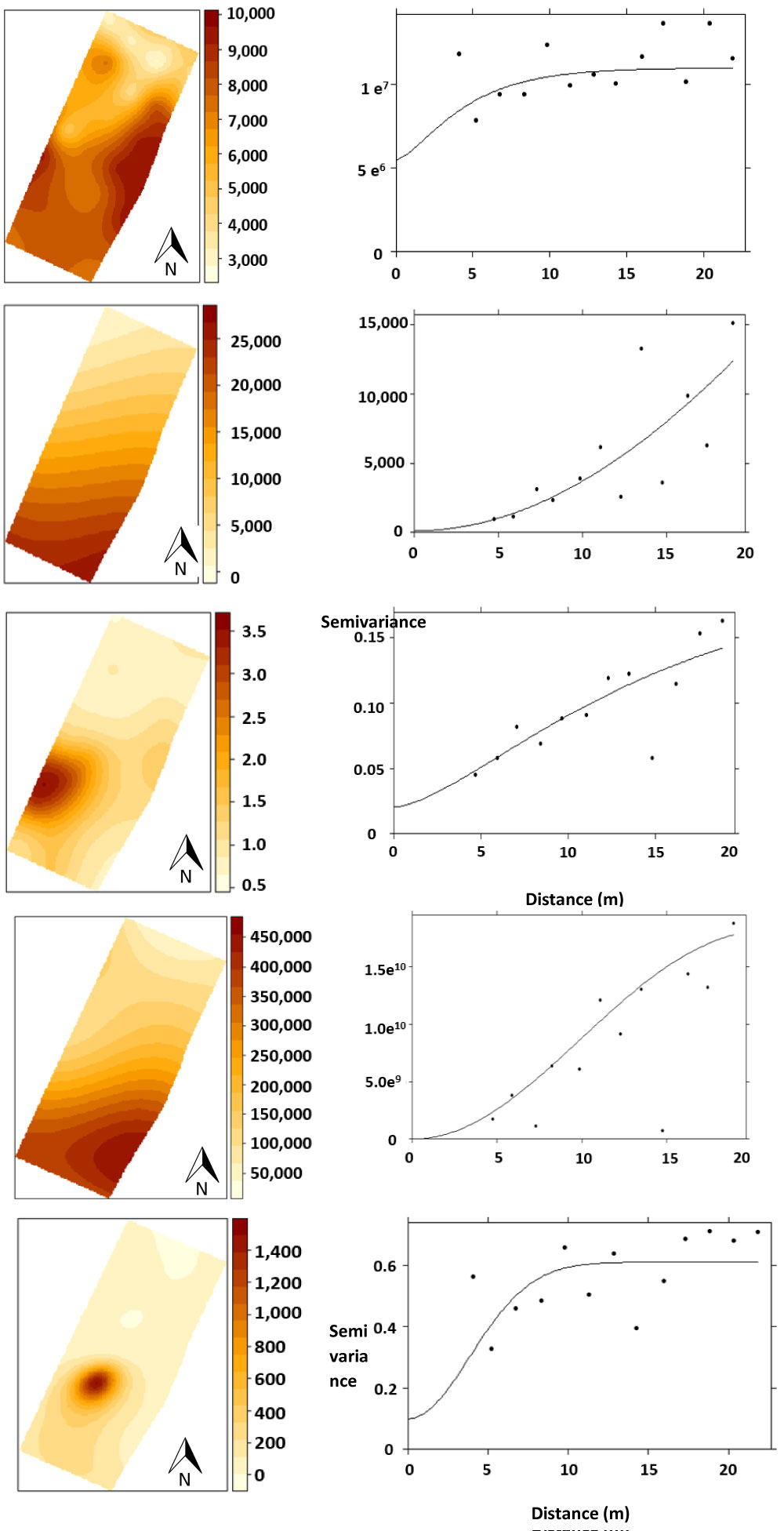
Standarized semivariogram was calculated to describe the way the spatial variation of Pb and Cu soil concentrations changes along the distance separation any two points varies. Ordinary block Kriging was used to estimate the values at unsampled places, and their validations were performed with semivariograms. Graphic representations of Pb and Cu concentration in all the terrain were generated (Figure 1). The statistical package used was R 3.1.3 with the libraries gstat, sp, maptools, gr Devices and raster [23].
Data analysis
To evaluate the potential risk of Pb, Cu and Cd accumulated in the top soil, the potential mobile fractions (PMF) weakly bound to soil components was calculated. High values suggested the tendency entering to the food chain.
Speciation in saturation paste extracts was carried out simulating the soil solution using Geochemist's Work bench 10 software. Correlations were performed with Spearman or Pearson method depending on the assumptions satisfied by the variables. P-values were used to validate these correlations. The statistical software used was R 3.1.3 [23].
Results
Contrasting to the landscape, the soil was completely naked, even this very close to the river; no vegetation was growing, with salt spots on the soil surface.
Soil chemical properties
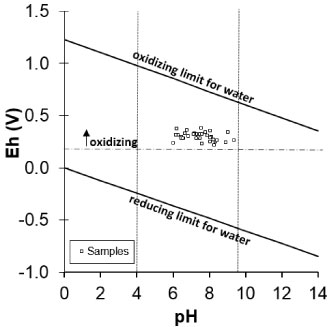
Soil pH was alkaline, where 68% of the samples had pH > 7 and 9% pH > 8.5 (Table 1). The ground showed oxidizing conditions (ORP: 300-600 mV; Figure S1).The soil was saline (EC > 4 dS m-1). In high proportion (55%) of the samples the PO43-, SO42-, Cl- and Na+ concentrations were high, contributing to the EC increment. Taking into account SAR and EC values at the LABR site, 57% of the soil surface was saline-sodic; 34% is sodic and only 9% was not affected by salts nor Na (Table 1).
Organic matter (OM) content varied from 0.8% to 13.4%.The carbonate content was from 0.2% to 3.6%; the highest concentrations were found on the eastern part of the site. Plant-available PO43- and SO42- were detected in high concentrations; they ranged from 19 to 1,354 mg kg-1 and 3.8 to 34,086 mg kg-1, respectively. Available N was low in all samples, from 0 mg kg-1 to 5 mg kg-1of NH4+ and from 0 mg kg-1 to 5 mg kg-1 of NO2- + NO3-. However, NH4+ was detected only in samples from the north part of the field. The site reaction was alkaline and have poor fertility [24, 25].
Total heavy metal concentrations
The total metal concentration decreased as follows Pb>Fe>Cu>Zn>Mn>Ni>Cd (Table 2). These values were higher than the concentrations of the non-polluted sites in the vicinity of the LABR site; and up to 1,451, 782, 19 and 15 times for Pb, Cu, Cd, and Ni, respectively (Figure 1a). The soil is polluted with Pb, Fe, Cu and Zn. Apparently, these elements were introduced as a result of the dispersion of the battery slurry (Figure 1).
Heavy metal fractionation
The highest Pb concentrations were observed in the exchangeable fraction (Table 2). The general sequence in soil fractions decreased in the following order: PbEX>PbS>PbWOM>PbFeMn>PbR>PbSOM. Exchangeable Pb was spatially distributed with a pattern similar to total Pb concentrations. Lead concentration decreased gradually from the south to the north side of the site (Figure 1a, Figure 1b and Table 2).
Positive correlations of PbEX with SO42- (0.528, p = 0.024), PO43- (0.749, p = 0.001) and OM (0.641, p = 0.004) were found. Strongly bound to OM Pb was correlated positively with pH (0.492, p = 0.039) and similarly PbFeMn with SO42- (0.510, p = 0.024). PbWOM ranged from 0.1% to 31.6%. In contrast, low concentration of PbSOM was observed (< 2% of total Pb).
Copper concentrations showed the following distribution: CuS>CuWOM>CuFeMn>CuR>CuEX>CuSOM. A positive correlation was found between CuEX and soil OM content (0.71, p = 0.001); CuFeMn with OM (0.72, p = 0.001) and with SO42- (0.79, p = 0.001). Similarly, CuSOM was correlated with OM content (0.67, p = 0.002) and CuS with SO42- concentrations (0.680, p = 0.001).
Concentrations of Cd decreased as follows: CdS>CdFeMn>CdEX>CdWOM = CdR>CdSOM; where CdEX was up to 63% of the total Cd. Concentrations of Fe and Mn forming oxides (FeFeMn and MnFeMn) varied from 74 to 30,130 mg kg-1 for Fe and 30 to 221 mg kg-1 for Mn. Up to 21% and 63% of total Pb and Cu was bound to Fe and Mn oxides (Table 2).
Nickel concentration in soil fractions decreased as follows NiFeMn>NiS>NiEX>NiWOM>NiR>NiSOM. The Zn concentration followed this order: ZnEX>ZnFeMn>ZnWOM>ZnS>ZnR>ZnSOM, where ZnEX ranged from 24 to 2,337 mg kg-1.
DTPA-extractable metals
DTPA-extractable concentrations were variable: Pb concentrations varied from 154 to 12,000 mg kg-1 and Cu ranged from 0.01 to 1,936 mg kg-1, with similar distribution patterns (Figures 1c and Figure 1d). The highest values were at the south of the site. DTPA-extractable Cu concentrations correlated negatively with OM contents (-0.722, p = 0.005). Bioavailability index ranged 1% to 66% for Pb, 1% to 54% for Cu, 1% to 25% for Zn, 1% to 8% for Ni, 0% to 10% for Mn, 0% to 25% for Cd and 0% to 0.4% for Fe. Besides, bioavailability index of Pb was negatively correlated with OM (-0.570, p = 0.021). Low concentrations of DTPA-extractable Fe, Mn, Zn, Ni, and Cd were found in all samples; soluble concentrations of Mn, Zn, Fe and Cu in some samples were lower than detection limits.
Chemical speciation of Pb and Na in the soil solution
Soluble Pb concentrations ranged from 0.024 to 4.650 mg kg-1, where higher concen trations were detected in the southern part of the site (Figure 1e). The Pb2+ ion was present in all pH values tested and was the predominant species at low pH. SO42-, CO32- and Cl- had a strong influence on Pb speciation in solution. Formation of Pb-Cl complexes can be observed at the highest Pb concentrations, and Pb-SO42- at the lowest pH values. In the soil solution with the lowest Pb concentrations (at pH = 8.25), 100% of the Pb was bound to CO32- (PbCO3 (aq) and Pb(CO3)22), (Table S1).
The highest Na concentration in soil solution (33,101 mg kg-1) corresponded to the lowest pH (6.0) at the site. Na was present mainly as a free ion (Na+ = 97%) and in minor proportion was associated with Cl-, CO3- and OH-. The lowest sodium concentration was observed at the higher pH (8.5): The free ion was predominant and the second species was Na associated to Cl-, CO32- and PO43-.
Discussion
Soil fertility, pH and salinity
Low concentrations of N (0 to 5 mg kg-1) and OM (1.7%) showed poor soil fertility [25]. Therefore, it is needed to amend soil to support plant growth. The soil was alkaline (pH up to 9.4, Table 1) despite the parent material of the area (welded tuff and volcanic ashes) [26]. High concentrations of Cl- at the site (8,050 mg kg-1; Table 1) increase pH, apparently because Cl- replaces OH- ions on positively charged sites of soil particles, and OH- remains free [27]. High Na concentrations (33,101 mg kg-1) can also contribute to alkalinizing the soil.
The source of Na is probably for the use of NaOH to neutralize H2SO4 in the slurry to Na2SO4 during the SO42-recuperationin LAB recycling [28]. Sodium bicarbonate (Na2CO3) is used to transform PbSO4 to PbCO3 [29]. These processes also explain the high SO42- concentrations (up to 47,130 mg L-1, Table 1). Inadequate management of old batteries and Na compounds used for recycling and scant disposal of residues were performed by the LABR factory which operated at the site. Furthermore, it is well known that Na+ excess promotes soil particles deflocculation and breakdown of aggregates [14]. Particle dispersion influences Pb mobilization by wind or rain which may easily transport the soil particles.
Phosphorus concentrations (4.1-7.2 mg L-1) were above the typical soil concentrations (0.05 to 0.5 mg L-1) [30]. Similarly, plant available PO43- concentration (19 to 1,354 mg kg-1) were also excessive (> 50 mg kg-1) [30]. These concentrations may be due to the use of H3PO4 as the electrolyte in some batteries. Other important parameters concerning soil salinity are SAR and PAR. At the LABR site, these parameters were above recommended values (Table 1). High SAR values suggest that there is not enough Ca and Mg to compete with Na on the exchange surfaces in the soil [15]. That means 85% of the samples had SAR > 102, which is the maximum value proposed for very tolerant crops [31]. Moreover, PAR gives structural stability and K retention in soils. Therefore, the poor balance of Na, K, Ca and Mg ions in the site difficult the LABR soil management.
Total metal concentrations
Total concentrations of Pb, Cu, Cd, and Ni in the LABR soil were up to 8,882, 50, 29 and five times higher, respectively than values referenced by Kabata-Pendias [32] for agricultural lands (Table 2). Possibly, high Cu, Cd and Ni concentrations (up to 3911, 57 and 408 mg kg-1, respectively) come from alloys, batteries grids, and poles. In the same way, total Pb concentrations (up to 444,105 mg kg-1) exceeded regulatory limits of the country by 27 - 1110 times. The thresholds for residential land use in the USA range from 500 to 1000 mg kg-1. While in Canada this is 375 mg kg-1, in The Netherlands is between 50 to 600 mg kg-1 and in England is 500 mg kg-1 [33].
Hence, total Pb concentrations at LABR soil are comparable with soils polluted by LABR in other regions of the world. Some of the most polluted sites have up to 400,000 mg kg-1 (40%) of Pb [5, 7]. Similarly, Mohammed, et al. [4] found a soil containing up to 70% of Pb. Wasay, et al. [34] observed 24,600 mg kg-1 (2.46%) of Pb in soil due to the disposal of wastes of a LABR in Canada. In the same way, Yeh, et al. [35] reported a value of 3,590 mg kg-1 Pb in soil near a LABR factory in Taiwan. Rodríguez, et al. [36] found 1,050 mg kg-1 of Pb total concentration near a LABR facility in Argentina. Total soil Pb concentrations in LABR site makes necessary intervention to reduce the risk for public health and environmental damage.
The observed total Pb, Cu, Ni, Cd and Zn concentrations in LABR soil suggest the impact of the free recycling process and inadequate waste management from this factory. Furthermore, the spatial total Pb distribution (Figure 1a) shows that the south zone of the site was the most polluted. Probably used slurry was disposed of in this part of the terrain.
Soil heavy metals fractionation
High PbEX concentrations in the soil (up to 284,580 mg kg-1; Table 2) makes this metal the main risk in the site. The major Pb proportion was found in an exchangeable form (44 to 97%). In another soil affected by LABR, Rodríguez, et al. [36], found up to 640 mg kg-1 of PbEX at approximately 100 m away from a LABR facility. The potential mobile fraction (PMF) of lead was between 5.7 and 59.3%, which suggested easy enter to the food chain.
Organic matter content, available SO42-(0.528, p = 0.024) and PO43-(0.749, p = 0.001) concentrations were correlated positively with PbEX. This can indicate the presence of available forms of Pb bound to the mentioned anions. The positive correlation between PbEX and OM (0.641, p = 0.004) probably shows an electrostatic Pb-OM association. The OM ability to sequestrate heavy metals may explain the correlation, which has been reported [37,38] and can also explain the Pb concentrations observed as PbSOM (up to 7,506 mg kg-1) and PbWOM (up to 73,642 mg kg-1). A Pb proportion was found as PbFeMn (up to 21% of the total Pb). The behavior of heavy metals with stable oxidation states depends indirectly on Fe compounds [27,39]. Pb may be adsorbed on the Fe and Mn oxides surfaces [40,41]. Pb fixation on oxides reduces PbEX concentration in the LABR soil.
Regarding Cu fractionation, the negative correlation found between OM and CuEX (-0.71, p = 0.001) can be explained in terms of the high affinity of Cu by OM. Cu is strongly associated, so Cu electrostatically retained decreased. The PMF was lower than 20.6%. Other metals found in high concentration were ZnEX (1,984 mg kg-1) PbEX (284,580 mg kg-1) NiEX (63 mg kg-1) and CdEX (11 mg kg-1; Table 2). The PMF was also low (2.8 to 17.2%). Cadmium is considered a very hazardous metal due to its toxicity; but, an important proportion of Cd is associated to OM: CdSOM (up to 37%), CdWOM (up to 29%), which ameliorates the potential effects of Cd. In this soil the PMF was high (4.1 to 39.8%). Similarly, Ni concentrations bound to OM (NiWOM up to 62 mg kg-1 and NiSOM up to 7 mg kg-1) may contribute to the depletion of NiEX concentrations.
Furthermore, heavy metals concentrations bound to Fe and Mn oxides were up to 21%, 63%, 46%, 40% and 46% of their total concentrations for Pb, Cu, Ni, Zn, Cd, and Ni respectively (Table 2). Apparently, Fe and Mn oxides may be contributing to heavy metal fixation in LABR site, which has been reported before [42].
DTPA-extractable metals
A similar spatial pattern of Pb (DTPA-extractable, extractable, water-soluble and total) and DTPA-extractable Cu (Figure 1) shows that south part of the site was the most affected by heavy metal pollution. Possibly due that to the slurry disposal was performed at the south side of the terrain.
The negative correlation found between Pb bioavailability index and OM content (-0.570, p = 0.021) may be due to the influence of OM on Pb bioavailability in LABR soil. Lead can be strongly adsorbed by OM and fixed as non-extractable forms or complexed with humic substances [43]. The addition of organic amendments to the soil may be a strategy to reduce Pb availability. Nevertheless, high DTPA-extractable Pb concentrations are observed in the LABR site; which may be due to the saturation of OM adsorbent sites.
Pb and Na chemical speciation in the soil solution
Oxidizing (ORP: 300-600 mV; Table 1) and alkaline (pH > 7.4) conditions were predominantly observed in soil samples. Although the presence of [PbOH]+ and [PbO2] species [43] in LABR soil solution was expected. The pH only allows the PbOH+ species (3.51% of the total Pb in solution) in the paste saturation extract (Table S1).
The Pb concentration in soil solution was low the significant proportion of this metal was in the saturation paste extract (up to 85% of the Pb in solution) remained as a free divalent ion (Pb2+). Other chemical species such as PbOH+, Pb(CO3)22-, Pb(OH)2(aq) and Pb(SO4)2- were in minor concentrations. The occurrence of different Pb species is related to Pb mobility in soil. For instance, Weng [44] found that Pb2+ is less adsorbed on soils than Pb(OH)+. In the same way, Nedwed and Clifford [34] mentioned that CO32- or CaO could lead to the formation of Pb3(CO3)2(OH)2, PbCO3 or Pb4SO4(CO3)2(OH)2; these species could reduce Pb mobility. In LABR site, CO32- was found in all samples and Pb associates with the mentioned anion at high pH (8.25 and 9.38; Supplementary Table 1). In contrast, in the soil with the lowest pH (6.05), Pb was associated predominantly to SO42- (55.76% and 39.27% of the total Pb in the solution for Pb(SO4)2- and PbSO4(aq) respectively). However, despite high SO42- concentration in saturation paste extract (47,130 mg kg-1), low concentrations of Pb(SO4)2- and PbSO4(aq) (1.27 and 0.89 mg kg-1, respectively) may be explained by the low solubility of compounds formed between Pb and SO42-; and Pb competition with Ca2+, Na+ and K+, cations with more affinity for SO42-. Other possible reaction of SO42- is the reduction as sulfides, which can sequestrate heavy metals. Adsorption and precipitation of Pb on SO42- compounds are important buffer mechanisms for fixation and controlling the migration of Pb [45]. The fractionation shows a high proportion of Pb bound to sulfides (PbS); up to 39% of the total.
Sodium ion was the predominant species in soil solution (Table S1), but Na associated with CO32-, SO42-, PO43-, Cl- was also present. There is scarce information regarding the effect of Na on speciation in soils metal bioavailability. Ghallab and Usman [46] found increased concentrations of Cd in solution due to NaCl additions; apparently, Cd was displaced by Na from exchange surfaces. In LABR soil solution, NaCl was present in high concentration (up to 943.8 mg L-1). Hence, the influence of Na on heavy metals solubility should be further studied in LABR soil.
High Na+, Cl-, PO43- and SO42- concentrations (Table 1) increase soil solution ionic strength (IS) in the LABR site. The IS ranged from 0.005 to 1.185 M values. While, in non-polluted soils, the IS ranged from 0.003 to 0.016 M [47]. Ionic strength leads to an increment of metal solubility because cations compete with metals for exchange sites and formation of metal-salt species with no charge or negative charge such as metal -Cl2o, metal -Cl3- and metal -Cl4- complexes [48]. At the LABR soil, some Cl- metal species were found (Table S1). Hence, Na+, Cl-, PO43- and SO42- could contribute to metal solubility.
Conclusions
The LABR activities at this site resulted in a heavily polluted soil due to: 1) Very high total Pb concentrations; which makes this site similar to others reported in the literature as the most world polluted sites by Pb; 2) Elevated total concentrations of Cu. High DTPA-extractable and exchangeable concentrations of Pb and Cu are very risky for plant establishment; and 3) Alkaline conditions and high concentrations of plant available SO42-, PO43- in the soil solution, as well as water-soluble Cl- and Na+. These hazards represent a potential risk to human health and the environment. One possible method to reduce concentrations of salts and heavy metals from solution is by leaching the soil. However, the process might demand large volumes of water and the resulting leachate will require treatment.
The fertility of the soil should be improved, due to the low N concentration. An organic addition could improve physical and chemical soil conditions, and fertility, besides could reduce availability and mobility of heavy metal. At this time, long time phytoremediation and biofuel experiments in the LABR site are being performed taking into account the information provided by the present research.
Acknowledgments
The facilities to access the LABR area from PROFEPA from the State of Mexico and Tepetlaoxtoc Municipality are recognized. Partial financial support work was provided by CONACYT-Mexico as a Masters Fellowship.
References
- Q Zhang (2013) The current status on the recycling of lead-acid batteries in China. Int J Electrochem Sci 8: 6457-6466.
- EPA (Environmental Protection Agency) (1991) States' Efforts to Promote Lead-Acid Battery Recycling, Environmental Protection Agency.
- MA Kreusch, MJJS Ponte, HA Ponte, et al. (2007) Technological improvements in automotive battery recycling. Resour Conserv Recycl 52: 368-380.
- TI Mohammed, I Chang-Yen, I Bekele (1996) Lead pollution in East Trinidad resulting from recycling and smelting activities, Environ. Geochem. Health 18: 123-128.
- Blacksmith Institute, Green Cross Switzerland (2013) The World's Worst Pollution Problems: Assessing Health Risks at Hazardous Waste Sites.
- ATSDR (2017) Priority list of hazardous substances.
- Pure Earth/Green Cross Switzerland, (2015) Top ten: Toxic pollution problems. Green Cross Switzerland and Pure Earth, New York.
- R Gilbert (1987) Statistical methods for environmental pollution monitoring. John Wiley & Sons Inc.
- TS Light (1972) Standard solution for redox potential measurements. Anal Che 44: 1038-1039.
- SR Olsen, LE Sommers, (1982) Phosphorus. In: CA Black, Part 2: Chemical and Microbiological Properties. Agronomy 9, Methods of Soil Analysis, 1159.
- DR Walker (1972) Soil sulfate I. Extraction and measurement. Can J Soil Sci 52: 253-260.
- JM Bremner (1965) Inorganic forms of nitrogen. in: CA Black (Edn) Agronomy 9. Methods of Soils Analysis. Part 2: Chemical and Microbiological Properties, American Society of Agronomy and American Society for Testing and Materials, 1179-1237.
- RH Loeppert, DL Suarez (1996) Carbonate and gypsum. In: D.L. Sparks, Methods of soil analysis. Part 3 Chemical methods, SSSA Book Series, Madison, 437-474.
- DL Rowell (1994) Soil Science: Methods and Applications. Longman Scientific & Technical/John Wiley & Sons.
- USDA (1954) Diagnosis and Improvement of Saline and Alkaline Soils. Agriculture United States Department of Agriculture.
- US EPA (1986) Method 9038: Sulfate (Turbidimetric).
- BS Chauhan (2013) Engineering Chemistry (MTU). Laxmi Publications.
- HD Chapman, PF Pratt (1986) Métodos de Análisis para Suelos. Plantas y Aguas, Trillas.
- US EPA (1996) Method 3050B: Acid digestion of sediments, sludges and soils. Environmental Protection Agency.
- WL Lindsay, WA Norvell (1978) Development of a DTPA soil zinc, iron, manganese and copper. Soil Sci Soc Am J 42: 421-428.
- B Chen, X Shan Han, J Qian (1996) Bioavailability index for quantitative evaluation of plant availability of extractable soil trace elements. Plant Soil 186: 275-283.
- F Pagnanelli, E Moscardini, V Giuliano, et al. (2004) Sequential extraction of heavy metals in river sediments of an abandoned pyrite mining area: Pollution detection and affinity series. Environ Pollut 132: 189-201.
- R Development Core Team (2015) R: A language and environment for statistical computing. The R fundation for Statistical Computing, Vienna, Australia.
- P Marschner Z Rengel (2012) Nutrient Availability in Soils. (3rd edn) In: Marschner P, Marschner's Mineral Nutrition of Higher Plants. Elsevier Ltd, 315-330.
- DE Smiles, CJ Smith (2004) A survey of the cation content of piggery effluents and some consequences of their use to irrigate soils. Aust J Soil Res 42: 231-246.
- FJ Cortés (1970) Estudio pedogenético y distribución de los vertisoles en la cuenca de México. Engeneer thesis. Escuela Nacional de Agricultura, México.
- G Xu, Magen H, Tarchitzky J, et al. (2006) Advances in chloride nutrition. Adv Agron 68: 97-150.
- K Ramus, P Hawkins (1993) Lead/acid battery recycling and the new Isasmelt process. J Power Sour 42: 299-313.
- M Stevenson (2004) Recovery and Recycling of Lead-Acid Batteries. in: PT Moseley, CDP Garche, DAJ Rand, Valve-Regulated Lead-Acid Batteries. Elsevier, 491-512.
- DA Horneck, DM Sullivan, JS Owen, (2011) Soil Test Interpretation Guide. Oregon State University. Extension Service.
- H Hazelton, B Murphy (2007) Interpreting Soil Test Results: What Do All the Numbers Mean? (2nd edn), CSIRO Publishing, Oxford.
- A Kabata-Pendias (2011) Trace Elements in Soils and Plants. (4th edn), CRC Press/Taylor & Francis Group, USA.
- DA Nedwed, A Clifford (1998) A survey of lead battery recycling sites and soil remediation processes. Waste Manag 17: 257-269.
- SA Wasay, WJ Parker, PJ Van Geel (2001) Contamination of a calcareous soil by battery industry wastes I Characterization. Can J Civ Eng 28: 341-348.
- CY Yeh, HY Chiou, RY Chen, et al. (1996) Monitoring lead pollution near a storage battery recycling plant in Taiwan, Republic of China. Arch Environ Contam Toxicol 30: 227-234.
- JH Rodríguez, MJ Salazar, L Steffan, et al. (2014) Assessment of Pb and Zn contents in agricultural soils and soybean crops near to a former battery recycling plant in Córdoba, Argentina. J Geochemical Explor 145: 129-134.
- Y Yin, CA Impellitteri, SJ You, et al. (2002) The importance of organic matter distribution and extract soil: Solution ratio on the desorption of heavy metals from soil. Sci Total Environ 287: 107-119.
- YF Zhou, RJ Haynes, R Naidu (2012) Use of inorganic and organic wastes for in situ immobilization of Pb and Zn in a contaminated alkaline soil. Environ Sci Pollut Res 19: 1260-1270.
- YN Vodyanitskii (2010) The role of iron in the fixation of heavy metals and metalloids in soils : A Review of Publications. Eurasian Soil Sci 43: 519-532.
- A Suda, T Makino (2016) Functional effects of manganese and iron oxides on the dynamics of trace elements in soils with a special focus on arsenic and cadmium: A review. Geoderma 270: 68-75.
- MD Royer, A Selvakumar, R Gaire (1992) Control technologies for remediation of contaminated soil and waste deposits at superfund lead battery recycling sites. J Air Waste Manage Assoc 42: 970-980.
- L Wang, B Yan, L Zhu, et al. (2015) The effect of reclamation on the distribution of heavy metals in saline-sodic soil of Songnen Plain, China. Environ Earth Sci 73: 1083-1090.
- N Takeno (2005) Atlas of Eh-pH diagrams: Intercomparison of thermodynamic databases, Natl Inst Adv Ind Sci Technol.
- CH Weng (2004) Modeling Pb(II) adsorption onto sandy loam soil. J Colloid Interface Sci 272: 262-270.
- PE Jensen, LM Ottosen, AJ Pedersen (2006) Speciation of Pb in industrially polluted soils. Water Air Soil Pollut 170: 359-382.
- A Ghallab, ARA Usman (2007) Effect of sodium chloride-induced salinity on phyto-availability and speciation of Cd in soil solution. Water Air Soil Pollut 185: 43-51.
- CD Edmeades, DM Wheeler, E Clinton (1985) The chemical composition and ionic strength of soil solutions from New Zealand topsoils. Aust J Soil Res 23: 151-165.
- G Petruzzelli, G Guidi, L Lubrano (1985) Ionic strength effect on heavy metal adsorption by soil. Commun Soil Sci Plant Anal 16: 971-986.
- M Radojević, VM Bashkin (1999) Practical Environmental Analysis. Royal Society of Chemistry, Cambridge.
Corresponding Author
Rogelio Carrillo-González, Soil and Environmental Chemistry Lab, Colegio de Postgraduados, km 36.5 Carretera México-Texcoco. 56230, Mexico, Tel: +52- 5558045900, Email: crogelio@colpos.mx.
Copyright
© 2019 Carrillo-González R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.