Postoperative Analgesia in Laparoscopic and Robotic Rectal Cancer Surgeries Compared to Laparotomy Approach: A Retrospective Analysis
Abstract
Purpose: Although epidural analgesia (EA) is recommended for laparotomic (LT) rectal surgery, there is no consensus regarding pain management in laparoscopic or robotic (LR) rectal cancer surgery. According to our local guidelines, EA is usually chosen for LT rectal procedures and intravenous analgesia for LR rectal procedures.
Methods: This retrospective study included patients who underwent rectal cancer surgery in our center from January 2016 to February 2020 using either laparotomy (LT) or laparoscopic or robotic (LR) techniques. Analgesia technique varied according to surgery technique, the choice of patient and anesthesia provider. Data were acquired from electronic databases and consisted of pain scores in the recovery room and the three postoperative days, morphine consumption and total length of hospital stay.
Results: 151 patients were included: 92 in the LR group and 59 in the LT group. Epidural analgesia was used for 8/92 LR patients and of 48/59 LT patients. Pain scores were comparable regardless of the surgical and analgesic technique in the three postoperative days, but patients with epidural analgesia used significantly less morphine, even in the LR group. After correction for multiple pairwise comparisons, our results showed that in laparoscopic rectal surgery, the use of epidural analgesia was associated with significantly lower morphine consumption (corrected p = 0.01, reduction over 3 days: 25 mg).
Conclusion: Compared to other analgesia regimens, perioperative epidural analgesia for LR rectal surgery was associated with significantly less postoperative morphine consumption to achieve the same pain scores. EA appears to be beneficial even in laparoscopic rectal surgery.
Keywords
Robotic rectal surgery, Postoperative analgesia, Rectal cancer, Epidural analgesia, Rectal surgery, Postoperative pain
Abbreviations
ASA: American Society of Anesthesiologists; BIS: Bispectral Index; CI: Confidence Interval; EA: Epidural Analgesia; EMR: Electronic Medical Record; ICU: Intensive Care Unit; LR: Laparoscopic or Robotic Surgery; LT: Laparotomic; NRS: Numeric Rating Scale; PACU: Postanesthesia Care Unit; PCA: Patient Controlled Analgesia; PCEA: Patient Controlled Epidural Analgesia; PPC: Postoperative Pulmonary Complication; RF: Respiratory Failure; SpO2: Peripheral Blood Oxygen Saturation By Pulse Oximetry; SPS: Subjective Pain Score
Introduction
Rectal cancer surgery has traditionally been performed under laparotomy (LT), which carries a high risk of postoperative morbidity and high level of postoperative pain [1,2]. Over the past two decades, laparoscopic procedures (with or without robotic assistance) have become popular in colorectal surgeries due to their shorter incision, reduced blood loss, faster recovery of bowel motion, decreased length of hospital stay, and improved recovery time [3-5].
For surgical treatment of rectal cancer, total mesorectal excision is recommended to reduce the local recurrence rate and obtain optimal oncological results [6]. This makes laparoscopic rectal surgery more difficult than laparoscopic colon surgery. Robotic technologies have been developed to overcome the limitations of laparoscopic surgery. A surgical robot can be manipulated by the surgeon, allowing three-dimensional vision and finer dissection while providing the operator with better comfort in terms of ergonomics and tremor control [7,8].
Postoperative pain management is the cornerstone of enhanced recovery after surgery. For colorectal cancer resection surgeries, epidural analgesia (EA) is recommended by European Society of Regional Anesthesia when surgery is performed with laparotomy techniques. No consensus exist for patients undergoing specifically laparotomic rectal cancer surgeries, but EA is usually proposed to then, like other colon cancer surgeries. For laparoscopic colorectal surgery, some authors recommend EA [9,10], whereas others report a comparable quality of analgesia when using an intravenous analgesic strategy [11]. These conflicting results may be partly related to the inclusion in the studies of patients who underwent recto-colic as well as colic surgery. We think that these two populations (rectal surgery vs colon surgery) should be differentiated and pain control after rectal cancer surgery to be addressed separately. We think that including rectal surgeries with colon surgeries is a cause of heterogeneity in the results of the pain control studies found in the literature.
Our local guidelines for postoperative analgesia favored intravenous intraoperative analgesia (and not epidural analgesia) for robotic and laparoscopic surgery in accordance with international recommendations [12]. After several years of experience, it is necessary to assess the effectiveness of this strategy. To this end, we conducted a retrospective evaluation to compare opioid consumption and postoperative pain intensity in patients undergoing either laparotomy or laparoscopic rectal surgery performed under general anesthesia, with or without epidural analgesia.
Methods
This retrospective study included patients who underwent rectal cancer surgery at our center from January 2016 to February 2020. At our institution, each patient provided written consent at the first consultation and accepted that anonymized data from medical records could be used for research purposes. In addition, health data collection and use were declared to the French Data Protection Authority [enregistration number: 2224445]. Treatment and surgical techniques were decided mainly based on surgical and oncological data during a multidisciplinary meeting that included surgeons, chemotherapists, and radiotherapists. The protocols did not include medical contraindications for laparoscopic or robotic surgeries. All surgeries were performed by surgeons experienced in digestive and laparoscopic surgeries.
In all surgical groups, anesthesia was maintained using halogenated agents after induction with propofol (2-2.5 mg/kg) to obtain a bispectral index (BIS) of 40-60. A non-depolarized muscle relaxant agent was continuously infused, with rate adjustments according to quantitative monitoring (Train of Four, Philips) and the demand of the operator. Patients received intraoperative ketamine infusion (a bolus of 0.3 mg/kg, followed by 0.15 mg/kg/h), which was stopped at the beginning of closure.
According to our local guidelines, EA was favored in the LT group and not in the LR group. The analgesia technique varied according to the type of surgery and the choice of patient and anesthesia provider. When EA was used in association with general anesthesia, epidural infusion of 2% ropivacaine was started before incision or wound closure. To reduce the risk of respiratory depression, intravenous (IV) morphine administration was avoided during the postoperative epidural infusion mixture of ropivacaine 0.2% (10 mg/h) and morphine (125 µg/h). When no EA was used, patients received intraoperative continuous IV infusion of 2% lidocaine (a bolus of 1.5 mg/kg, followed by 1.5 mg/kg/h, then 1.3 mg/kg/h for 45 min before recovery) along with target-controlled infusion of remifentanil adjusted for hemodynamic parameters. This protocol was also applied to patients in the laparotomy group who had either a contraindication to or failure of EA.
From medical records, we extracted demographic data, age, weight, height, medical and surgical antecedents (cardiorespiratory history), and preoperative treatments (particularly analgesic and neuropsychiatric treatments). Anesthesia data were recorded using an automatic record-keeping system and stored in a relational database (Centricity Anesthesia General Electric). This tool has recently been specifically evaluated and missing data was less than 5% [13,14]. Data regarding events, blood loss, and drug injections were entered by the anesthesia team in structured fields, with fixed information regarding the average dose and units. The injected quantities of remifentanil, ketamine, and ropivacaine were directly recorded from the pump through an interface. During anesthesia and in the Post-Anesthesia Care Unit (PACU), the administered amounts of morphine and other analgesic agents (paracetamol, nefopam, and tramadol) were recorded using the same computerized tool.
Data concerning the duration of surgery and anesthesia, as well as pain scores at the arrival and exit of the PACU, were extracted from our hospital software. In the PACU, a subjective pain scale (SPS) of 0-4 was used: 0 = no pain, 1 = mild pain, 2 = strong pain, 3 = intense pain, and 4 = excruciating pain. After the patients exited the PACU, pain intensity was measured on a numerical rating scale (NRS) of 0-10. These data, as well as the total consumption of morphine and other analgesics, were extracted using Medasys France DxCare software.
The two primary study endpoints were pain scores on the three postoperative days, as well as postoperative morphine consumption, in the LT and LR groups and in patients with or without EA. The secondary endpoint was hospital length of stay. In the exploratory approach, a subgroup analysis was performed between robotic and laparoscopic surgery.
All statistical analyses were performed using the R statistical software (version 4.0.2). Categorical variables were compared using the χ2 test, and continuous variables were compared using the Student's t-test. The ANOVA test was used to compare different means, and the Kruskal-Wall is test was used when conditions of normality were not met. Statistical significance was set at P < 0.05. When the ANOVA test was significant, the Games-Howell post-hoc test was used to perform pairwise comparisons, with Tukey's method correction for multiple comparisons. When the Kruskal-Wall is test was significant, the pairwise Wilcoxon test was used to compare all possible combinations of group differences, with Bonferroni correction for multiple comparisons. This paper follows the standards for reporting observational studies outlined therein (https://www.strobe-statement.org/checklists/).
Results
Among 155 patients who underwent rectal surgery during the study period, 151 were included in this study: 92 in the LR group and 59 in the LT group. Four patients were excluded because hyperthermic intraperitoneal chemotherapy was associated with surgical resection. Table 1 presents the patients' general characteristics according to surgery type (LR vs. LT). Three laparoscopy cases and one robotic case were converted to a laparotomy. Medical history, cardiopulmonary disease, age, sex, and the presence of preoperative pain did not differ between the two groups (LR and LT) (Table 1). The surgery duration was significantly longer (P = 0.005) in the LR (343.09 ± 106 min) than in the LT (290.66 ± 118 min). As expected, EA was significantly more frequent (P < 0.001) in LT (81%) than in LR (11%) patients.
Table 2 presents the preoperative and intraoperative variables according to the type of surgery (LR vs. LT) and presence or absence of epidural analges (PCEA).
In patients who did not receive epidural analgesia, continuous intravenous lidocaine was administered to 74.4% in the LR group and 63.6% in the LT group (p = 0.694). Patients in the PCEA group received less remifentanil (P < 0.001).
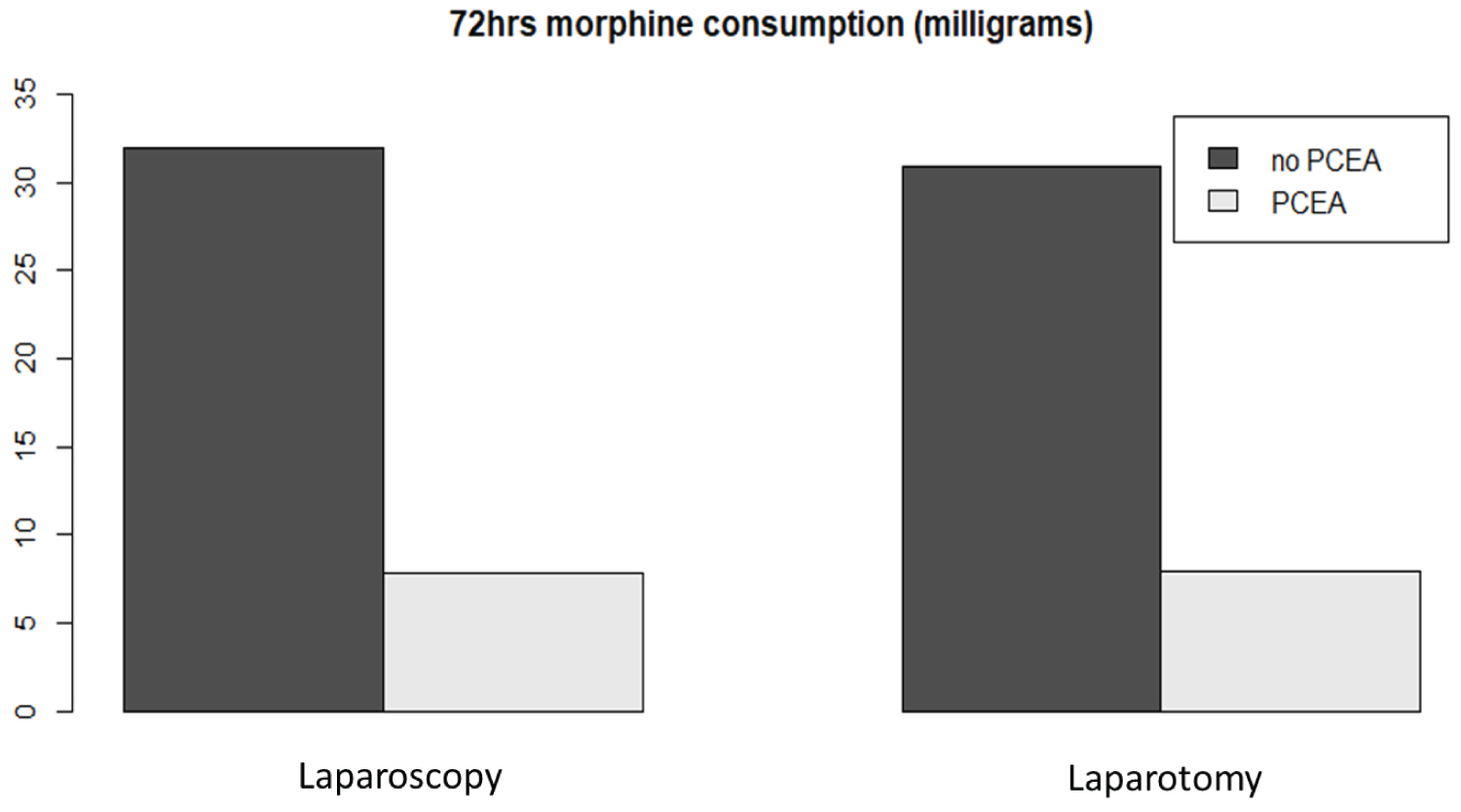
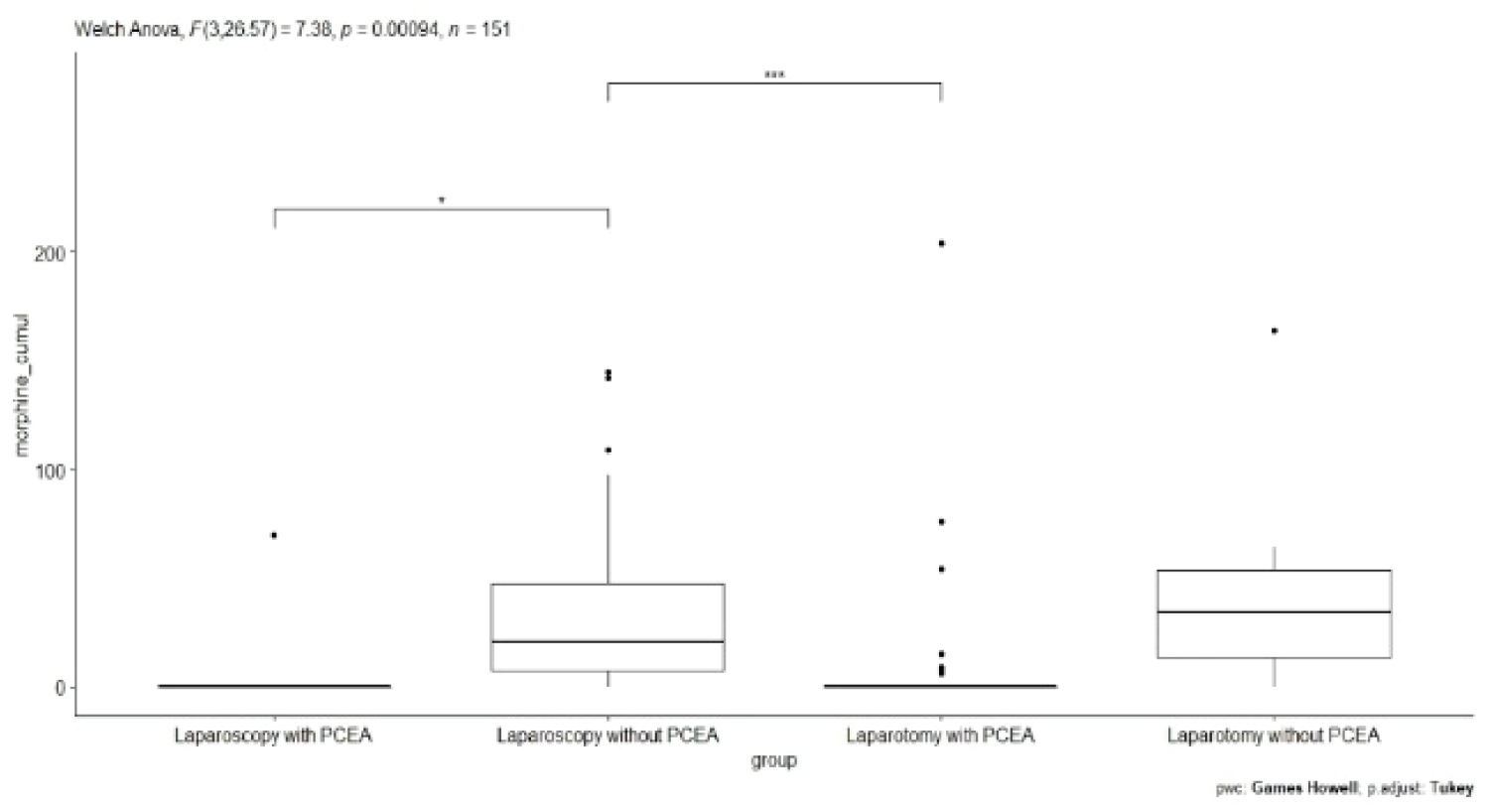
Postoperative pain scores on the first three postoperative days were comparable regardless of the surgical or analgesic technique, with no significant differences in pain scores among the groups (Table 3). Despite similar maximal pain scores, morphine consumption was significantly different between the groups (Table 3 and Figure 1). High standard deviations suggest high interindividual variations in morphine consumption. After pairwise comparisons with correction for multiple comparisons, patients who underwent laparoscopic rectal surgery with PCEA used significantly less morphine than those who underwent laparoscopic surgery without PCEA (p = 0.01, morphine reduction over 3 days: 25 mg) (Figure 2 and Table 4). The patients in both groups showed no difference in maximal pain scores. Interestingly, patients who underwent laparotomy with PCEA used significantly less morphine than those who underwent laparoscopy without PCEA (adjusted p-value = 0.000503). Interestingly, the length of hospital stay was shorter in the LR group (median = 10 days) than in the LT group (median = 14 days), without a significant role for the presence or absence of EA.
In the laparoscopic group (LR), a subgroup exploratory analysis was performed between patients who underwent robotic surgery and those who underwent classic laparoscopic surgery. No differences were found among the different variables (pain score and morphine consumption) except for the duration of surgery (Table 5). In the PACU, postoperative pain was low and did not differ significantly between the two groups (Table 5).
Very few patients experienced severe or excruciating pain in the PACU: 8 in the LR group and 3 in the LT group. The majority of patients received no rescue analgesic treatment (ketamine or lidocaine) in the PACU, indicating reasonably good pain control during this period. Among the 58 patients with PCEA, two experienced PCEA failure with a very high pain level (score 3-4) that justified setting up morphine PCA after titration: Early after PACU admission.
Discussion
Based on the previously determined endpoints, the level of pain during the first three days postoperatively was comparable in both surgical groups, regardless of the analgesic technique used. Nevertheless, morphine consumption was significantly lower when PCEA was used to control postoperative pain regardless of the surgical technique used. These results are in agreement with the literature, which describes better efficacy of EA after LT, all else being equal [15]. Although the data available from previous studies seem to be sufficient to abandon EA for laparoscopic colon resections, the evidence is insufficient to do so for rectal resections due to the small number of rectal resections included in randomized trials [16,17].
Our present results are consistent with the meta-analysis findings that laparoscopy had no benefit in terms of postoperative pain compared with laparotomy for rectal surgery [18]. The presently observed increase in pain scores upon return to the ward, especially on postoperative day 1, indicates an alteration of care in all groups, which is in agreement with observations after rectal surgery in prior studies. Once a patient is in the surgical ward, support analgesia is often not adjusted in patients with high pain by increased flow of EA or the addition of other analgesic drugs. Support from a nurse specializing in pain management would be useful for identifying patients whose pain level warrants a change in treatment.
In agreement with previous findings, we observed large individual variations in pain and morphine consumption, regardless of the surgical technique. Locoregional analgesia during and after surgery reportedly allows for a reduction in intraoperative and postoperative opioid consumption [19]. We found that EA was effective in the vast majority of patients who did not require postoperative morphine injections, as previously reported [20]. Among patients with PCEA failure, the average pain level was > 5 on postoperative day 3, despite larger morphine doses. Except for patients with PCEA failure, none of the patients with EA received IV morphine or reported a pain level > 4 over 10. The average dose of morphine decreased over time after postoperative day 1, but the individual variations were substantial. As epidural failure has major clinical consequences, particular attention should be paid to holding the catheter in place and to quickly manage pain in the event of failure, for example, via installation of a new catheter or setup of a morphine PCA. When EA was efficient, morphine was not necessary during the first postoperative day. Most previous studies have allowed morphine administration in cases of insufficient EA and have used daily morphine consumption as the primary endpoint. This was not the case in our present study because the systematic use of epidural morphine prevents the addition of more intravenous morphine. The confrontation between the pain location and the extension of the sensory block enables the option of ropivacaine reinjection.
A prospective cohort study compared open versus laparoscopic surgery and reported that perioperative administration of intravenous lidocaine and ketamine to opioids did not improve postoperative pain perception or decrease morphine equivalents. Notably, pain peaks remained early after minimally invasive surgery and after epidural removal for open surgery [21]. Levy, et al. [22] conducted a meta-analysis and suggested that the none of the analgesia protocols were shown to be clearly superior. Moreover, Kehlet, et al. [23] demonstrated that EA may not be necessary in laparoscopic colorectal surgery and can be replaced with non-opioid analgesia. However, this was not confirmed in a more recent study that demonstrated that thoracic EA provided better analgesia than intravenous lidocaine in patients undergoing laparoscopic rectal surgery [10].
Laparoscopic and robotic patients received intraoperative ketamine/lidocaine infusion followed by morphine titration, which provided comparable analgesia quality on average, but with high variability in pain levels and morphine consumption. A study of a similar patient population also showed this variability among patients and from one day to another in the same patient [16,17]. These differences can be induced by acute pain during early mobilization. Better pain control should be expected in the PCA group, either through better handling of the PCA or the prescription of additional agents. These findings support individual analgesic adjustments by acute pain services in the wards.
With regard to a morphine-sparing strategy to limit potential cancer risk recurrence [24] and to avoid opioid-induced hyperalgesia [19] or dependency, it seems logical to prefer EA over intravenous analgesia. This choice may be reinforced by the fact that intraoperative intravenous lidocaine administration was identified as an independent predictive factor for increased postoperative morbidity [25]. The authors of a meta-analysis also attempted to compare the effects of lidocaine infusion with placebo or EA [26], but the methodological shortcomings of several studies prevented clear conclusions.
Our study has several limits: It is a single-center retrospective study, the small number of patients in the "laparotomy, no epidural analgesia" subgroup do not allow generalizability, but it is a real-life situation because the vast majority of patients underhoing laparotomy procedures in our center have epidural analgesia offered (due to pre-established protocols as well as the European Society of Regional anesthesia recommandations). Another important limitation is the absence of available data on postoperative morbidity, e.g., postoperative complications including anastomotic insufficiency, wound infection, ileus or urinary tract infections.
The advantage of this type of study is that it describes real-life situations, including hazards of daily care. Similarly, according to the pre-established protocols, the vast majority of laparotomy patients were treated with EA, whereas patients undergoing other surgery types were treated with morphine PCA.
This retrospective study was performed using structured information contained in the computerized records. The data completeness at our institution was evaluated to be > 90% [13]. However, the bias of the missing data cannot be completely eliminated. Additionally, predefined protocols limit the freedom of individual prescriptions, with most patients receiving standardized analgesic treatment. Individual variability in pain scores can be linked to the choice of the maximum daily NRS value. Unfortunately, we didn't have separate pain scores, such as rest and during mobilization, neither prevalence of morphine related side effects, such as nausea, vomiting or urine retention in both groups, to do finer analysis.
In conclusion, our retrospective study showed that perioperative analgesia with intravenous ketamine/lidocaine infusion during laparoscopic or robotic surgery was associated with the same postoperative pain scores as epidural analgesia in laparotomy, but at the expense of higher morphine consumption. These findings suggest that EA could reduce morphine consumption, along with known or suspected adverse effects, in rectal cancer patients. The use of epidural analgesia appears to play a major role in a morphing-sparing strategy, even in rectal laparoscopic surgery. Randomized controlled clinical trials are needed to validate these results. For all analgesic strategies, high interindividual variability of pain intensity and morphine consumption suggests prompt pain management in cases of analgesia failure.
Disclosures
Xiao Wang: This author helped with data acquisition for the study, wrote the manuscript, revised it critically for important intellectual content, and approved the final version to be published; Jamie Elmawieh: This author made the statistical analysis, participated in the writing of the article, and approved the final version to be published; Philippe Sitbon: This author helped with data analysis for the study, revised the study critically for important intellectual content, and approved the final version to be published; Jean-Louis Bourgain: This author helped with data acquisition for the study, revised the study critically for important intellectual content, and approved the final version to be published.
Funding
No disclosure of funding.
Conflicts of Interest
None.
References
- Johnson MD, Walsh RM (2009) Current therapies to shorten postoperative ileus. Cleve Clin J Med 76: 641-648.
- Story SK, Chamberlain RS (2009) A comprehensive review of evidence-based strategies to prevent and treat postoperative ileus. Dig Surg 26: 265-275.
- Abraham NS, Young JM, Solomon MJ (2004) Meta-analysis of short-term outcomes after laparoscopic resection for colorectal cancer. Br J Surg 91: 1111-1124.
- Jacobs M, Verdeja JC, Goldstein HS (1991) Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc 1: 144-150.
- Strohlein MA, Grutzner KU, Jauch KW, et al. (2008) Comparison of laparoscopic vs. open access surgery in patients with rectal cancer: A prospective analysis. Dis Colon Rectum 51: 385-391.
- Wibe A, Syse A, Andersen E, et al. (2004) Oncological outcomes after total mesorectal excision for cure for cancer of the lower rectum: anterior vs. abdominoperineal resection. Dis Colon Rectum 47: 48-58.
- Corcione F, Esposito C, Cuccurullo D, et al. (2005) Advantages and limits of robot-assisted laparoscopic surgery: Preliminary experience. Surg Endosc 19: 117-119.
- Ng KH, Lim YK, Ho KS, et al. (2009) Robotic-assisted surgery for low rectal dissection: From better views to better outcome. Singapore Med J 50: 763-777.
- Levy BF, Scott MJ, Fawcett W, et al. (2011) Randomized clinical trial of epidural, spinal or patient-controlled analgesia for patients undergoing laparoscopic colorectal surgery. Br J Surg 98: 1068-1078.
- Wongyingsinn M, Baldini G, Charlebois P, et al. (2011) Intravenous lidocaine versus thoracic EA: A randomized controlled trial in patients undergoing laparoscopic colorectal surgery using an enhanced recovery program. Reg Anesth Pain Med 36: 241-248.
- Neudecker J, Schwenk W, Junghans T, et al. (1999) Randomized controlled trial to examine the influence of thoracic EA on postoperative ileus after laparoscopic sigmoid resection. Br J Surg 86: 1292-1295.
- Gustafsson UO, Scott MJ, Schwenk W, et al. (2013) Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS((R))) Society recommendations. World J Surg 37: 259-284.
- Motamed C, Bourgain JL (2016) An anaesthesia information management system as a tool for a quality assurance program: 10years of experience. Anaesth Crit Care Pain Med 35: 191-195.
- Weil G, Motamed C, Eghiaian A, et al. (2015) The use of a clinical database in an anesthesia unit: Focus on its limits. J Clin Monit Comput 29: 163-167.
- Sitbon P (2017) Place de l'analgésie péridurale en 2016 en dehors de l'obstétrique. Anesth Réanim 3: 135-146.
- Kang SB, Park JW, Jeong SY, et al. (2010) Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): Short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 11: 637-645.
- Tolstrup R, Funder JA, Lundbech L, et al. (2018) Perioperative pain after robot-assisted versus laparoscopic rectal resection. Int J Colorectal Dis 33: 285-289.
- Memon MA, Yunus RM, Memon B, et al. (2018) A Meta-Analysis and Systematic Review of Perioperative Outcomes of Laparoscopic-assisted Rectal Resection (LARR) Versus Open Rectal Resection (ORR) for Carcinoma. Surg Laparosc Endosc Percutan Tech 28: 337-348.
- Fletcher D, Martinez V (2014) Opioid-induced hyperalgesia in patients after surgery: A systematic review and a meta-analysis. Br J Anaesth 112: 991-1004.
- Motamed C, Farhat F, Remerand F, et al. (2006) An analysis of postoperative EA failure by computed tomography epidurography. Anesth Analg 103: 1026-1032.
- Grass F, Cachemaille M, Martin D, et al. (2018) Pain perception after colorectal surgery: A propensity score matched prospective cohort study. Biosci Trends 12: 47-53.
- Levy BF, Tilney HS, Dowson HM, et al. (2010) A systematic review of postoperative analgesia following laparoscopic colorectal surgery. Colorectal Dis 12: 5-15.
- Kehlet H (2008) Fast-track colorectal surgery. Lancet 371: 791-793.
- Wuethrich PY, Hsu Schmitz SF, Kessler TM, et al. (2010) Potential influence of the anesthetic technique used during open radical prostatectomy on prostate cancer-related outcome: A retrospective study. Anesthesiology 113: 570-576.
- Maggiori L, Rullier E, Lefevre JH, et al. (2017) Does a combination of laparoscopic approach and full fast track multimodal management decrease postoperative morbidity? A Multicenter Randomized Controlled Trial. Ann Surg 266: 729-737.
- Weibel S, Jelting Y, Pace NL, et al. (2018) Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery in adults. Cochrane Database Syst Rev 6: CD009642.
Corresponding Author
Jamie ELMAWIEH, Department of Anesthesiology, Gustave Roussy Cancer Campus, Paris Saclay University, Villejuif, France
Copyright
© 2022 WANG X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.