Algal and Cyanobacterial Polysaccharides as Promising Antiviral Agents against Human Viruses
Abstract
Recently the morbidity, mortality and economic losses due to rapid transmission of Coronavirus (COVID-19) worldwide has incentivatedan urgent call for the scientists to discover efficient antiviral drugs for human health. Marine polysaccharides have long been recognized for their biological activities with huge application potentials in biomedical field. Their antiviral activities have been well documented against various types of infectious viruses and new studies proving their antiviral potential are frequently coming out. This paper is an attempt to compile the latest developments in research regarding the antiviral potential of algal polysaccharides, including macroalgae (red, brown, green), microalgae and cyanobacteria, against infectious human viruses. Furthermore, their possible mechanisms of antiviral actions will also be discussed briefly. This information will provide an insight for the utilization of algal polysaccharides to prevent the virulent viruses which are yet to be well treated.
Keywords
Algal polysaccharides, Antiviral activity, Mechanisms, Drug development, Sulfated polysaccharide
Introduction
Algae, have great potential as the producers of commercially significant natural compounds with numerous nutraceutical and pharmaceutical applications [1-3]. Algae are a diverse group of oxygen-generating photosynthetic organisms with simple reproductive system which play a key role in ecosystems as primary producers. Macroalgae are multi cellular organisms, on the basis of pigmentation they are generally classified as Phaeophyta (brown algae) Rhodophyta (red algae) and Chlorophyta (green algae) [4]. Both macro and microalgae are significant owing to their capability to accumulate a huge array of compounds with variety of biological activities. At cellular level, algae produce primary and secondary metabolites including complex organic compounds, polyunsaturated fatty acids, phytopigments (xanthophylls and carotenoids), phenolic substances, docosahexaenoic acid, carbohydrates, vitamins, tannins, peptides and Terpenoids [5-10]. The chemical composition of algae varies greatly among species with growth habitats, environmental conditions, seasonality and geographic areas. Algae are remarkable living cells as a source of valuable bioactive molecules since several species are Generally Recognized as Safe (GRAS) attributable to lack of harmful toxins and other pathogenic microbes [11]. The FDA (Food and Drug Administration) has approved several marine-derived natural compounds as therapeutic drugs, and many of them are currently in various stages of clinical trials [12]. Around 40 compounds of sulfated polysaccharides from marine algae are commercially available in the market, and many more are being implemented at the preclinical or clinical stages of human trials [13].
Polysaccharides, as natural bioactive compounds with useful chemical and biological characteristics, are gaining extensive research interests in recent times. Polysaccharides have also been broadly investigated for their biomedical and pharmaceutical application potentials [14]. Marine sulfated polysaccharides are being investigated as a possible source of biologically active compounds for drug development [15]. Algal polysaccharides have been known to have antitumor, antiviral, antioxidant, antimicrobial, anticoagulant, and anti-inflammatory effects [13,16].
In recent years, despite the development of vaccines and drugs to treat various infectious and/or harmful viruses, rapid spread of viral diseases are striking the world to a great extent. For instance, Human Immunodeficiency Virus (HIV) and Hepatitis C Virus (HCV) are serious threat to human health worldwide. Some viruses, such as Herpes Simplex Viruses (HSV-1 and HSV-2), dengue virus, and most respiratory-tract viruses, have no reliable vaccines [17]. Moreover, new viral infectious diseases have emerged in recent times. Ongoing worldwide pandemic of COVID-19 since the end of last year is a fitting example to quote here. It is found that the virus mutations are constantly occurring in infection, therefore, new antivirals are indispensable [18]. Toxicity, drug efficacy, and costs of the vaccines are still the unsolved problems, which are predominantly higher in developing world due to the inaccessibility of drugs [19]. Likewise, the emerging resistance to existing antiviral treatments by many viruses such as HIV type 1 or HSV-1 is becoming a major impediment in the treatment of viral diseases, encouraging the search for more efficient antiviral agents [17]. Nowadays, microalgal and cyanobacterial compounds have already gained considerable attention as potential antiviral agents. These natural compounds from marine sources are deliberated as the most auspicious antiviral agents with minimum toxicity to host cells. For instance, sulfated polysaccharides including fucoidans, p-KG03, heparin and dextran sulfate are known for their broad-spectrum antiviral activity against several types of viruses [20]. This review work is thus carried out to present the updated information about the antiviral activities of marine algae (macro, microalgae and cyanobacteria), by focusing on their polysaccharides. This review focuses on reporting the main advances published over the last 15-20 years.
Potential Antiviral Algal Polysaccharides
Macroalgae polysaccharides
Polysaccharides are abundant in macroalgae, accounting for up to 70% of their dry weight. In recent time, special research consideration has been given to the isolation and characterization of marine macroalgae due to their biological activities and plentiful health benefits in food and medical sectors. Among the algae polysaccharides, macroalgae polysaccharides were extensively studied, compared to their unicellular microalgal and cyanobacterial counterparts. Polysaccharides are complex and heterogeneous macromolecules made up of long chains of monosaccharides that are joined together by glycosidic linkages. They break down into its constituent oligosaccharides or monosaccharides when hydrolyzed. They have a variety of structures, ranging from heavily branching to linear. Algal polysaccharides mostly include mucopolysaccharides, storage, and cell wall polysaccharides. Laminarin, alginic acid, sargassan, and fucodin are found in brown algae. Carrageenans, agars, xylans, galactan, floridean, and porphyrin as a mucopolysaccharide are found in red algae. Whereas, green algae contain galactans, xylans and sulfuric acid polysaccharides [21]. The algal polysaccharides are species-specific and grouped into sulfated and non-sulfated categories [22, 23]. Their composition and proportion varies greatly among species with growth, morphological stage of algal life cycle and environmental conditions, seasonality and geographic areas [24, 25]. Sulfated polysaccharides (S-PS) areglycosaminoglycans, contains hemi-ester sulfate groups in the sugar residues, which play storage and structural roles in algae. The biological activities of S-PS normally depends on their complex interaction of structural features such as, molecular weight, sulfation, branching, glycosidic linkages, sulfate content, sugar composition and stereochemistry [1]. Therefore, understanding of their structures may lead to successful elucidation of their biological activities. Many species of marine algae contain significant amount of S-PS that have been shown invivo/invitro activity against a broad range of viruses such as HIV, herpes, tagoviruses, paramyxoviruses, rhadoviruses and dengue virus [26-28]. Polysaccharides from various marine algae have been extensively investigated for their biological activities [1, 21, 29].
Red algae (Rhodophyta)
Red algae are one of the ancient and largest groups of eukaryotic algae that are known as the source of unique sulfated galactans. The red algae usually contain hydrocolloids (carrageenan and agar), xylans, floridian, starch, cellulose and mannans [30, 31]. Among them, carrageenans are extensively studied for their antiviral potential against a variety of viruses.
Carrageenan is an anionic S-PS with high molecular weight (MW), composed of alternate units of α-d-galactose or 3,6-anhydro-α-d-galactose and β-d-galactose, linked together by α-(1,3) and β-(1,4) glycosidic bound [32]. Carrageenan is most widely studied polysaccharide of red algae which is mostly extracted from the genera Chondrus, Gigartina, Hypnea, and Eucheuma. Based on sulfate content and position, carrageenans categorized into kappa (κ), iota (ι), and lambda (λ) carrageenans [3, 33]. The bioactivity of carrageenans have been demonstrated against many viruses, for instance, herpes simplex virus (HSV-1 and HSV-2), human immunodeficiency virus (HIV), Human cytomegalovirus and vesicular stomatitis virus (VSV) [33]. In a recent report, Song et al. (2020) [34] found strong antiviral effects of ι-carrageenan extracted from red algae against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Rothan and Yusof (2020) [35] also reported the potential of carrageenan against Japanese Encephalitis Virus. In a study by Girond et al. (1991) [36] the researchers found a significant antiviral activity of ι-, λ- and κ-carrageenans against hepatitis A virus (HAV) because of their inhibitory action on the viral replication Moreover, Gomaa and Elshoubaky (2016) [37] reported the inhibition of Rift valley fever virus (RVFV) and HSV-1 replication by carrageenan obtained from a red alga Acanthophora specifira. In another study, ι-carrageenan was reported to inhibit Influenza A Virus by successfully inhibiting virus adsorption to the host cells [38]. Furthermore, a nasal spray containing ι-carrageenan had showed promising results for the successful treatment of initial common cold symptoms [39]. Several other studies also demonstrated the inhibition effect of ι-carrageenan against non-enveloped human viral pathogens such as HAV and papilloma viruses invitro [40]. The antiviral effects of carrageenans primarily linked with their MW and the degree of sulfation [26]. Due to high MW and reduced tissue-penetrating capability of carrageenan, its antiviral applications are limited [19]. However, the lower MW carrageenan oligosaccharides obtained by enzymatic or chemical degradation can easily contact to the virus, resulting in an increased bioactivity. More recently, Tang, et al. (2013) [41] investigated the invivo anti-influenza virus activity of low molecular weight carrageenans, their acetylated derivatives, and an acetylated and sulfated derivative in FM1-induced pulmonary oedema model. According to the findings, the antiviral activity of carrageenan was enhanced after the addition of an acetyl group and sulfation indicating that proper acetylation degree and sulfation degree demonstrating that the degree of acetylation and sulfation is critical for antiviral properties of S-PS. Moreover, Wang, et al. (2011) [42] found that low MW oligosaccharides of κ-carrageenan have shown significant antiviral effect against influenza A H1N1 virus by inhibiting the viral replication both invivo and invitro. Yamada, et al. (2000) [43] also reported the promising ability of depolymerized sulfated kappa- and iota-carrageenan to inhibit replication of HIV.
Galactan is another important sulfated polysaccharides commonly found in red alga. It has been demonstrated in the earlier studies that galactans have antiviral efficacy against HIV and HSV, pseudo rabies virus and human cytomegalovirus. Galactan can inhibit the viral entry to the host and also can suppress the viral replication [44]. Galactan can also block the replication of virus and the syncytium formation between uninfected and infected cells (Exploring algae and cyanobacteria as a promising natural source of antiviral drug against SARS-CoV-2). Bouhlal, et al. (2011) [45] have isolated sulfated polysaccharides from red algae (Boergeseniella thuyoides and Sphaerococcus coronopifolius) and reported their antiviral activities against HIV and HSV. They further reported that S-PS have inhibitory effects on invitro replication of the HIV at 12.5 μg/mL concentration, while they inhibited the HSV-1 replication in Vero cells at 4.1 and 17.2 μg/mL concentration.
Brown algae (Phaeophyceae)
Marine derived S-PS from brown algae, have gained increasing attention as potential antiviral drugs. Fucoidans are anionic polymers produced by brown algae and they contain L-fucose, sulfate groups and other monosaccharides [29, 46]. Their biological properties are associated with their physical characteristics such as structure of the main chain, sulfation pattern, MW, monosaccharide composition and acetate groups [47]. In a recent study, Sun, et al. (2020) [48] Purified fucoidans (SHAP-1 and SHAP-2) which possess high amount of sulfate groups from brown algae Sargassum henslowianum and evaluated their inhibitory activity against both HSV-1 and HSV-2 with no cytotoxicity. Sanniyasi, et al. (2019) [49] purified fucoidan from two brown algae, evaluated their anti-HIV activity, and reported that the IC50 value of the purified fucoidan extracts of Turbinaria decurrens and Dictyota bartayesiana were 131.7ng/ml and 57.6ng/ml, respectively. An earlier study also testified that the fractions of fucoidans extracted from Leathessia marina possess good anti-HSV-1, HSV-2 and anti-human cytomegalovirus potential by inhibiting the viral adsorption [50]. Likewise, the antiviral activity of the native and modified fucoidans (modified with enzyme) from Fucus evanescens against HSV-1, HSV-2, HIV-1 and enterovirus on Vero and human MT-4 cell lines were studied and the results indicated that native fucoidan more effectively inhibited the HSV (HSV-1 and HSV-2) replication than modified fucoidans. Whereas, both native and modified fucoidans showed similar antiviral activity against enterovirus and HIV-1 [47]. Wang, et al. (2017b) [51] demonstrated the potent antiviral activity of fucoidan derived from brown algae Kjellmaniella crassifolia against Influenza A Virus and reported that fucoidan efficiently blocked influenza virus infection invitro with low toxicity. In addition, polysaccharides extracted from Sargassum fluitans showed antiviral effects against HSV-1 invitro without cytotoxicity [52].
Laminaran is a non-sulfated molecule, however it can exist in sulfated form, primarily present in brown algae including Ascophyllum, Fucus, Saccharina. Laminaran is classified into two groups: M-series with D-mannitol units and G-series with glucose units in chain [33]. Laminarans were previously reported to inhibit viral replication, proliferation and reverse transcriptase activity of HIV [53].
Alginate is a non-sulfated anionic hydrophilic polymer normally obtained from brown algae including Laminaria, Macrocystis, Ascophyllum, Sargassum, Durvillaea, Lessonia, Eckloniaspecies. It has been frequently investigated for its biological activities [54, 55] and used for various applications in food, cosmetics, paper, and pharmaceutical industry [21]. Alginate is known to have antiviral activity against HIV infection mostly via the strong attachment of gp120 protein with CD4 molecules on the surface of T-cells [56]. It is a heteropolysaccharide consisting of 1,4-linked β-D-mannuronic acid (M) and 1,4 α-L-guluronic acid (G block) residues arranged in homogenous (poly-G, poly-M blocks) or heterogenous (GM blocks) block-like patterns [57]. The use of alginate in wound dressing products is becoming more prevalent. Alginate has also been identified as an immunogen that protects against infectious diseases in both animals and humans. In a semi-dilute state, aqueous solutions of these alginate derivatives demonstrated the usual rheological features of physically cross linked, gel-like networks, which may be beneficial for cartilage repair and regeneration. Furthermore, alginate hydrolysates and derivatives demonstrate exceptional bioactivities, including stimulation of human keratinocytes, acceleration of plant root growth, migration of human endothelial cells, and enhancement of penicillin synthesis from Penicillium chrysogenum [58]. The alginate polysaccharide 911 was reported to inhibit HIV reverse transcriptase along with DNA polymerase activity of Hepatitis B virus (HBV) [59,60].
Green algae (Chlorophyta)
Ulvan is the most important sulfated polysaccharide of green algal cell wall such as Ulva [61]. Ulvan is a sulfated hetero-polysaccharide comprised of disaccharide units of glucuronic acid or iduronic acid attached by a sulfated 1,4 L-rhamnose unit with trace amounts of xylose and glucose [62]. Many studies reported the potent antiviral activity of ulvan and other sulfated polysaccharides of green algae against HSV, Japanese encephalitis virus (JEV), dengue virus (DENV), measles virus (MeV) and influenza virus H1N1 [26, 63-66]. Ivanova, et al. (1994) [67] also reported dose dependent and strain-specific significant antiviral effect of the ulvan extracted from green algae on influenza A virus. Aside from above mentioned polysaccharides, many studies reported the antiviral activities of other sulfated polysaccharides, such as, rhamnan sulfate extracted from different marine algae. For example, Lee, et al. (2004) [68] extracted different S-PS from various green algae and assayed for anti-HSV-1 activity and demonstrated the significant antiviral effects with IC50 values of 0.38 - 8.5 micro g/mL. In a recent study, Terasawa, et al. (2020) [69] demonstrated the antiviral activity of rhamnan sulfate derived from the green alga Monostroma nitidum against influenza A virus and reported the inhibition of both virus adsorption and entry steps. In addition, Rhamnan sulfate also shown inhibition effects against HIV, human cytomegalovirus, HSV-1, HSV-2, measles virus, mump virus and human corona virus [68-71]. Antiviral studies of algal polysaccharides including red, brown and green algae against infection viruses along with their reported mechanisms of action are summarized in the Table 1.
Antiviral activity of eukaryotic microalgal polysaccharides
Microalgae have ability to produce a variety of valuable polysaccharides. These polysaccharides serve mainly as structural and storage molecules. During invitro cultivation of algal cells in broth medium, soluble fraction of polysaccharides dissolve from the cell surface to the medium, while the bound fraction consisting of 50-70% of polysaccharides remain attached to the cells. The microalgal polysaccharides protect the cells against desiccation and ensure its stability against environmental stress (temperature, pH, and salinity, oxidative stress). Algal polysaccharides have unique characteristics including, composition, structure, stability and fluid dynamics. Above all, the bioactivities of polysaccharides is a vital character to make them value-added products with various potential applications [72].
Various studies have verified the antiviral activities of polysaccharides obtained from marine microalgae against various types of infections viruses as presented in Table 2. Huheihel et al. (2002) [73] Reported impressive antiviral activity of sulfated polysaccharides from red microalga against HSV-1 and HSV-2. The findings also provided an indirect evidence for a strong interaction between the polysaccharide and HSV, and a weak interaction with the cell surface. Santoyo, et al. (2012) [74] Studied the antiviral activity of microalgal extracts against HSV-1 at different stages during viral infection. The results revealed that the extracts were effective against HSV-1 intracellular replication [74]. Hasui, et al. (1995) [75] Reported the antiviral efficiency of extracellular sulfated polysaccharides from Cochlodinium polykrikoides against different viruses with no cytotoxic effects to the host cells. The inhibition of viruses was attained at concentrations that were not noticeably inhibitory to the blood coagulation process. Furthermore, Kim, et al. (2012) [20] found that a sulfated polysaccharide (p-KG03) extracted from the marine microalga Gyrodinium impudium displayed significant antiviral effect on influenza A virus. Further studies disclosed that the involved mechanism of antiviral action was the inhibition of virus replication. The inhibition was maximized when p-KG03 is added within 6 hours after viral infection, indicating that primarily the viral attachment and internalization processes were targeted by algal polysaccharide.
Cyanobacterial polysaccharides
Cyanobacteria are a large and well-known group of microorganisms that are capable of performing oxygenic photosynthesis and also possess some typical prokaryotic features [76]. Cyanobacteria are capable of producing a large chemo-diversity of polysaccharides, playing different roles within the cells. These polysaccharides have gained increasing interests due to a very large variety of chemical structures and as a consequence, a great variety of physicochemical and potential original biological properties [76]. Chitin, cellulose, glycogen, starch, agar and carrageenan are some most abundant natural polysaccharides produced by cyanobacteria. A sulfated polysaccharide known as calcium spirulan composed of rhamnose, 3-O-methyl-rhamnose, 2,3-di-O-methyl-rhamnose, 3-O-methylxylose, uronic acids, sulfate groups and calcium ions was found in Arthrospira platensis. Nostoflan, another cyanobacterial polysaccharide, found in terrestrial cyanobacterium, Nostoc flagelliforme contains glucose, xylose, galactose, mannose and glucuronic acid on the non-reducing ends. Nostoflan has low cytotoxicity and a broad spectrum of antiviral activity as well as immunostimulatory activity [77]. Ca-spirulan showed potent and broad-spectrum activity against HIV-1, HIV-2, influenza and several other enveloped viruses. They inhibit the reverse transcriptase activity of HIV-1 and also prevent virus-cell attachment. They also inhibit the fusion between HIV-infected and uninfected CD4+ lymphocytes, a mechanism that greatly enhances viral infectivity [78].
Antiviral potential of cyanobacterial polysaccharides against different viruses along with their mechanism of action is summarized in Table 3. Intracellular polysaccharide produced by Arthrospira platensis, has been seen to exhibit antiviral activity against several viruses invitro by inhibiting the penetration of the virus into the host cells used [79]. Radonić, et al. (2011) [80] also showed that the anionic exopolysaccharide TK V3 produced by cyanobacteria A. platensis exhibited antiviral activity both invitro and invivo against two strains of Vaccinia virus and an Ectromelia virus. Moreover, an acidic polysaccharide nostoflan was reported to have antiviral activity against HSV-1, HSV-2, human cytomegalovirus, and influenza A virus [81]. Polysaccharides from Spirulina maxima and S. platensis have also shown antiviral potential against different viruses [82]. These findings suggest that cyanobacterial polysaccharides are having good potential to be used as starting material for antiviral drug development. Therefore, the identification of more antiviral cyanobacterial polysaccharides and to determine their potential as effective antiviral therapeutics are prerequisite.
Mechanism of Antiviral Activity
The mechanisms for antivirus activity of polysaccharides are yet to be completely understood however, few possible antiviral mechanisms have been proposed frequently. In this section, various antiviral mechanisms are briefly discussed on the basis of previous reports in the literature.
Inhibition of viral adsorption
The attachment of virus to host cells by electrostatic interactions is the primary step of viral incursion into the hosts. The following procedure is attained by converting the unstable reversible electrostatic interactions into stable irreversible binding. Algal polysaccharides have ability to inhibit viral infection by targeting the viral adsorption. Generally, the polysaccharides adopt two main strategies to inhibit viral attachment; interacting directly with virions, stimulating the viral protein to bind to the particular host cell receptors [83]. The blockage of viral attachment or internalization is the general antiviral mechanisms of these polysaccharides. Algal polysaccharides can inhibit viral adsorption and attachment process by direct interaction with virus particles or by attaching with specific viral receptors on the host cell surface. Inhibition of viral attachment by polysaccharides is attributed to their interaction with positive charges on the virus or on the cell surface which inhibits penetration of virus into the hosts [12, 19, 27, 84]. Additionally, polysaccharides can inhibit reverse transcriptase to prevent the production of new viral particles. However, the exact stage of viral replication with which they interfere remains to be expounded [18]. In sulfated polysaccharides, the negatively charged sulfated groups can play a role in antiviral activity. Therefore, the size and degree of sulfation of these compounds can be correlated relatively well with their capacity to inhibit viral infection of cells. Furthermore, diversity of the linkage chemistry and composition of the sugar units are factors that define not only the functional properties, but also the target specificity of sulfated polysaccharides [20].
The potential of algal polysaccharides against viruses was first reported by Ginsberg, et al. (1947) [85] and Gerber, et al. (1958) [86] who observed the inhibitory effect of polysaccharides extracted from Gelidium cartilagenium and Carrageenin on growth of mumps and influenza B virus in embryonated eggs. Furthermore, Ehresmann, et al. (1977) [87] observed the inhibitory effect of polysaccharides extracted from Farlowia mollis and Constantinea simplex on herpes simplex and hypothesized that herpes virus inhibition was due to the blockage of viral adsorption. Since then, several studies have reported the inhibition of viral entry as mechanism of action of algal polysaccharides towards various viruses [27,80,81,88,89]. In a recent finding, Terasawa, et al. [69] noted the inhibition of influenza A virus adsorption by rhamnan sulfate produced by Monostroma nitidum [69]. Carrageenan extracted from red edible seaweeds was recently reported to inhibit early stages of Japanese Encephalitis Virus infection and attachment [35]. A recent study revealed that sulfated polysaccharides bind tightly to the S-protein of SARS-CoV-2 invitro, demonstrating that they can act as decoys to interfere with S-protein binding to the heparan sulfate co-receptor in host tissues, inhibiting viral infection [90].
Inhibition of viral internalization
The internalization of most viruses frequently comprises of three steps: (a) uptake of virus by endocytoses, (b) vesicular transport through the cytoplasm, (c) transfer to endosomes and intracellular organelles. The uncoating of virus usually occurs after the completion of viral internalization process into host cells. Various algal polysaccharides, particularly sulfated polysaccharides, can interfere with virus internalization and uncoating by blocking the allosteric process of viruses [19]. Up until now, the antiviral approach targeting viral internalization and the uncoating stage is normally the interference with the release of DNA and RNA from the endosome by blocking the structural alterations in the viral glycoprotein [83]. For example, Buck, et al. (2006) [40] reported that carrageenans extracted from Eucheuma denticulatum can constrain the viral infection by binding directly to the capsid of human papillomavirus, which in turn, inhibited the interactions between the capsid and host cell receptors. According to Talarico, et al. (2007) [91] and Talarico, et al. (2011) [92] ι-carrageenans could inhibit not only viral adsorption, but also viral internalization of HSV and the Dengue virus. Moreover, in a study divers classes of sulfated algal polysaccharides were found to inhibit dengue virus internalization [27]. Polysaccharides can prevent the membrane fusion activity during virus-host interaction by interfering with membrane proteins responsible for fusion events. Polysaccharides can bind to fusion proteins and inactivate them by declining their hydrophobic properties. They can also bind with sugar groups linked to the polypeptide chains of the virus thus inhibiting their penetration [12].
Inhibition of viral replication
The replication of viruses consists of the synthesis of viral messenger RNA from "early" genes (with exceptions for positive sense RNA viruses), viral protein synthesis, possible assembly of viral proteins, and viral genome replication facilitated by early or regulatory protein expression. Algal polysaccharides have been observed to hinder the viral transcription and replication by direct intrusion with viral replication enzymes or inhibition on other intracellular targets [83]. Nakashima, et al. (1987) [93] were the first to observe that extract from the marine red alga Schizymenia pacifica showed antiviral activity against HIV due to either the inhibition of viral attachment/penetration, or the selective inhibition of HIV reverse transcriptase and replication. Various studies have reported the inhibition of viral replication by algal polysaccharides against different viruses [28,37,94,95]. In a study, Girond, et al. (1991) [36] Showed a potent inhibitory effect of ι, λ and κ-carrageenans on the replication of hepatitis A virus (HAV). Polysaccharide extracted from Sphaerococcus coronopifolius belong to the family of λ-carrageenans exerts its antiviral effect by effectively inhibiting HSV-1 adsorption to host cells and a direct inhibitory effect on HIV-1 replication [45]. Moreover, Gomaa and Elshoubaky (2016) [37] reported the inhibition of HSV-1 and RVFV replication by carrageenan polysaccharides isolated from red alga Acanthophora specifira. Viral replication of rhinovirus (HRV) was also inhibited by ι-carrageenans [96]. Furthermore, ι-carrageenans significantly blocked viral replication process and increased the survival of host cells containing influenza virus H1N1 strain [38].
Immuno-stimulatory Effects
As the first mechanism of defense, infection of viruses may usually induce the antiviral immune responses of host cells in which the interferons play a vital role in protection of host cell and viral inactivation process. It is believed that the interferons are able to enhance the antiviral activity since they can elicit the production of antiviral and immune-modulating proteins by interacting with cell surface receptors. The process can increase the activity of macrophages, natural killer cells and T lymphocytes which are key weapons of the immunoregulatory system [83].
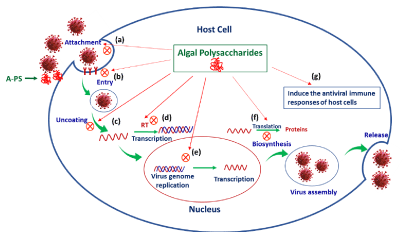
Studies have been demonstrated that algal polysaccharides can stimulate the immune response by binding to recognition receptors (mannose receptors or Toll-like receptors (TLR)) on the surface of macrophages this binding of polysaccharides to cell receptors might be possible due to their structural similarities with bacterial lipopolysaccharides [97]. Recently, a few studies reported the immune-stimulatory effects of algal polysaccharides that help in inhibition of viral and speed up viral clearance. Kim, et al. (2011) [98] assessed the antiviral activity of water-soluble sulfated polysaccharides extracted from Ulva prolifera and reported that the sulfated polysaccharide could modulate the immune responses. Zhang, et al. (2015) [99] studied the immune functions of four algal (Ascophyllum nodosum, Macrocystis pyrifera, Undaria pinnatifida and Fucus vesiculosus) fucoidans and the results indicated that fucoidans have strong immune-modulation effect through the activation of T cells and NK cells. They further suggested that fucoidans could be possible therapeutic agents for infectious diseases and an excellent resource for vaccine production. An acidic polysaccharide from brown algae Laminaria japonica was found to inhibit apoptosis and inducing IFN-β expression against enterovirus 71 [100]. In another study, polysaccharide from Laminaria japonica has shown its role in up-regulation of IRF3 signaling-mediated IFN-α production against respiratory syncytial virus [101]. These findings established that algal polysaccharides could not only inhibit viral entry and replication, but also stimulate the immune system to cope with infectious pathogens. Possible antiviral mechanisms of algal polysaccharides discussed above are presented in Figure 1.
Antiviral activity of the S-PS differs quantitatively as well as qualitatively in dependence on their structure, Algal S-PS consisting of 35-60 sulfate groups per hundred sugar residues have shown the best antiviral activity [102]. The degree of sulfation has a key influence on the antiviral activity of a polysaccharide, higher the degree of sulfation, the better their antiviral potency. Some other characteristics of polysaccharides, including the degree of polymerization, branching and chemical composition of carbohydrate moieties might also play an important role in their antiviral properties. Additionally, structural diversity, molecular mass, the position of sulfate groups and polymeric backbone, charge density, and molecular composition of uncharged portions can also influence on the antiviral potential of polysaccharides [84]. The degree of sulfation, acetylation, and other chemical changes can be successfully adopted to enhance the antiviral activity and immune-stimulatory effect of polysaccharides. For instance, desired molecular weight of polysaccharides can be achieved by depolymerization, allowing them to penetrate more efficiently into the cell and exert greater antiviral activity. Furthermore, enveloped viruses are more susceptible to polyanionic inhibitors than non-enveloped viruses. Another crucial aspect in maintaining antiviral action for a longer period of time is the slower breakdown of polyanions contained in the PS [12]. Invivo antiviral activity of S-PS is typically depends on their capabilities to block the attachment of virus to the host cells, however, irreversible interaction with virions leading to virucidal action plays an additional role [102].
Conclusion
In recent years, much consideration has been given to the discovery of natural antiviral compounds particularly from marine sources. Marine algae are considered as valued sources of a huge array of bioactive compounds from the blue water, and polysaccharides are one of them. The information compiled in this review depicts clearly the astonishing potential of algal polysaccharides against variety of infection caused by virulent viruses. However, the invivo mechanism of their antiviral actions is not fully understood and a lot of research is still to be done to discover specific antiviral mechanisms. Antiviral polysaccharides of microalgae and cyanobacteria are less explored in comparison to macroalgae. Therefore, it may be an imperative area to focus in future research. Algal polysaccharide antiviral mechanisms suggest that these natural compounds can be used in both the prevention and treatment of viral infections. For instance, the role of polysaccharides in preventing viral attachment and entry demonstrated that viruses can be inactivated before infection begins. On the other hand, polysaccharides have the potential to stimulate the immune system, which may aid in the treatment of viral infections by removing the virus from affected cells through the immunological defense systems. Algal polysaccharides should be further investigated as antiviral agents through animal studies and clinical trials. These polysaccharides have a number of advantages over other antiviral drugs, such as, safety, low production costs, broad spectrum of antiviral activity, unique mode of actions and low risk of viral drug resistance. Furthermore, these polysaccharides are not only bioactive but also non-toxic, biocompatible and chemically modifiable. They can be exploited as candidate drugs, healthcare products, vaccine adjuvants, nanomaterials, drug delivery systems, dietary supplements and food additives. Choosing the most promising drug candidates from the vast array of available polysaccharides will be a challenge. The literature reporting invivo antiviral effects of S-PS is limited, therefore, extensive studies regarding the invivo performance of antiviral polysaccharides are indispensable before their utilization in controlling emerging infectious viruses.
Declarations
The authors declare no conflict of Interest.
Author contributions
Conceptualization, Mahammed Ilyas Khazi, Fakhra Liaqat, Pengcheng Fu; formal analysis, Mahammed Ilyas Khazi, Fakhra Liaqat, Chenshuo Li; investigation, Mahammed Ilyas Khazi, Fakhra Liaqat, Chenshuo Li; writing-original draft preparation, Mahammed Ilyas Khazi, Fakhra Liaqat; writing-review and editing, Mahammed Ilyas Khazi, Fakhra Liaqat, Jian Li, and Pengcheng Fu; supervision, Pengcheng Fu; project administration, Pengcheng Fu; funding acquisition, Pengcheng Fu. All authors have read and agreed to the published version of the manuscript.
Acknowledgement
This study was supported by Talented Young Scientists Program, Ministry of Science and Technology, China, and Research start-up funds from Hainan University (KYQD_ZR2017271), China. We would like to thank the Hainan University for the support provided to Mahammed Ilyas Khazi during his research work.
References
- de Jesus Raposo MF, De Morais AMB, De Morais RMSC (2015) Marine polysaccharides from algae with potential biomedical applications. Marine drugs 13: 2967-3028.
- Wang HMD, Li XC, Lee DJ, et al. (2017a) Potential biomedical applications of marine algae. Bioresource technology 244: 1407-1415.
- Tanna B, Mishra A (2019) Nutraceutical potential of seaweed polysaccharides: Structure, bioactivity, safety, and toxicity. Comprehensive Reviews in Food Science and Food Safety 18: 817-831.
- Kasimala MB, Mebrahtu L, Magoha PP, et al. (2015) A review on biochemical composition and nutritional aspects of seaweeds. Caribbean journal of Science and Technology 3: 789-797.
- de Jesus Raposo MF, de Morais RMSC, de Morais AMMB (2013) Health applications of bioactive compounds from marine microalgae. Life sci 93: 479-486.
- Michalak I, Chojnacka K (2015) Algae as production systems of bioactive compounds. Engineering in Life Sci 15: 160-176.
- da Silva Vaz B, Moreira JB, de Morais MG, et al. (2016) Microalgae as a new source of bioactive compounds in food supplements. Current Opinion in Food Science 7: 73-77.
- Sudhakar M, Kumar BR, Mathimani T, et al. (2019) A review on bioenergy and bioactive compounds from microalgae and macroalgae-sustainable energy perspective. Journal of Cleaner Production 228: 1320-1333.
- Ameen F, AlNadhari S, Al-Homaidan AA (2020) Marine microorganisms as an untapped source of bioactive compounds. Saudi J Biol Sci 28: 224-231.
- Levasseur W, Perré P, Pozzobon V (2020) A review of high value-added molecules production by microalgae in light of the classification. Biotechnol Adv 41: 107545.
- Rosales-Mendoza S, García-Silva I, González-Ortega O, et al. (2020) The potential of algal biotechnology to produce antiviral compounds and biopharmaceuticals. Molecules 25: 4049.
- Andrew M, Jayaraman G (2021) Marine sulfated polysaccharides as potential antiviral drug candidates to treat Corona Virus disease (COVID-19). Carbohydr Res 505: 108326.
- Hans N, Malik A, Naik S (2020) Antiviral activity of sulfated polysaccharides from marine algae and its application in combating COVID-19: Mini review. Bioresour Technol Rep 13: 100623.
- Belhaj D, Frikha D, Athmouni K, et al. (2017) Box-Behnken design for extraction optimization of crude polysaccharides from Tunisian Phormidium versicolor cyanobacteria (NCC 466): Partial characterization, Invitro antioxidant and antimicrobial activities. Int j biol macromol 105: 1501-1510.
- Hans N, Naik SN, Malik A (2019) Platform molecules from algae by using supercritical CO2 and subcritical water extraction. In. (1st Edn) Handbook of Algal Technologies and Phytochemicals. CRC Press 229-243.
- Fedorov SN, Ermakova SP, Zvyagintseva TN, et al. (2013) Anticancer and cancer preventive properties of marine polysaccharides: Some results and prospects. Marine drugs 11: 4876-4901.
- Dewi IC, Falaise C, Hellio C, et al. (2018) Anticancer, antiviral, antibacterial, and antifungal properties in microalgae. In. Microalgae in Health and Disease Prevention. Elsevier 235-261.
- Amaro HM, Guedes AC, Malcata FX (2011) Antimicrobial activities of microalgae: an invited review. Science against microbial pathogens: communicating current research and technological advances 3: 1272-1284.
- Wang W, Wang SX, Guan HS (2012) The antiviral activities and mechanisms of marine polysaccharides: an overview. Marine drugs 10: 2795-2816.
- Kim M, Yim JH, Kim SY, et al. (2012) Invitro inhibition of influenza A virus infection by marine microalga-derived sulfated polysaccharide p-KG03. Antiviral research 93: 253-259.
- Kraan S (2012) Algal polysaccharides, novel applications and outlook. IntechOpen.
- Patil NP, Le V, Sligar AD, et al. (2018) Algal polysaccharides as therapeutic agents for atherosclerosis. Front cardiovasc med 5: 153.
- Venugopal V (2019) Sulfated and non-sulfated polysaccharides from seaweeds and their uses: An overview. EC Nutr 14: 126-141.
- Bruhn A, Janicek T, Manns D, et al. (2017) Crude fucoidan content in two North Atlantic kelp species, Saccharina latissima and Laminaria digitata-seasonal variation and impact of environmental factors. Journal of applied phycology 29: 3121-3137.
- friso AA, Gallo M, Baldi F (2017) Seasonal variation and yield of sulfated polysaccharides in seaweeds from the Venice Lagoon. Botanica Marina 60: 339-349.
- Yamada T, Ogamo A, Saito T, et al. (1997) Preparation and anti-HIV activity of low-molecular-weight carrageenans and their sulfated derivatives. Carbohydrate Polymers 32: 51-55.
- Pujol CA, Ray S, Ray B, et al. (2012) Antiviral activity against dengue virus of diverse classes of algal sulfated polysaccharides. Int j biol macromol 51: 412-416.
- Lopes N, Ray S, Espada SF, et al. (2017) Green seaweed Enteromorpha compressa (Chlorophyta, Ulvaceae) derived sulphated polysaccharides inhibit herpes simplex virus. Int j biol macromol 102: 605-612.
- Usman A, Khalid S, Usman A, et al. (2017) Algal polysaccharides, novel application, and outlook. In. Algae based polymers, blends, and composites. Elsevier 115-153.
- Usov AI (2011) Polysaccharides of the red algae. In. Advances in carbohydrate chemistry and biochemistry. Elsevier 65: 115-217.
- Lee C (2020) Carrageenans as Broad-Spectrum Microbicides: Current Status and Challenges. Mar Drugs 18: 435.
- Bilal M, Iqbal H (2019) Marine seaweed polysaccharides-based engineered cues for the modern biomedical sector. Mar drugs 18: 7.
- Ahmadi A, Zorofchian Moghadamtousi S, Abubakar S, et al. (2015) Antiviral potential of algae polysaccharides isolated from marine sources: a review. BioMed res int 2015: 825203.
- Song S, Peng H, Wang Q, et al. (2020) Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct 11: 7415-7420.
- Rothan HA, Yusof R (2020) Antiviral and Virucidal activities of sulphated polysaccharides against Japanese Encephalitis Virus. Trop Biomed 37: 13-721.
- Girond S, Crance J, Van Cuyck-Gandre H, et al. (1991) Antiviral activity of carrageenan on hepatitis A virus replication in cell culture. Res virol 142: 261-270.
- Gomaa HH, Elshoubaky GA (2016) Antiviral activity of sulfated polysaccharides carrageenan from some marine seaweeds. International Journal of Current Pharmaceutical Review and Research 7: 34-42.
- Leibbrandt A, Meier C, König-Schuster M, et al. (2010) Iota-carrageenan is a potent inhibitor of influenza A virus infection. PloS one 5: e14320.
- Eccles R, Winther B, Johnston S, et al. (2015) Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: the ICICC trial. Respir res 16: 121.
- Buck CB, Thompson CD, Roberts JN, et al. (2006) Carrageenan is a potent inhibitor of papillomavirus infection. PLoS pathog 2: e69.
- Tang F, Chen F, Li F (2013) Preparation and potential in vivo anti-influenza virus activity of low molecular-weight ?-carrageenans and their derivatives. Journal of Applied Polymer Science 127: 2110-2115.
- Wang W, Zhang P, Hao C, et al. (2011) Invitro inhibitory effect of carrageenan oligosaccharide on influenza A H1N1 virus. Antiviral res 92: 237-246.
- Yamada T, Ogamo A, Saito T, et al. (2000) Preparation of O-acylated low-molecular-weight carrageenans with potent anti-HIV activity and low anticoagulant effect. Carbohydrate Polymers 41: 115-120.
- Sangtani R, Ghosh A, Jha HC, et al. (2020) Potential of algal metabolites for the development of broad-spectrum antiviral therapeutics: Possible implications in COVID-19 therapy. Phytother Res.
- Bouhlal R, Haslin C, Chermann JC, et al. (2011) Antiviral activities of sulfated polysaccharides isolated from Sphaerococcus coronopifolius (Rhodophytha, Gigartinales) and Boergeseniella thuyoides (Rhodophyta, Ceramiales). Mar drugs 9: 1187-1209.
- Luthuli S, Wu S, Cheng Y, et al. (2019) Therapeutic effects of fucoidan: A review on recent studies. Mar drugs 17: 487.
- Krylova NV, Ermakova SP, Lavrov VF, et al. (2020) The comparative analysis of antiviral activity of native and modified fucoidans from brown algae Fucus evanescens invitro and invivo. Mar drugs 18: 224.
- Sun QL, Li Y, Ni LQ, et al. (2020) Structural characterization and antiviral activity of two fucoidans from the brown algae Sargassum henslowianum. Carbohydr polym 229: 115487.
- Sanniyasi E, Venkatasubramanian G, Anbalagan MM, et al. (2019) Invitro anti-HIV-1 activity of the bioactive compound extracted and purified from two different marine macroalgae (seaweeds)(Dictyota bartayesiana JV Lamouroux and Turbinaria decurrens Bory). Sci rep 9:12185.
- Feldman S, Reynaldi S, Stortz C, et al. (1999) Antiviral properties of fucoidan fractions from Leathesia difformis. Phytomedicine 6: 335-340.
- Wang W, Wu J, Zhang X, et al. (2017b) Inhibition of influenza A virus infection by fucoidan targeting viral neuraminidase and cellular EGFR pathway. Scientific reports 7: 1-14.
- Bedoux G, Caamal-Fuentes E, Boulho R, et al. (2017) Antiviral and cytotoxic activities of polysaccharides extracted from four tropical seaweed species. Natural Product Communications 12.
- Muto S, Niimura K, Oohara M, et al. (1988) Polysaccharides from marine algae and antiviral drugs containing the same as active ingredients. Eur Patent EP295956.
- Szekalska M, Pucilowska A, Szymanska E, et al. (2016) Alginate: current use and future perspectives in pharmaceutical and biomedical applications. International Journal of Polymer Science 2016.
- Besednova NN, Zvyagintseva TN, Kuznetsova TA, et al. (2019) Marine algae metabolites as promising therapeutics for the prevention and treatment of HIV/AIDS. Metabolites 9: 87.
- Satpati GG (2020) Algal Sulfated Polysaccharides: Potent immunomodulators against COVID-19 in pandemic 2020. Biosciences Biotechnology Research Asia 17: 601-605.
- Lee KY, Mooney DJ (2012) Alginate: properties and biomedical applications. Prog polym sci 37: 106-126.
- Rajendran I (2020) Marine algal polysaccharides and their applications. Encyclopedia of Marine Biotechnology 2: 1195-1208.
- Xianliang X, Meiyu G, Huashi G, et al. (2000) Study on the mechanism of inhibitory action of 911 on replication of HIV-1 Invitro. Zhongguo hai Yang yao wu=Chinese Journal of Marine Drugs 19: 15-18.
- Jiang B, Xu X, Li L, et al. (2003) Study on-911? anti-HBV effect in HepG2. 2.15 cells culture. Modern Preventive Medicine 30: 517-518.
- Rioux LE, Turgeon SL (2015) Seaweed carbohydrates. In. Seaweed sustainability. Elsevier, 141-192.
- Cunha L, Grenha A (2016) Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar drugs 14: 42.
- Chiu YH, Chan YL, Li TL, et al. (2012) Inhibition of Japanese encephalitis virus infection by the sulfated polysaccharide extracts from Ulva lactuca. Mar biotechnol 14: 468-478.
- Jiao G, Yu G, Wang W, et al. (2012) Properties of polysaccharides in several seaweeds from Atlantic Canada and their potential anti-influenza viral activities. Journal of Ocean University of China 11: 205-212.
- Hardouin K, Bedoux G, Burlot AS, et al. (2016) Enzyme-assisted extraction (EAE) for the production of antiviral and antioxidant extracts from the green seaweed Ulva armoricana (Ulvales, Ulvophyceae). Algal Research 16: 233-239.
- Morán-Santibañez K, Cruz-Suárez LE, Ricque-Marie D, et al. (2016) Synergistic effects of sulfated polysaccharides from Mexican seaweeds against measles virus. BioMed res int 2016: 8502123.
- Ivanova V, Rouseva R, Kolarova M, et al. (1994) Isolation of a polysaccharide with antiviral effect from Ulva lactuca. Prep Biochem 24: 83-97.
- Lee JB, Hayashi K, Maeda M, (2004) Antiherpetic activities of sulfated polysaccharides from green algae. Planta med 70: 813-817.
- Terasawa M, Hayashi K, Lee JB, et al. (2020) Anti-influenza A virus activity of rhamnan sulfate from green algae Monostroma nitidum in mice with normal and compromised immunity. Mar Drugs 18: 254.
- Suzuki K, Terasawa M (2020) Biological Activities of Rhamnan Sulfate Extract from the Green Algae Monostroma nitidum (Hitoegusa). Mar Drugs 18: 228.
- Wang S, Wang W, Hou L, et al. (2020). A sulfated glucuronorhamnan from the green seaweed Monostroma nitidum: Characteristics of its structure and antiviral activity. Carbohydr polym 227: 115280.
- Arad SM, Levy-Ontman O (2010) Red microalgal cell-wall polysaccharides: Biotechnological aspects. Curr opin biotechnol 21: 358-364.
- Huheihel M, Ishanu V, Tal J, et al. (2002) Activity of Porphyridium sp. polysaccharide against herpes simplex viruses Invitro and in vivo. J biochem biophys methods 50: 189-200.
- Santoyo S, Jaime L, Plaza M, et al. (2012) Antiviral compounds obtained from microalgae commonly used as carotenoid sources. Journal of applied phycology 24: 731-741.
- Hasui M, Matsuda M, Okutani K, et al. (1995) Invitro antiviral activities of sulfated polysaccharides from a marine microalga (Cochlodinium polykrikoides) against human immunodeficiency virus and other enveloped viruses. Int J Biol Macromol 17: 293-297.
- Phélippé M, Gonçalves O, Thouand G, et al. (2019) Characterization of the polysaccharides chemical diversity of the cyanobacteria Arthrospira platensis. Algal Research 38: 101426.
- Mazur-Marzec H, Ceglowska M, Konkel R, et al. (2021) Antiviral cyanometabolites-A review. Biomolecules 11: 474.
- Singh RK, Tiwari SP, Rai AK, et al. (2011) Cyanobacteria: An emerging source for drug discovery. J antibiot 64: 401-412.
- Rechter S, König T, Auerochs S, et al. (2006) Antiviral activity of Arthrospira-derived spirulan-like substances. Antiviral res 72: 197-206.
- Radonic A, Thulke S, Achenbach J, et al. (2011) Anionic polysaccharides from phototrophic microorganisms exhibit antiviral activities to Vaccinia virus.
- Kanekiyo K, Lee JB, Hayashi K, et al. (2005) Isolation of an antiviral polysaccharide, nostoflan, from a terrestrial cyanobacterium, Nostoc f lagelliforme. J nat prod 68: 1037-1041.
- Hernández-Corona A, Nieves I, Meckes M, et al. (2002) Antiviral activity of Spirulina maxima against herpes simplex virus type 2. Antiviral Res 56: 279-285.
- Shi Q, Wang A, Lu Z, et al. (2017) Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr res 453: 1-9.
- He X, Fang J, Guo Q, et al. (2020) Advances in antiviral polysaccharides derived from edible and medicinal plants and mushrooms. Carbohydr polym 229: 115548.
- Ginsberg HS, Goebel WF, Horsfall Jr FL (1947) Inhibition of mumps virus multiplication by a polysaccharide. Proc Soc Exp Biol Med 66: 99-100.
- Gerber P, Dutcher JD, Adams EV, et al. (1958) Protective effect of seaweed extracts for chicken embryos infected with influenza B or mumps virus. Proc Soc Exp Biol Med 99: 590-593.
- Ehresmann D, Deig E, Hatch M, et al. (1977) Antiviral substances from California marine algae 1. Journal of phycology 13: 37-40.
- Mazumder S, Ghosal PK, Pujol CA, et al. (2002) Isolation, chemical investigation and antiviral activity of polysaccharides from Gracilaria corticata (Gracilariaceae, Rhodophyta). International journal of biological macromolecules 31: 87-95.
- Montanha JA, Bourgougnon N, Boustie J, et al. (2009) Antiviral activity of carrageenans from marine red algae. Lat Am J Pharm 28: 443-448.
- Kwon PS, Oh H, Kwon SJ, et al. (2020) Sulfated polysaccharides effectively inhibit SARS-CoV-2 Invitro. Cell dis 6: 50.
- Talarico LB, Duarte ME, Zibetti RG, et al. (2007) An algal-derived DL-galactan hybrid is an efficient preventing agent for Invitro dengue virus infection. Planta Med 73: 1464-1468.
- Talarico LB, Noseda MD, Ducatti DR, et al. (2011) Differential inhibition of dengue virus infection in mammalian and mosquito cells by iota-carrageenan. J Gen Virol 92: 1332-1342.
- Nakashima H, Kido Y, Kobayashi N, et al. (1987) Inhibition of avian myeloblastosis virus reverse transcriptase and human immunodeficiency virus by sulfated polysaccharides extracted from sea algae: purification and characterization of a reverse transcriptase inhibitor. Antimicrob Agents Chemother 31: 1524-1528.
- Haslin C, Lahaye M, Pellegrini M, et al. (2001) Invitro anti-HIV activity of sulfated cell-wall polysaccharides from gametic, carposporic and tetrasporic stages of the Mediterranean red alga Asparagopsis armata. Planta Med 67: 301-305.
- Wang S, Wang W, Hao C, et al. (2018) Antiviral activity against enterovirus 71 of sulfated rhamnan isolated from the green alga Monostroma latissimum. Carbohydr polym 200: 43-53.
- Grassauer A, Weinmuellner R, Meier C, et al. (2008) Iota-Carrageenan is a potent inhibitor of rhinovirus infection. Virol j 5: 107.
- Chen D, Wu X, Wen Z (2008) Sulfated polysaccharides and immune response: promoter or inhibitor? Panminerva med 50: 177-183.
- Kim JK, Cho ML, Karnjanapratum S, et al. (2011) Invitro and in vivo immunomodulatory activity of sulfated polysaccharides from Enteromorpha prolifera. Int J Biol Macromol 49: 1051-1058.
- Zhang W, Oda T, Yu Q, et al. (2015) Fucoidan from Macrocystis pyrifera has powerful immune-modulatory effects compared to three other fucoidans. Mar drugs 13: 1084-1104.
- Yue Y, Li Z, Li P, et al. (2017) Antiviral activity of a polysaccharide from Laminaria japonica against enterovirus 71. Biomed Pharmacother 96: 256-262.
- Cao Yg, Hao Y, Li Zh, et al. (2016) Antiviral activity of polysaccharide extract from Laminaria japonica against respiratory syncytial virus. Biomed Pharmacother 84: 1705-1710.
- Ghosh T, Chattopadhyay K, Marschall M, et al. (2009a) Focus on antivirally active sulfated polysaccharides: from structure-activity analysis to clinical evaluation. Glycobiology 19: 2-15.
- Pujol C, Coto C, Damonte E (1995) Determination of the antiviral activity of a naturally occurring sulfated xylomannan under various experimental conditions. Rev Argent microbiol 27: 91-98.
- Duarte M, Noseda DG, Noseda MD, et al. (2001) Inhibitory effect of sulfated galactans from the marine alga Bostrychia montagnei on herpes simplex virus replication Invitro. Phytomedicine 8: 53-58.
- Pujol C, Estevez J, Carlucci M, et al. (2002) Novel DL-galactan hybrids from the red seaweed Gymnogongrus torulosus are potent inhibitors of herpes simplex virus and dengue virus. Antivir Chem Chemother 13: 83-89.
- Ghosh T, Pujol CA, Damonte EB, et al. (2009b). Sulfated xylomannans from the red seaweed Sebdenia polydactyla: structural features, chemical modification and antiviral activity. Antivir Chem Chemother 19: 235-242.
- Bandyopadhyay SS, Navid MH, Ghosh T, et al. (2011) Structural features and Invitro antiviral activities of sulfated polysaccharides from Sphacelaria indica. Phytochemistry 72: 276-283.
- Thuy TTT, Ly BM, Van TTT, et al. (2015) Anti-HIV activity of fucoidans from three brown seaweed species. Carbohydr polym 115: 122-128.
- Dinesh S, Menon T, Hanna LE, et al. (2016) Invitro anti-HIV-1 activity of fucoidan from Sargassum swartzii. Int j biol macromol 82: 83-88.
- Li H, Li J, Tang Y, et al. (2017) Fucoidan from Fucus vesiculosus suppresses hepatitis B virus replication by enhancing extracellular signal-regulated Kinase activation. Virol j 14: 178.
- Kim H, Lim CY, Lee DB, (2020) Inhibitory effects of laminaria japonica fucoidans against noroviruses. Viruses 12: 997.
- Lee JB, Hayashi K, Hayashi T, et al. (1999) Antiviral activities against HSV-1, HCMV, and HIV-1 of rhamnan sulfate from Monostroma latissimum. Planta Med 65: 439-441.
- Huleihel M, Ishanu V, Tal J, et al. (2001) Antiviral effect of red microalgal polysaccharides on Herpes simplex and Varicella zoster viruses. Journal of applied phycology 13: 127-134.
- Talyshinsky MM, Souprun YY, Huleihel MM (2002) Anti-viral activity of red microalgal polysaccharides against retroviruses. Cancer Cell Int 2: 8.
- Yim JH, Kim SJ, Ahn SH, et al. (2004) Antiviral effects of sulfated exopolysaccharide from the marine microalga Gyrodinium impudicum strain KG03. Mar biotechnol 6: 17-25.
- Lee JB, Hayashi K, Hirata M, et al. (2006) Antiviral sulfated polysaccharide from Navicula directa, a diatom collected from deep-sea water in Toyama Bay. Bio Pharm Bull 29: 2135-2139.
- Santoyo S, Plaza M, Jaime L, et al. (2010) Pressurized liquid extraction as an alternative process to obtain antiviral agents from the edible microalga Chlorella vulgaris. J agric food chem 58: 8522-8527.
- Hayashi K, Hayashi T, Kojima I (1996) A natural sulfated polysaccharide, calcium spirulan, isolated from Spirulina platensis: Invitro and ex vivo evaluation of anti-herpes simplex virus and anti-human immunodeficiency virus activities. AIDS res hum retroviruses 12: 1463-1471.
Corresponding Author
Pengcheng Fu, State Key Laboratory of Marine Resource Utilization in South China Sea, Hainan University, Haikou, China, Tel: +86-898-6629-2060.
Copyright
© 2022 Khazi MI. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.