Prognostic Significance of MIPI and MIPI-B Scoring Systems for Mantle Cell Lymphoma in the Turkish Population
Abstract
Aim
Our objective in this study is assessment of Mantle Cell Lymphoma International Prognostic Index (MIPI) and biological MIPI (MIPI-B) scores as prognostic markers based on real-life data of patients diagnosed with mantle cell lymphoma (MCL) in Turkey.
Materials and methods
A total of 54 patients above 18 years of age who were diagnosed with MCL were included in the study. Patients' demographical data, chronic diseases, treatments, and outcomes were reviewed. Cox regression analysis was used for evaluation of potential risk factors that were statistically associated with mortality and Kaplan-Meier analysis was used for evaluation of survival.
Results
A total of 54 patients, 35 male and 19 female, who were diagnosed with MCL were included in the study. Mean age was 62.2 years and mortality rate was 44.4% (n: 24). Among the deceased patients, beta-2 microglobulin level, CNS involvement, presence of B symptoms, bone marrow involvement (87.0% vs. 26.7%), and rate of extranodal involvement (100% vs. 20%) were determined to be high. Among the surviving patients, the rate of patients whose initial response to treatment was complete remission was higher (66.7% vs. 25%). Among the deceased patients, the median Ki-67 proliferation index level (70 vs. 22.5), median International Prognostic Index level (4.5 vs. 3), median MIPI level (8 vs. 5), and median MIPI-B level (8.1 vs. 4. 1) were high.
Conclusion
This study is the first study demonstrating that MIPI and MIPI-B scores are important prognostic markers in patients diagnosed with MCL in the Turkish population.
Keywords
Mantle cell lymphoma, Prognostic factors
Introduction
Mantle cell lymphoma (MCL) is a mature B-cell non-Hodgkin lymphoma, which is described as an aggressive B-cell lymphoma by the World Health Organization [1-3]. Diagnosis of MCL is established by lymph node, tissue, or bone marrow biopsy and the presence of t(11,14) aids the diagnosis. CD20, CD5, and cyclin D1 are immunophenotypically expressed. The presence of SOX11 is also a significant diagnostic marker. High Ki-67 proliferation index is closely associated with aggressive course [4,5].
The age before treatment, performance, and stage are important for determination of treatment. As prognostic factors determining the course of the disease, scoring systems including the International Prognostic Index (IPI), Follicular Lymphoma International Prognostic Index (FILIPI), and Mantle Cell Lymphoma International Prognostic Index (MIPI) are used. The common parameters included in all these indices are age, lactate dehydrogenase (LDH) level, and stage [6].
The MIPI is the first disease-specific prognostic scoring system. Parameters indicating poor survival in the MIPI scoring system are determined to be older age, poor Eastern Cooperative Oncology Group (ECOG) performance score, high LDH, and high white blood cell count at the time of diagnosis. The MIPI score is based on logarithmic measurements of continuous variables. Based on the scores, groups are defined as follows: 0-3, low-risk; 4-5, moderate-risk; and 6-11, high-risk. By adding Ki-67 proliferation index to the MIPI scoring, the biological MIPI (MIPI-B) score is obtained, and the MIPI-B may improve the prediction of survival [7].
The most commonly used treatment regimens are rituximab and the CHOP combination. In addition, different rituximab-based treatment regimens are also used. Furthermore, autologous stem cell transplantation following an induction treatment administered to patients above 65 years of age has significantly increased the duration of progression-free survival due to short durations of remission [8,9].
The defined prognostic scoring systems for MCL are based on data obtained from studies of European populations. Our objective in this study is the assessment of MIPI and MIPI-B scores as prognostic markers based on real-life data of patients diagnosed with MCL in Turkey.
Material and Method
Study population
This study was conducted in Ankara Numune Training and Research Hospital between 2008 and 2017. A total of 54 patients above 18 years of age who were diagnosed with MCL were included in the study.
In addition to the demographical data, patients' chronic diseases and accompanying comorbidities were also recorded. Diagnosis of MCL was confirmed from tissue and blood samples by immunohistochemical staining, fluorescence in situ hybridization, translocation (11;14) analysis, and flow cytometry. The tissue samples were examined by a hematopathologist in accordance with World Health Organization's 2008 classification. The Ki-67 proliferation index and SOX11, cyclin D1, and translocation (11,14) tests could not be applied for all patients due to technical reasons.
Approval from the Ankara Numune Training and Research Hospital ethics committee was received.
Prognostic factors
The IPI, MIPI, and MIPI-B were used as prognostic classifications applied to the patients. All criteria included in these prognostic scores were evaluated. The Ki-67 proliferation indices of the patients were evaluated from tissue samples for pathology. In addition to this, serum albumin and beta-2 microglobulin levels were also included in the evaluation.
Treatment
Given the post-treatment clinical and accompanying comorbid conditions, standard chemotherapy protocols for the patients and, for appropriate patients, consolidated autologous stem cell transplants (ASCTs) were administered after induction treatment. Chemotherapy protocols administered were R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone), R-hyper-CVAD (rituximab plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with rituximab plus high-dose methotrexate and cytarabine), RCVP (rituximab, cyclophosphamide, vincristine, prednisone), R-CHOP/R-DHAP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone/rituximab, dexamethasone, high-dose cytarabine, cisplatin).
Statistical analysis
Statistical evaluation was performed using SPSS 20 for Windows (IBM Corp., Armonk, NY, USA). Normal distribution of the data was evaluated using the Kolmogorov-Smirnov test. Numerical variables exhibiting normal distribution were represented as mean ± standard deviation; numerical variables not exhibiting normal distribution were represented as median (min-max). Categorical variables were given as count and percentage. Potential risk factors that were statistically associated with mortality were evaluated using Cox regression analysis. Those considered to be significant were included in multivariate Cox regression analysis and potential risk factors predicting mortality were determined. Survival graphics were created using Kaplan-Meier analysis. In statistical analyses, p < 0.05 was considered to be significant.
Results
The study population comprised a total of 54 patients who were being followed due to diagnosis of MCL, 35 being male and 19 being female. The mean age of the patients was 62.2 ± 12.1 years. Duration of follow-up of the patients ranged between 1 and 147 months and the mortality rate was 44.4% (n: 24). Although mean age and ratio of females for the deceased patients were determined to be higher, no association with survival was demonstrated. Compared to the surviving patients, the rates of diabetes mellitus (DM) (41.7% vs. 16.7%), hypertension (HT) (20.8% vs. 13.3%), and coronary artery disease (CAD) (16.7% vs. 6.7%) were higher among the deceased patients and these were also determined to be risk factors for mortality (p < 0.05). The site of diagnosis was lymph node in the majority of the patients, with no association found between site of diagnosis and mortality (Table 1).
While median albumin level was lower (3.7 ± 0.4 vs. 4 ± 0.5) among the deceased patients, median C-reactive protein (CRP) (36 vs. 6) and median beta-2 microglobulin (5.7 vs. 2.8) levels were determined to be higher (p < 0.05), and these were determined to be risk factors for mortality (p < 0.05). Other laboratory results did not differ significantly (Table 2).
Among deceased patients, the presence of B symptoms (100% vs. 26.7%), central nervous system (CNS) involvement (37.5% vs. 3.3%), bone marrow involvement (87.5% vs. 26.7%; p < 0.001), and presence of extranodal involvement (100% vs. 20%) were determined to be higher and were determined to be risk factors for mortality (p < 0.05) (Table 3).
Initial treatment outcomes did not significantly differ between the deceased patients and those who survived. The complete remission (CR) rate (66.7% vs. 25%) was higher among the surviving patients, while the partial remission (PR) rate did not significantly differ between the deceased patients and those who survived (16.7% vs. 23.3%). The stable disease (SD) response rate (16.7% vs. 0%) and progressive disease (PD) response rate (41.7% vs. 10%) were determined to be higher among the deceased patients. SD and PR responses were determined to be risk factors for mortality. The majority of the surviving patients (80%) were not given a second-line treatment, while this rate was 29.2% among deceased patients. Of the surviving patients that received a second-line treatment, 66.7% showed CR and 33.3% showed PR response. Of the deceased patients that received a second-line treatment, PR response was observed in 64.7%, SD response in 11.8%, and PD response in 23.5%. Rate of autologous transplantation was determined to be lower among the deceased patients (12.5% vs. 66.7%) (Table 4).
The ratio of those with ECOG scores of 0-1 was higher among surviving patients compared to deceased ones (70% vs. 4.2%); among deceased patients, however, the rates of ECOG 2 (41.7% vs. 10%), ECOG 3 (29.2% vs. 20%), and ECOG 4 (25% vs. 0%) were determined to be higher compared to those of surviving patients. While the ratio of patients with stage 2-3 was higher among surviving patients compared to deceased patients (70% vs. 8.3%), the ratio of those with stage 4 was higher among deceased patients (91.7% vs. 30%). The median Ki-67 proliferation index level (70 vs. 22.5), median IPI level (4.5 vs. 3), median MIPI level (8 vs. 5), and median MIPI-B level (8.1 vs. 4.1) were determined to be higher among deceased patients, while the MIPI rate (87.5% vs. 23.3%) and MIPI-B rate (83.3% vs. 16.7%) were also determined to be higher compared to surviving patients (Table 5).
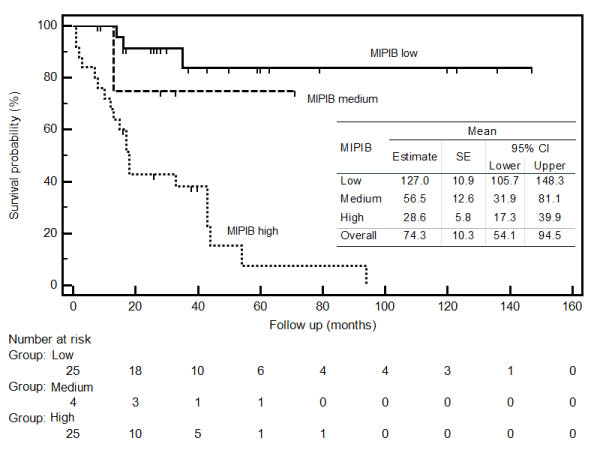
In the multivariate regression analysis model, in which potential risk factors associated with mortality were included, MIPI-B score, bone marrow involvement, and response to initial treatment parameters were determined to be independent risk factors that increased the risk of mortality. While no difference was determined in regard to mortality between patients with MIPI-B scores of moderate risk compared to those with low risk (HR = 2.25; p = 0.497), risk of mortality was determined to be 5.97-fold higher among patients with high risk (HR = 5.97; p < 0.001) (Figure 1). Risk of mortality was 4.37-fold higher among patients with bone marrow involvement (HR = 4.37; p = 0.025). While no statistical difference was determined between patients whose response to initial treatment was CR or PR and risk of mortality (HR = 1.28; p = 0.722), risk of mortality was determined to be 6.32-fold higher among patients whose response to initial treatment was SD (HR = 6.32; p = 0.012) and 5.51-fold higher among those with PD response (HR = 5.51; p = 0.004) (Table 6).
Discussion
In MCL treatment, very favorable outcomes have been obtained with the recently developed targeted treatments. Despite these outcomes, ASCT after high-dose chemotherapy is still considered to be one of the best options for patients who are young and whose performance is favorable. In 2008, the MIPI score was developed to aid prediction of response to treatment and to determine the prognosis of patients with MCL, and then the MIPI-B score was developed through the addition of the Ki-67 proliferation index to this score. In previous studies, it has been observed that both scores are a guide for determination of prognosis in patients with MCL. This study is the first study in which MIPI and MIPI-B scores were evaluated and the other factors influencing mortality were investigated in patients diagnosed with MCL in the Turkish population [10].
Mean albumin level was determined to be lower and mean CRP and median beta-2 microglobulin levels were determined to be higher among deceased patients, and these were determined to be risk factors for mortality (p < 0.05). In a study that evaluated beta-2 microglobulin among patients with MCL, beta-2 microglobulin was reported to be associated with tumor burden and advanced disease [11].
When patients' response to treatment was evaluated, while the ratio of patients whose response to initial treatment was CR was higher among survivors, the PR response rate did not significantly differ between deceased and surviving patients. In deceased patients, however, the SD response rate and PD response rate were determined to be higher. Lack of response to initial treatment was observed to be a significant risk factor for mortality among patients with MCL. All of our patients had received a rituximab-based chemotherapy regimen. Our outcomes were consistent with those of similar studies in which responses to rituximab-supported treatment were examined among patients with MCL. The rate of ASCT was statistically significantly higher among survivors, in parallel with similar studies [12-14].
When patients' performance and accompanying comorbidities were evaluated, it was determined that ECOG scores of survivors were 0-1 and the rate of ECOG scores of 3-4 was higher among deceased patients. With these outcomes, ECOG performance was shown to be associated with mortality in our study. Rates of DM, HT, and CAD were determined to be higher among deceased patients and these were determined to be significant risk factors for mortality. These outcomes were thought to be obtained because of treatment-associated toxicity, failure to administer high-dose chemotherapy, and lack of currently available targeted treatments. Townsley and colleagues obtained similar outcomes in patients with ECOG performance scores of 3 and above [15]. In studies conducted by Hamaker, et al. in which age and other comorbidities were evaluated, similar outcomes were obtained in the patient group due to advanced age, patients not being suitable for treatment due to their comorbidities, and treatment-associated toxicity [16,17]. In the study conducted by Cohen, et al. comorbidities were also shown to be associated with mortality. When association of the Ann Arbor stage with mortality was reviewed, the rate of stage 4 was determined to be higher among deceased patients and the Ann Arbor stage was thought to be associated with mortality [18]. Also, in other previous studies on non-Hodgkin lymphomas, advanced Ann Arbor stage was proven to be associated with poor prognosis, which is consistent with the outcomes of our study [17,18].
In our study the presence of bone marrow involvement was observed to increase the risk of mortality 4.37-fold, and when deceased patients were evaluated, the rate of CNS involvement, rate of B symptoms, rate of bone marrow involvement, and rate of extranodal involvement were determined to be higher. Furthermore, these findings were determined to be risk factors for mortality. Consistently with the study conducted by Cohen, et al. the presence of extranodal disease and B symptoms were proven to be negative prognostic indicators in our study, as well [18].
When the prognostic scores of our patients were evaluated, the median MIPI-B, MIPI, IPI, and Ki-67 proliferation index levels were determined to be higher among deceased patients. MIPI and MIPI-B rates were also observed to be higher among deceased patients. MIPI-B, bone marrow involvement, and rate of response to initial treatment were determined to be independent risk factors that increased the risk of mortality. Consistently with the results of the study conducted by Yoo, et al. the Ki-67 proliferation index, which was also used as a biological marker in our study, was determined to be a significant prognostic marker [11]. In general, the data that we obtained from our study were consistent with those of other prognostic studies on MCL [19-21].
Limitations of this study included it being a retrospective study; differences in chemotherapies administered, despite the inclusion of rituximab treatment in the treatment schemes of all patients; and lack of a control group in regard to patients' ages, treatments, and stages.
In conclusion, this study is the first study to reveal that MIPI and MIPI-B scores are important prognostic markers in patients diagnosed with MCL in the Turkish population. Additionally, direct association of the presence of bone marrow and CNS involvement and failure to respond to initial treatment with mortality has been demonstrated. For patients with MCL who have these findings, high-dose chemotherapy, ASCT, and new agents must be considered. This group of patients should be closely monitored for relapse-refractory courses. In order to obtain better outcomes, multi-center prospective studies in which randomization in regard to age, stage, prognostic score, performance, and treatment is ensured and new agents are included are needed.
References
- Swerdlow SH, Campo E, Harris NL, et al. (2008) WHO classification of tumors of the hematopoietic and lymphoid tissues. IARC, Lyon, France.
- (1997) A clinical evaluation of the international lymphoma study group classification of non-hodgkin's lymphoma. The non-hodgkin's lymphoma classification project. Blood 89: 3909-3918.
- Tiemann M, Schrader C, Klapper W, et al. (2005) Histopathology, cell proliferation indices and clinical outcome in 304 patients with mantle cell lymphoma (MCL): A clinicopathological study from the European MCL Network. Br J Haematol 131: 29-38.
- Mozos A, Royo C, Hartmann E, et al. (2009) SOX11 expression is highly specific for mantle cell lymphoma and identifies the cyclin D1-negative subtype. Haematologica 94: 1555-1562.
- Bernard M, Gressin R, Lefrère F, et al. (2001) Blastic variant of mantle cell lymphoma: A rare but highly aggressive subtype. Leukemia 15: 1785-1791.
- Herrmann A, Hoster E, Zwingers T, et al. (2009) Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol 27: 511-518.
- Hoster E, Dreyling M, Klapper W, et al. (2008) A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood 111: 558-565.
- Howard OM, Gribben JG, Neuberg DS, et al. (2002) Rituximab and CHOP induction therapy for newly diagnosed mantle-cell lymphoma: Molecular complete responses are not predictive of progression-free survival. J Clin Oncol 20: 1288-1294.
- Dreyling M, Lenz G, Hoster E, et al. (2005) Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: Results of a prospective randomized trial of the European MCL Network. Blood 105: 2677-2684.
- Abrahamsson A, Albertsson-Lindblad A, Brown PN, et al. (2014) Real world data on primary treatment for mantle cell lymphoma: A nordic lymphoma group observational study. Blood 124: 1288-1295.
- Yoo C, Yoon DH, Kim S, et al. (2016) Serum beta-2 microglobulin as a prognostic biomarker in patients with mantle cell lymphoma. Hematol Oncol 34: 22-27.
- Hermine O, Hoster E, Walewski J, et al. (2012) Alternating courses of 3x CHOP and 3x DHAP plus rituximab followed by a high dose ARA-C containing myeloablative regimen and autologous stem cell transplantation (ASCT) increases overall survival when compared to 6 courses of CHOP plus rituximab followed by myeloablative radiochemotherapy and ASCT in mantle cell lymphoma: Final analysis of the MCL younger trial of the European mantle cell lymphoma network (MCL net). Blood 120.
- Geisler CH, Kolstad A, Laurell A, et al. (2008) Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: A nonrandomized phase 2 multicenter study by the Nordic lymphoma group. Blood 112: 2687-2693.
- Griffiths R, Mikhael J, Gleeson M, et al. (2011) Addition of rituximab to chemotherapy alone as first-line therapy improves overall survival in elderly patients with mantle cell lymphoma. Blood 118: 4808-4816.
- Augustin A, Le Gouill S, Gressin R, et al. (2018) Survival benefit of mantle cell lymphoma patients enrolled in clinical trials; a joint study from the LYSA group and French cancer registries. J Cancer Res Clin Oncol 144: 629-635.
- Hamaker ME, Stauder R, van Munster BC (2014) Exclusion of older patients from ongoing clinical trials for hematological malignancies: An evaluation of the National Institutes of Health Clinical Trial Registry. Oncologist 19: 1069-1075.
- Christian Schmidt, Sebastian Fetscher, Christian Görg, et al. (2011) Treatment of indolent lymphoma in Germany - results of a representative population-based survey. Clin Lymphoma Myeloma Leuk 11: 204-211.
- Cohen JB, Han X, Jemal A, et al. (2016) Deferred therapy is associated with improved overall survival in patients with newly diagnosed mantle cell lymphoma. Cancer 122: 2356-2363.
- Bea S, Valdes-Mas R, Navarro A, et al. (2013) Landscape of somatic mutations and clonal evolution in mantle cell lymphoma. Proc Natl Acad Sci U S A 110: 18250-18255.
- Zhang J, Jima D, Moffitt AB, et al. (2014) The genomic landscape of mantle cell lymphoma is related to the epigenetically determined chromatin state of normal B cells. Blood 123: 2988-2996.
- Hoster E, Klapper W, Hermine O, et al. (2014) Confirmation of the mantle-cell lymphoma international prognostic index in randomized trials of the European mantle-cell lymphoma network. J Clin Oncol 32: 1338-1346.
Corresponding Author
Mehmet Ali Uçar, MD, Department of Hematology, University of Mersin, Ankara Numune Training and Research Hospital, Turkey, Tel: 0905057591874.
Copyright
© 2019 Uçar MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.