Probiotic Potential of Indian Traditional Fermented Foods to Combat Listeriosis
Abstract
Due to their great nutritional content, certain traditional fermented foods and beverages from India have gained recognition on a global scale. In northeast India, fermented foods like pickles and bamboo beverages are widely consumed and well-liked. Probiotics found in fermented foods have been linked to better health. Probiotics are living bacteria that offer a number of health advantages when taken in the appropriate amounts. Beneficial bacteria improve immunity and digestion, which helps to avoid cancer and metabolic diseases like diabetes. The most widely used probiotics that have been identified from fermented foods include Bifidobacteria species, Lactococcus lactis, and Lactobacillus acidophilus. As fundamental characteristics, probiotics have antibacterial action, acid tolerance, and bile tolerance. Inhibitory substances like bacteriocins, organic acids are produced and secreted by probiotics to prevent the colonisation of harmful pathogens. By removing harmful microorganisms, the intestinal barrier is strengthened, which modifies the gut flora. The primary pathogen that causes listeriosis, Listeria monocytogenes (LM), has a special physiology that allows it to grow even in subfreezing temperatures and tolerating harsh conditions. Foods with a dairy or beef origin are specifically contaminated by the LM. Pregnant women, new babies, and the elderly in general are the pathogen's main targets. The current review focuses on worldwide LM infection-related abortions during pregnancy and foetal fatalities. The study also considers Indian fermented foods and drinks as potential sources of new probiotics because of their anti-listerial capabilities.
Keywords
Fermented Foods, Probiotics, Listeria monocytogenes, LAB
Introduction
Food is a symbol of a people's history, culture, and unique identity. Traditional knowledge, generational experience, agro-climatic conditions, and the availability of food resources have all influenced the development of fermented foods. Their development is influenced by conventional ideas, racial preferences, religious convictions, socioeconomic systems, gastronomic traditions, and social restrictions imposed over time by successive rulers. Non-fermented foods and fermented foods (including alcoholic beverages) are the two divisions of ethnic foods [1]. Food fermentation is an ancient technique in India, where uncontrolled or reverse fermentation is practiced as a domestic skill. Worldwide, there are more than 5000 different varieties of fermented foods and alcoholic beverages [2].
In the northeastern part of India, more than 250 different kinds of fermented foods and alcoholic beverages are produced and consumed [3]. Due to its probiotic properties, improved shelf life, protection, sensory benefits, and nutritional value, fermented foods, beverages have grown in popularity and are used more regularly [4]. Probiotics, or fermented meals with living bacteria, have gained appeal to improve human health in recent years [5]. The WHO (World Health Organization) defines probiotics as "live bacteria that, when administered in suitable amounts, confer a health benefit on the host" [6,7]. They serve as the foundational cultures in ethnic cuisine and stimulate numerous productive and profitable enterprises. They are used as a starter culture in ethnic foods, boost a variety of functional and economic activities. Probiotics aid in food preservation, and LAB's functional antibacterial activity reduces the number of undesirable microbes in milk products, making them safe for human consumption [8].
Fermented foods have been shown to include lactic acid bacteria (LAB) such as Lactobacillus plantarum, Lactobacillus acidophilus [9], Lactobacillus mesenteroides, Lactobacillus lactis, and Pentobacillus pentosaceus [10]. Fermented soybean meal contains Bacillus spp. as well [11]. Yeast and fungi considerably facilitate fermentation [9]. Pathogenic organisms like B. cereus, S. aureus, and Enterobacteriaceae have been found in fermented foods like ngari, hentak, and tungtap [12]. However, dominant lactic acid bacteria prevented these harmful strains from multiplying, resulting in reduced CFU (colony forming units) units and decreased pathogenicity [13]. Ocins, which are proteinaceous antibacterial compounds, are produced and secreted by probiotics. Ocins, proteinaceous antibacterial compounds, are produced and secreted by probiotics. Listeria monocytogenes (LM) , Listeria innocua, B. cereus, S. aureus S1, S. mutans DSM 6178, K. pneumonia, P. aeruginosa BFE162, E. bifidum BFE 282, and E. agglomerans BFE 154 are a few pathogenic Gram-positive and Gram-negative bacteria that have been deemed hostile [14,15].
LM is a serious contaminant that has caused the dairy industry to suffer enormous financial losses. Global listeriosis outbreaks have been documented, and LM is a concern for the food industry [16]. The northeast of India has very little LM, the cause of listeriosis, present or dispersed there [17]. The consumption of fermented foods rich in probiotics, which create ocins, may be the reason why these foods can affect infections like Listeria spp. LM is a zoonotic bacterium that can be found in food and can grow at temperatures between 4 and 37 °C. Listeria, which is brought on by consuming, causes septicemia, meningitis, stillbirths, and miscarriages.
Those who have compromised immune systems and pregnant women are more vulnerable to listeriosis. It is a serious foodborne infection that leads to health issues, including pregnancy-related abortions, in developing nations like India. Unlike bacteriocins or antimicrobials, the pathogen has developed resistance to antibiotics like daptomycin and tetracycline. Researchers are interested in the food spoilage bacteria's broad-spectrum antimicrobial activities, particularly against LM.
The major goal of the review is to investigate the health benefits of traditional fermented foods from India. The review then concentrates on listeriosis, its causes, and potential remedies. The greatest treatment and prevention method for listeriosis is to consume native fermented foods from India. According to historical records, listeriosis has never caused an abortion in a woman in northeast India. Locals in this region consume fermented foods because they've been proven to be healthier. We therefore want to investigate and link traditional fermented foods to listeriosis, especially in the northeastern section of the state.
Fermented Foods and Beverages
Around the world, everyone's diet contains a substantial amount of fermented foods and beverages. They have advantageous functional characteristics and are produced spontaneously as a preservation mechanism. They differ in their tastes, textures, enticing looks, and flavours as well as how they are used. They were made to give the body vitamins and minerals [18]. While spontaneous fermentation is employed in Asia and Africa, starter cultures are used in New Zealand, Europe, North America, and Australia to generate fermented foods [19]. India's traditional method of spontaneous fermentation is called backslopping [20]. The previous fermented product can be used in this process as an inoculum source to ferment the food samples [21]. Most fermented food producers and consumers are in Northeast India.
Ethnic groups made the traditional fermented foods utilising ingredients derived from animals or plants [3]. Probiotic bacteria included in fermented foods can act as a starter culture, changing substrates that are acceptable to consumers on a cultural and social level [22]. Probiotic attributes, such as fibrinolytic activity [23], antioxidant activities [24], antibacterial properties [25], and anti-nutritive chemical breakdown, were observed in the helpful bacteria found in fermented foods, according to Hill, et al. [7,26]. When choosing a starting culture to produce valuable meals, these traits serve as a defining attribute [27]. In general, the bacteria present in fermented foods enhance a variety of health benefits when consumed [28].
The "Kinema-natto-thua nao triangle" (KNT triangle) was created by Tamang (2015) and later expanded to Indonesia. It claims that naturally fermented soybean foods concentrated with bacteria and moulds are frequently consumed in India, Japan, and Thailand, the triangle's three vertices [29,30]. The main constituents are Pediococcus pentosaceous (P. pentosaceous), Lactobacillus plantarum, and Lactobacillus brevis. P. pentosaceous from Hamei, an alcoholic starter, produces bacteriocin against LM [31]. The production of Hawaijar, a traditional non-salted fermented soybean meal with Bacillus spp. as the main bacterium, is well-known in Manipur and has a considerable positive impact on human health [32].
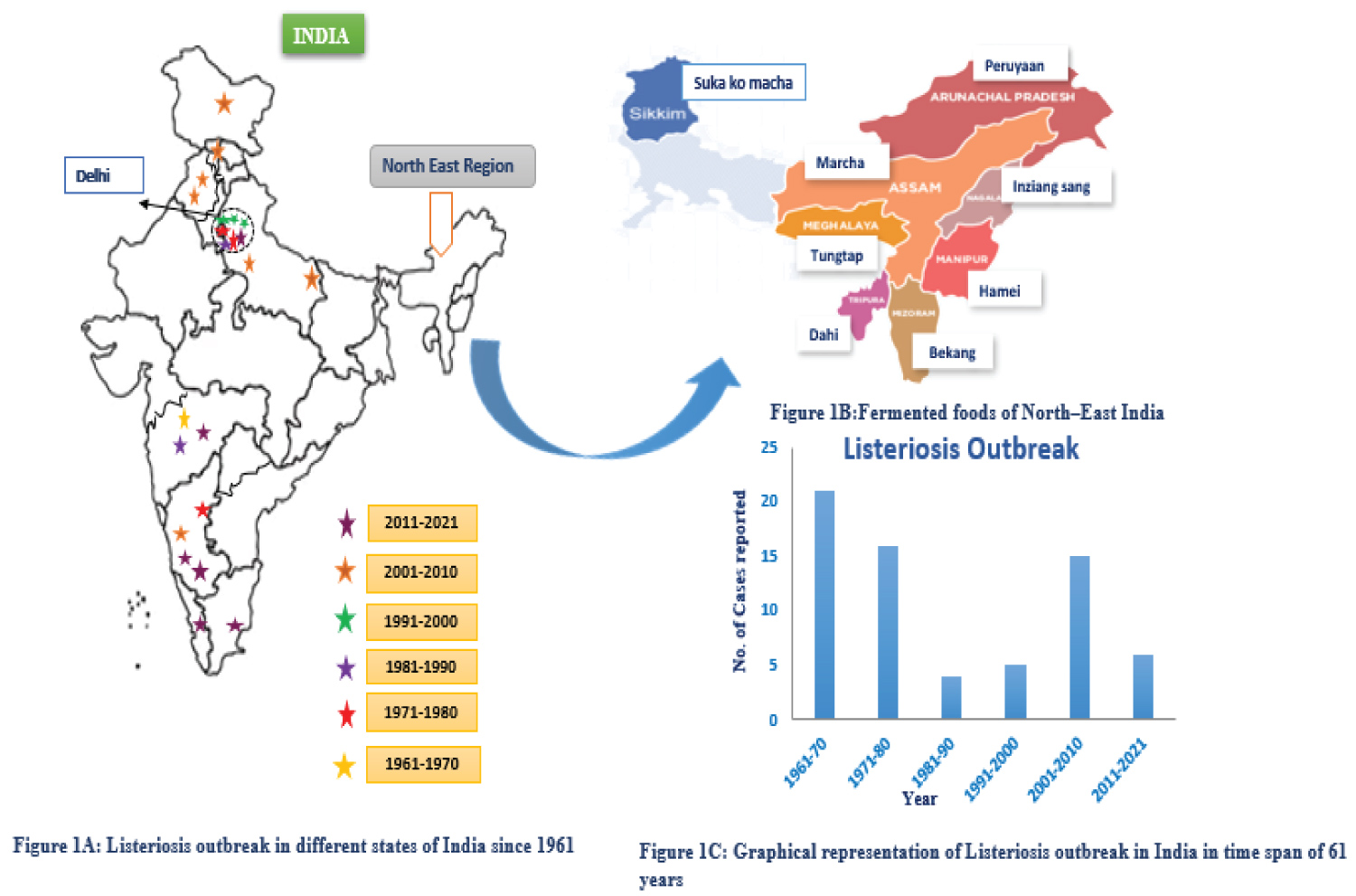
Table 1 [3,10-12,14,31-40] shows fermented foods, sources, probiotics, and India's biggest consumer state. Figure 1B shows fermented foods produced by northeastern states including significant LAB bacteriocin producers. In South India, fermented foods made from rice are the most popular. Idli, dosa, and uthappam are among the most very well delicious foods in South India. Mixed yeast and LAB cultures mediate the fermentation [40]. Most LAB strains and Bacillus spp. are hostile to food spoilage germs, which presents a risk to the food industry. A major source of worry is the possibility that listeriosis would impact the dairy, meat, and vegetable industries. The potential for infection to be fatal [41].
Probiotics and Their Attributes in Fermented Foods
Metchnikoff's research around the beginning of the twentieth century gave rise to the idea of probiotics. The findings of Ilya Ilyich Metchnikoff's research showed that LAB intake can enhance host health. By consuming fermented foods like yoghurt, cheeses, other fermented milk products, and fermented meat products, people can frequently find LAB, a well-known probiotic that includes Lactococcus and Lactobacillus, in their guts. Other popular probiotics include Bifidobacterium, Pediococcus, and Leuconostoc strains [42-44]. The classic example of a symbiotic connection is the gut microbiota, which is crucial to the maintenance of the host's physiology and health. The intestinal bacterial community must be maintained with a healthy, balanced diet. Over the past ten years, it has become clear that metabolic illnesses including type 2 diabetes and obesity are greatly influenced by the gut flora (T2D). Intestinal homeostasis has been disturbed by the westernized obesogenic diet, which is high in simple carbohydrates and saturated or trans fats, which results in insulin resistance [45]. One important risk factor for type 2 diabetes is obesity [46]. Hyperglycemia, a metabolic condition marked by elevated blood glucose levels, is a feature of T2D [47].
According to several investigations, LAB has anti-diabetic potential. In high-fat diet animals demonstrating biochemical alterations, probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei prevented the progression of diabetes [48]. According to a different study, some LAB strains may act as bio-therapeutics for the treatment of diabetes by promoting the production of gut hormones like GLP-1 and GIP [49]. Additionally, LAB strains exhibit the highest levels of -glucosidase inhibition activity, which lowers blood glucose levels and glucose absorption to help prevent T2D [50]. VSL#3, a probiotic blend of eight distinct strains, is efficient in both the management and prevention of diabetes and obesity [51].
Serum cholesterol levels are lowered when fermented milk containing large amounts of Lactobacillus and Bifidobacterium is consumed [52]. By permeating the intestinal mucosal layer and promoting phagocytic activity in the spleen and other organs, Lactobacilli and Bifidobacterium, for instance, can assist in regulating the host immune response [53]. By lowering bacterial toxins, probiotics have positive effects in the treatment of liver illnesses [54]. Healthy adults who consume Lactobacillus paracasei for four weeks see a decrease in Escherichia coli in their faeces and ammonia levels as well as an increase in Lactobacillus, Bifidobacterium, acetic acid, and butyric acid [55].
Exopolysaccharides, bacteriocins, organic acids, and antibacterial substances are only a few of the various molecules that probiotics produce. In the gut, dangerous microorganisms are eliminated by the bacteriocins and acids [56,57]. It is well known that LAB can sense its surroundings and create bacteriocins and organic acids that, by lowering pH and boosting peristalsis, prevent pathogen colonization. By keeping hazardous pathogens out, the intestinal barrier is strengthened, which directly affects the gut microbiota [58].
Mucin is secreted by intestinal epithelial cells to prevent the colonisation of microbial pathogens. Surface adhesins are secreted by certain Lactobacillus strains to aid in their ability to adhere to the mucosal layer [59-61]. The Caco-2 cell line has been successfully utilised for the past three decades to assess the probiotics' in vitro adhesion capacity. The ability of LAB strains to colonise intestinal epithelial cells with the maximum degree of adhesion makes them an effective barrier against harmful bacteria [47]. However, HT-29 cells can also be used to assess intestine adhesion capacity. The most significant amount of L. rhamnosus MG4502 adhered to HT-29 cells [46].
Listeria Monocytogenes (LM) and Listeriosis
Food safety is a serious concern in the modern public health world [62]. Disease outbreaks in a variety of foods have been linked to the genus LM of bacterial pathogens [63]. Food samples from around the world, including meat, milk, and dairy products, have all been shown to have Listeria spp [64-67]. LM is a zoonotic, non-sporulating, Gram-positive, rod-shaped, facultative, intracellular, virulent bacteria that thrives at temperatures between -2 °C and 50 °C, with optimal growth occurring between 30 °C and 37 °C [68,69]. It is a hazard to the food business and a pathogen that is found in food. It is a food-borne pathogen and a threat to the food industry, possessing attributes like the ability to grow even at 4.0 °C, resistance to extreme pH, metals, and disinfectants [70-72]. LM causes the invasive illness listeriosis [73].
LM infection
LM is connected to numerous public health problems all throughout the world. In addition to abortions, meningitis, meningoencephalitis, septicemia, and stillbirths, it is associated with visceral, neurologic, and reproductive clinical entities. A dangerous infection called listeriosis can harm expectant mothers, elderly people with compromised immune systems, newborns, people with kidney diseases, HIV patients, and even those who come into touch with animals [74-77].
It has been discovered that healthy people's intestines contain LM [78,79]. Contrary to typical foodborne pathogens like Salmonella spp., which seldom result in fatalities, listeriosis is now known to have a staggering fatality rate of up to 30% [78]. The common serotypes linked to human listeriosis include 1/2a, 1/2b, and 4b [80]. Since the placenta acts as a breeding ground for LM, pregnant women are more susceptible to infection. This can result in spontaneous miscarriages, neonatal infections, placental necrosis, stillbirths, severe necrotizing hepatitis, and a high risk of post-implantation failure [81,82]. Pregnant women with latent listeriosis are more likely to experience abortions, intrauterine deaths, and abnormalities in the foetus [83,84]. Pregnant women have a 17-fold higher prevalence of listeriosis than the general population [85]. The clinical condition known as granulomatosis infantiseptica is brought on by the foetus' increased susceptibility to infection during pregnancy [86]. Meningitis and hydrocephalus are caused by infected moms during childbirth in newborns [87]. The most recent publications emphasise the significance of the pathogen as a contributor to infant mortality and spontaneous abortions.
Pathogenesis density Global Scenario
Women from different countries, including India, who have had numerous abortions and have a poor obstetric history, have been discovered to have listeriosis [88-90]. According to the CDC, spontaneous abortion occurs in the second and third trimesters of pregnancy in one-third of all human listeriosis cases (Centre for Disease Control and Prevention). 10-20% of cases in England and Wales result in pregnancy and neonatal disease, while 15-25% of infections result in stillbirths and abortions [91]. The CDC has reported a Listeria strain outbreak in six American states as of November 9, 2022. A total of 16 persons became ill after consuming Listeria spp.- contaminated meat and cheese from deli corners, one of whom became ill while pregnant and miscarried. The CDC reported a Listeria outbreak on May 14, 2021, in four states of the United States of America due to Quesco Fresco that was tainted with Listeria spp. Four of the 13 affected individuals were ill during pregnancy, which led to two miscarriages and one early birth. The eating of Olaf ice cream tainted with Listeria monocytogenes resulted in the infection of a total of 28 persons, the majority of whom are residents of Florida. Seven women experienced illness during pregnancy, with one miscarriage.
An outbreak of LM was caused by consumption of Listeria spp.-contaminated enoki mushrooms, a Korean product, and 36 illnesses were documented. Two foetuses died in six of the cases, which were related to pregnancy. In Los Angeles and Orange counties, California, in the first six months of 1985, 86 instances of Listeria monocytogenes illness linked to Mexican-style cheese were documented. This occurred in 58 mother-infant pairings, resulting in 29 fatalities. A total of 5576 instances of listeriosis were recorded in the 10-year (2010-2019) case study in Germany, with 9% of the cases being connected to pregnancy.
A sentinel hospital in China recorded 211 occurrences of listeriosis between 2013 and 2017, with 55 resulting in foetal fatalities. Due to a multistate outbreak of Listeriosis in 1998-1999, the CDC reported that two pregnant women in the United States spontaneously aborted their unborn children. In one of the 178 cases that were documented, 36 moms (or around 20%) suffered spontaneous abortions, according to Mylonakis. In the remaining 142 cases, 97 neonates (about 68%) had the infection at birth. Because of this, obstetricians give a lot of thought to avoiding, identifying, and managing pregnancy.
LM Infection in India
In India, there is less evidence than in developed nations to support the correlation between pathogenic LM and spontaneous abortions. Because of its irregular occurrence in India, Chugh's [92] growing concern over the novel foodborne illness Listeriosis is due to this. According to Prem Saran Tirumalai's clinical research, the prevalence of listeriosis in the Indian Subcontinent amply demonstrates both newborn and maternal listeriosis [93]. Listeria in humans was originally discussed by Usha, et al. in 1966 [94,95]. Figure 1A shows the geographical risk map for listeriosis in India from 1961 (61 years). According to Bhujwala, et al. [96], LM is the etiological cause causing abortions and premature births in India. They isolated LM from 3 of 100 women having bad obstetric history. Later again in 1975, they isolated LM from 9 of 670 women possessing terrible obstetric history. Krishna, et al. later isolated LM from the cervix of 21 women with a poor obstetric history. Stephen, et al., [97], found 4 abortion cases out of 40 women in their study of diverse LM infections. According to Kaur, et al. [77], L. monocytogenes was found in 3.3% of spontaneous abortion cases. Children born to LM infected mothers had meningitis and hydrocephalus, according to Gogate and Deodhar [87].
In order to comprehend the epidemiology of listeriosis, serology is a crucial technique. However, the cross-reactivity with other related bacteria hinders the study. Serological assays can use virulence factors unique to LM as antigens to investigate the pathophysiology of listeriosis. Listerolysin O (LLO), its main virulence factor, is detected using ELISA-based methods to determine whether an animal or a human has listeriosis [98-100]. With the help of the study, we have reason to believe that India's north and west had experienced a listeriosis outbreak with high fatality rates. Although reversal was seen after eating fermented meals high in probiotics, which stop pathogen growth in food, it is probable that this is connected to eating contaminated foods. Compared to the rest of India, South India has experienced fewer breakouts. The listeriosis outbreak in India is detailed in Table 2 [77,87-89,96,97,101-113], and the sickness is graphically shown in Figure 1C.
LM in foods
In India, LM has been found in both clinical cases and foods derived from animals [77,93,114,115]. This pathogen can be found frequently in raw tropical seafood and ready-to-eat foods. This bacterium might be living in a "biofilm" -a coating of extracellular polysaccharide matrix- in food processing facilities [63,93]. Gulab Jamun, Rasagolla, curd, and payasam have been identified as sources of Listeria welshimeri, L. murrayi, and L. seeligiri [116]. LM can be used to treat human listeriosis because it is responsive to some medicines and resistant to others; nevertheless, multidrug-resistant species have evolved.
Antibiotic Susceptibility and Resistance
Antibiotics are chemicals made naturally, semi-synthetically, and synthetically that prevent bacterial growth (bacteriostatic) or eradicate bacteria (bactericidal) [117,118]. Depending on their method of action, they are categorised as bacteriostatic or bactericidal, or as narrow-spectrum or broad-spectrum drugs [119,120]. In order to help reduce food-borne illness, antibiotics are employed in the food business. The evolution of antibiotic resistance in bacteria that cause food deterioration has been linked to the usage of antibiotics in the food industry. In LM, resistance has grown to gentamicin, tigecycline, tetracycline, ciprofloxacin, ceftriaxone, and trimethoprim-sulfamethoxazole. Because the strain is primarily serotype IV sensitive, ampicillin, benzyl penicillin, linezolid, meropenem, rifampicin, and vancomycin can be employed to prevent food contamination [121]. Antibiotics are not thought to be safe in the food and cattle industries and have detrimental effects when consumed by humans. It is prohibited to utilise them in agriculture and the production of food. Pressure to limit antibiotic use in agriculture and the food sector has made antimicrobial peptides and potential antibiotic surrogates more prominent as effective natural alternatives to fight contamination and microbial infections [122,123]. Less time was spent researching safe medicinal chemicals such antimicrobial peptides (bacteriocins) and more time was spent reducing the usage of antibiotics [124,125].
Antimicrobial peptides (AMPs) or Ocins
The potential natural antimicrobials known as bacteriocins were first found by Gratia around a century ago [126]. They are proteinaceous, heterogeneous groups of cationic, amphiphilic, and/or hydrophobic antimicrobial peptides derived from both Gram-positive and Gram-negative bacteria unfriendly to closely related strains, and act at pico- to nano-molar doses [127,128]. Unlike antibiotics, which are ribosomally generated, antimicrobials also contain post-translational changes, also referred to as "postbiotics" [129].
Bacteriocins have both restricted and broad-spectrum activity as they inhibit a wide variety of bacteria, both related and unrelated [128]. They are known to be rapid to kill germs that are resistant to antibiotics [130]. These substances are GRAS (generally acknowledged as safe) microorganisms that are utilised as food biopreservative in the food industry. Perhaps they are used in place of conventional antibiotics to treat illnesses in humans and livestock used for food production. Bacteriocins play a significant role in the food industry since they are absorbed by the human gastrointestinal (GI) tract [131]. In recent years, a substantial number of LAB bacteriocins have been characterized [132].
By generating channels in the target cell membrane that allow low-molecular-weight ions to exit, they cause the proton motive force to collapse [133]. According to Klaenhammer [134], there are four different types of LAB bacteriocins: Class I, which is commonly referred to as lantibiotics and includes lanthionine, dehydrated residues, and methyl lanthionine; class II, which includes non-lanthionine peptides (less than 10 kDa); class III, which includes large heat-labile proteins (greater than 30 kDa); and class IV, which is bac NISIN, a class I lantibiotic produced by the LAB Lactococcus lactis (GRAS), is one of them and inhibits both Gram-positive and Gram-negative bacteria [135,136]. It is the only bacteriocin for the preservation of processed cheese that has been approved by the FDA [137]. It is the only bacteriocin for processed cheese preservation that has received FDA approval [137]. Pediococcus acidilactici produces Pediocin PA-1, a Class II bacteriocin, which prolongs the shelf life of ready-to-eat foods by inhibiting LM [138]. Bacillus subtilis Class I ocin subtilin suppresses LM in a variety of ways [139]. Both gram-positive and gram-negative bacteria, including Salmonella spp., Campylobacter spp., Escherichia coli, Vibrio spp., Brucella spp., and Yersinia spp., can result in food poisoning. Well-known pathogenic bacteria include B. cereus, C. botulinum, C. perfringens, B. anthracis, and S. aureus [41]. Because of recurring and severe listeriosis outbreaks, pathologists have been studying LM for the past decade [132]. The search for bacteriocin-producing LAB has since been turned to compounds that target Listeria spp., yielding a huge number of anti-listerial bacteriocins. These days, probiotics with lactic acid bacteria, such as Lactobacillus and Bifidobacterium, are widely used [140-143]. In fermented foods, they prevent potentially harmful bacteria from expanding, colonising, and multiplying [144]. There is a long history of safe usage of Bacillus strains in the food industry, and they produce a variety of antimicrobial peptides and proteins [139].
Ocins against LM
Bacteriocins that block LM are particularly interesting and have drawn a lot of research funding [145]. There aren't many published investigations on the antibacterial activity of LAB strains obtained from fermented foods against Listeria spp. P. pentosaceus HS: B1 isolated from Hamei produces bacteriocin that is effective against L. monocytogenes and L. innocua [31]. P. pentosaceus MA: C1, an isolate from Marcha, contained inhibition zones against Listeria spp [31]. It was discovered that Sukako Maacha LAB strains were effective against Listeria spp [14]. Isolates of fermented foods that are resistant to food-borne illnesses are listed in Table 3.
Consuming some Bacillus strains has been demonstrated to be healthy [32]. Bacillus subtilis produces the bacteriocin Subtilosin A, which has antibacterial activity against LM [146]. However, Subtilosin A1 (3412.5 Da), which is produced by the hemolytic mutant of Bacillus subtilis, has more antibacterial activity against LM [139,147]. Additionally, a number of clinically significant drug-resistant pathogens, including methicillin vancomycin antibiotic-resistant Staphylococcus aureus (MVRSA), vancomycin-resistant Enterococcus faecalis (VRE), and methicillin-streptomycin antibiotic-resistant Staphylococcus epidermidis (MRSE), are susceptible to the antimicrobial effects of Bacillus spp [130].
Host Immune Response against LM
The pathogen of the genus Listeria that causes listeriosis in humans is called Listeria monocytogenes (LM) [148,149]. Similar to human infection, the pathogen is ubiquitous, saprophytic, and opportunistic [149]. Meningoencephalitis, vomiting, diarrhoea, flu-like symptoms, and spontaneous abortions in pregnant women are all indications of the food-borne illness listeriosis [150]. Immune-compromised elderly people and pregnant women have died from listeriosis [149]. Since 1980, scientists have researched the molecular mechanisms behind LM's pathogenicity [151]. Understanding the immune response brought on by this intracellular infection is necessary to combat listeriosis [152]. Since the pathogen is present in both industrially produced and raw foods, LM consumption is prevalent. Considering both sporadic and epidemic cases, contaminated food is the major source of infection [67,153].
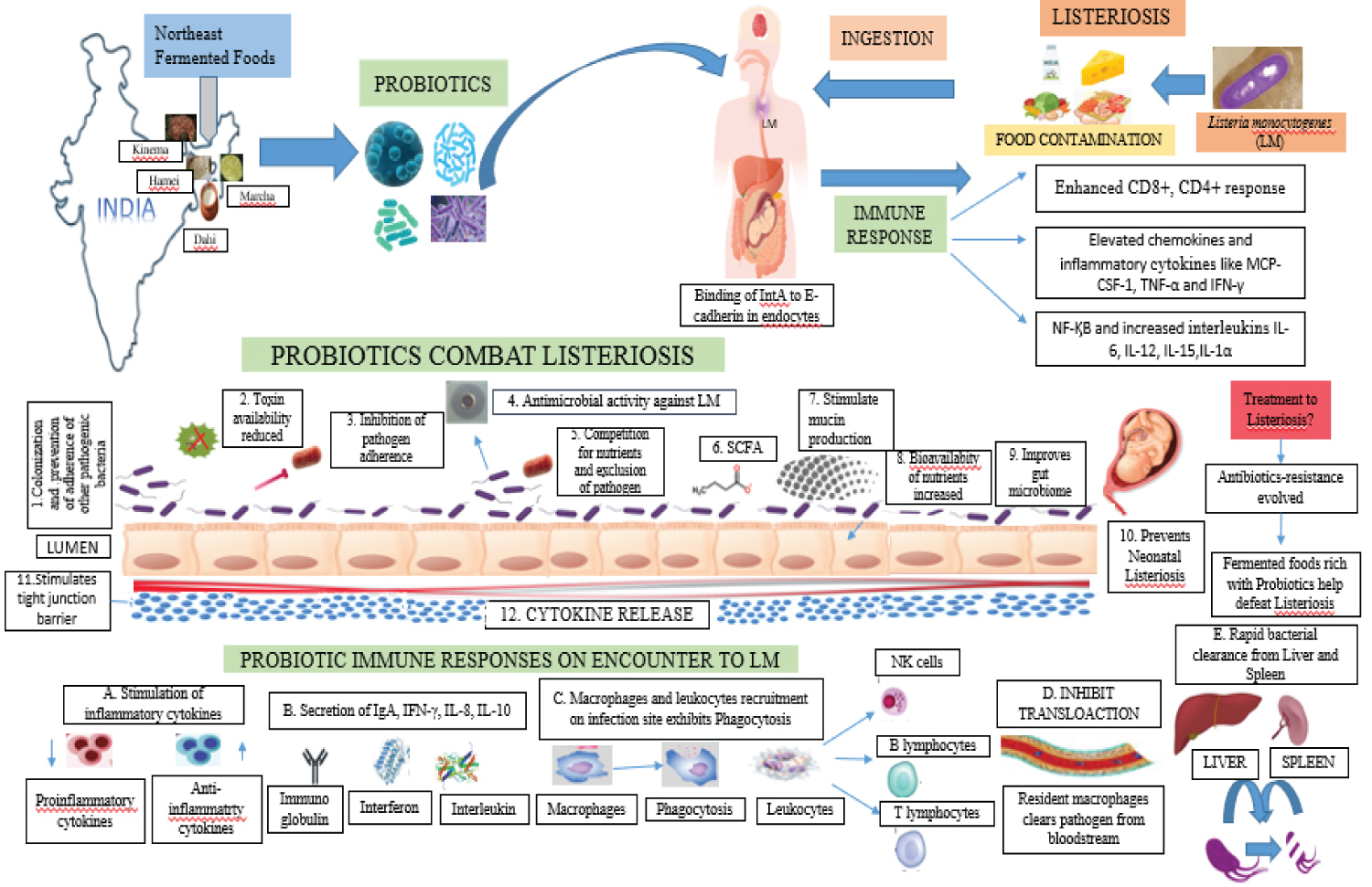
The number of bacteria in the liver rose, with the hepatocyte acting as the main site of infection. Kupffer cells (KC) in the liver gathered cytokine releases, T-lymphocyte proliferation, and anti-listerial immunity [154]. Macrophages, which are the major target of the innate immune response, cause the release of chemokines such interleukin-12 and tumour necrosis factor alpha (TNF-α) (IL-12). This triggers a bactericidal response by activating macrophages and triggering natural killer (NK) cells [155-157]. The host produces TNF-α, IL-6, IL-12, IL family, and gamma interferon (IFN-γ) as acute inflammatory cytokines in response to LM [155,158-160].
In the early stages of liver colonisation, IL-6 draws neutrophils to the sites of infection. Neutrophils then release chemokines like monocyte chemoattractant protein-1 (MCP-1) and colony stimulating factor-1 (CSF-1), which boost the number of macrophages at the localised infection site and kill LM-infected hepatocytes [161-163]. The entire clearance of the bacterial burden is aided by adaptive immunity, which is mediated by IFN-γ and CD-8, according to a week's worth of post-infection trials. TNF-α, IFN-γ, and IL-12 are produced by infected macrophages in addition to adaptive immunity [151]. In the hepatocytes of an immune-compromised host or a pregnant woman, bacteria grow without restriction before spreading hemogenously to the brain and other organs [164].
Studies on pregnant mice show that LM enters the foetus through haematogenous penetration of the placental barrier [151]. The placental villi are infected after the decidua basalis, causing necrosis and inflammation. Macrophages are not the primary participants in an immune response. To infected foci in the decidua basalis, CSF-1 draws neutrophils. LM may travel through the foetal bloodstream and cause stillbirth, early birth, or infection to the baby because to bacterial colonisation in the trophoblast layer and endothelial translocation. Due to high amounts of pregnancy hormones like oestrogen, the T-cell-mediated immune response is compromised during pregnancy. IFN-γ, IL-2, and IL-12 production are consequently decreased, all of which are necessary to remove infection. Consequently, increased susceptibility to LM was observed during pregnancy [165-167] in fetus and placenta due to local depression of cell-mediated response [168].
Although LM responds to a variety of drugs, listeriosis is challenging to treat. Only a small number of antibiotics have bactericidal qualities, while the majority have bacteriostatic properties [169]. Because of the rise of antibiotic resistance, several countries' antibiotic restrictions are a subject of concern [170]. LM is naturally resistant to antibiotics such cephalosporin, nalidixic acid, and fosfomycin [171]. As a result, as antibiotic resistance increases, antibiotic therapy fails [172]. Artificial preservatives may lower food quality because LM can flourish in any meal at any temperature [173]. Although the use of bacteriocins or other antimicrobial substances in the meat sector is well known, antimicrobial substances are currently being researched for use in the dairy industry. Bacteriocins from probiotics have been shown to stimulate an immune response against LM and so minimise the mortality rate from Listeriosis. Probiotics provide protection against intracellular bacterial infections when taken orally and function as an alternative to antibiotics [174]. Potential probiotic options Lactobacillus and Bifidobacterium are present in many foods, especially fermented dairy products [175]. Via preventing adhesion to intestinal epithelial cells by colonisation replacement, probiotics prevent the invasion of the intracellular pathogen [176,177].
By coming into touch with monolayers of intestinal epithelial cells, pre-treatment of enterocytes with probiotic bacteria prevents the invasion of Listeria [178]. By promoting mucin expression, maintaining tight junctions, and bolstering cytoskeletal integrity, the probiotics improve gut barrier function and decrease bacterial translocation [177,179-181]. Probiotic bacteria enhance the epithelial and mucosal barriers by releasing butyrate, a short-chain fatty acid produced during microbial fermentation [182]. Probiotic pre-treatment of monolayers prevents listeria infection by raising anti-inflammatory IL-10 cytokines and lowering pro-inflammatory IL-8 cytokines in the cells, according to in-vitro experiments using the C2eBb1 epithelial cell model [178].
The host response is changed by increased IgA production and decreased pro-inflammatory IFN-γ [183,184]. IL8 draws macrophages and leukocytes to the infectious inflammation region [185]. A visual illustration of listeriosis with Bacteriocin and the host immune response is shown in Figure 2. By raising TNF-α and IFN-γ levels, mono-association with LAB and subsequent Listeria infection strengthen mice's immune systems. By enhancing bacterial clearance from the liver and spleen, it also stops mice from dying when an illness lasts for a week. 2011 (Doss and associates). S-layer proteins, a type of probiotic bacterial cell component, neutralise toxins and prevent pathogen colonization [186]. Human beta-defensin-2 (hBD2) gene expression is induced by probiotics such as Pediococcus pentosaceus, Lactobacillus acidophilus, Lactobacillus fermentum, and the probiotic combo VSL#3, which results in the formation of defensins that improve mucosal barrier function. This was due to the signalling pathways of AP-1, NF-kB and mitogen-activated protein kinase (MAPK) [187].
The P. acidilactici UL5 strain of this LM inhibitor, which was isolated from fermented sausages, shows potential. According to in-vivo research, pre-treatment with Pediocin PA-1, followed by LM infection, causes the liver and spleen to be cleared of bacterial pathogens [188]. Oral administration of bioengineered Lactobacillus casei for the treatment of LM infection in pregnant and non-pregnant mice (BLP). The maternal mesenteric lymph node (MLN), liver, and spleen were infected in the BLP-fed guinea pig model, but no LM was identified in the placenta, maternal blood, lungs, kidney, or foetal liver. Pregnant BLP-fed models had a lower inflammatory response to LM, which led to a healthy pregnancy [189]. Probiotics generally contribute in the improvement of digestive processes, and the prevention of food poisoning.
Conclusion
Each society has a distinct culisnary tradition that includes fermented foods and drinks that highlight the history, social structure, and cultural characteristics of that society. Many traditional fermented meals have been produced and consumed all across the world for generations. The Eastern Himalayas are the only place in the world where soybeans are fermented into food. A range of helpful bacteria found in fermented foods have the power to enhance human health while eradicating harmful pathogenic microbes from the food supply. Probiotic strains such LABs, Bacillus spp., P. pentosaceous, and others are frequently found in fermented foods. These probiotic bacteria produce antimicrobial proteins and peptides and are antagonistic to a variety of food spoilage germs, especially LM. The recent decline in abortion rates in the Northeast and South India may be related to the region's high consumption of fermented foods. It has been discovered that fermented foods including Hamei, Marcha, and Sukako maccha contain LABs that inhibit Listeria spp. We conclude that abortion rates are reduced or non-existent in Northeast India, possibly as a result of the consumption of traditional fermented foods. Fermented foods rich in probiotics help foods develop immunity against LM, which lowers the rate of abortion. To fully understand the microbiota of all fermented foods from the Northeast, more study is required. It is necessary to record the ethnic tribal population of the region and their distinctive food customs. To fully comprehend the microbial repertoire in fermented foods, more study is required.
References
- Tamang JP (2009) Himalayan Fermented Foods: Microbiology, Nutrition, and Ethnic Values. (1st edn), CRC Press, United States.
- Tamang JP, Holzapfel WH, Shin DH, et al. (2017) Microbiology of ethnic fermented foods and alcoholic beverages of the world. Front Microbiol 8: 1377.
- Tamang JP, Tamang N, Thapa S, et al. (2012) Microorganisms and nutritional value of ethnic fermented foods and alcoholic beverages of North East India. I J Trad Knowl 11: 7-25.
- Hutkins RW (2018) Microbiology and Technology of Fermented Foods. (2nd edn), John Wiley & Sons, Ltd.
- Marco ML, Heeney D, Binda S, et al. (2017) Health benefits of fermented foods: Microbiota and beyond. Cur Opi Biotechnol 44: 94-102.
- FAO/WHO Expert Consultation Group (2007) Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Geneva: WHO.
- Hill C, Guarner F, Reid G, et al. (2014) Expert consensus document: The International Scientific Association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol Hepatol 11: 506-514.
- Mufandaedza J, Viljoen BC, Feresu SB, et al. (2006) Antimicrobial properties of lactic acid bacterial and yeast-LAB cultures isolated from traditional fermented milk against pathogenic Escherichia coli and Salmonella enteritidis strains. Int J Food Microbiol 108: 146-152.
- Rezac S, Kok, CR, Heermann M, et al. (2018) Fermented foods as a dietary source of live organisms. Front Microbiol 9: 1785.
- Jeyaram K, Romi W, Singh TA, et al. (2010) Bacterial species associated with traditional starter cultures used for fermented bamboo shoot production in Manipur state of India. Int J Food Microbiol 143: 1-8.
- Chettri R, Tamang JP (2015) Bacillus species isolated from tungrymbai and bekang, naturally fermented soybean foods of India. Int J Food Microbiol 197: 72-76.
- Thapa N, Pal, J, Tamang JP (2004) Microbial diversity in ngari, hentak and tungtap, fermented fish products of North-East India. World J of Microbiol and Biotechnol 20: 599-607.
- Adams MR, Nicolaides L (1997) Review of the sensitivity of different foodborne pathogens to fermentation. Food Control 8: 227-239.
- Thapa N. Pal J, Tamang JP (2006) Phenotypic identification and technological properties of lactic acid bacteria isolated from traditionally processed fish products of the Eastern Himalayas. Int J Food Microbiol 107: 33-38.
- Tamang JP, Tamang B, Schillinger U, et al. (2009) Functional properties of lactic acid bacteria isolated from ethnic fermented vegetables of the Himalayas. Int J of Food Microbiol 135: 28-33.
- CDC (2005) Listeriosis. Coordinating Centre for Infectious Diseases/Division of Bacterial and Mycotic Diseases, Centre for Disease Control and Prevention, Atlanta, GA30033.
- Saikia P, SR Joshi (2010) Retail market poultry meats of North-East India - A microbiological survey for pathogenic contaminants. Res J Microbiol 5: 36-43.
- Steinkraus KH (1994) Nutritional significance of fermented foods. Food Res Inter 27: 259-267.
- Tamang JP, Cotter PD, Endo A, et al. (2020) Fermented foods in a global age: East meets West. Compr Rev Food Sci Food Saf 19: 184-217.
- Sonar NR, Halami, PM (2014) Phenotypic identification and technological attributes of native lactic acid bacteria present in fermented bamboo shoot products from North-East India. J Food Sci Technol 51: 4143-4148.
- Dewan S, Tamang JP (2007) Dominant lactic acid bacteria and their technological properties isolated from the Himalayan ethnic fermented milk products. Antonie Van Leeuwenhoek 92: 343-352.
- Tamang JP, Watanabe K, Holzapfel WH (2016) Diversity of microorganisms in global fermented foods and beverages. Front Microbiol 7: 377.
- Kotb E (2012) Fibrinolytic bacterial enzymes with thrombolytic activity. In: Fibrinolytic Bacterial Enzymes with Thrombolytic Activity, SpringerBriefs in Microbiology. Berlin, Heidelberg, 1-74.
- Perna A, Intaglietta I, Simonetti A, et al. (2013) Effect of genetic type and casein halotype on antioxidant activity of yogurts during storage. J Dairy Sci 96: 1-7.
- Meira SMM, Daroit DJ, Helfer VE (2012) Bioactive peptides in water soluble extract of ovine cheese from southern Brazil and Uruguay. Food Res Int 48: 322-329.
- Babalola OO (2014) Cyanide content of commercial gari from different areas of Ekiti State, Nigeria. World J Nutri Health 2: 58-60.
- Badis A, Guetarni D, Henni DE, et al. (2004) Identification of cultivable lactic acid bacteria isolated from Algerian raw goat’s milk and evaluation of their technological properties. Food Microbiol 21: 343-349.
- Tamang JP, Shin DH, Jung SJ, et al. (2016) Functional properties of microorganisms in fermented foods. Front Microbiol 7: 578.
- Tamang JP (2015) Naturally fermented ethnic soybean foods of India. J Ethnic Food 2: 8-17.
- Tamang JP, Sarkar PK (1996) Microbiology of mesu, a traditional fermented bamboo shoot product. Int J Food Microbiol 29: 49-58.
- Tamang JP, Dewan S, Tamang B, et al. (2007) Lactic acid bacteria in hamei and marcha of North East India. Indian J Microbiol 47: 119-125.
- Jeyaram K, Singh WM, Premarani T, et al. (2008) Molecular identification of dominant microflora associated with ‘Hawaijar’- a traditional fermented soybean (Glycine max (L.)) food of Manipur, India. Int J Food Microbiol 122: 259-268.
- Sarkar PK, Tamang JP (1994) The influence of process variables and inoculum composition on the sensory quality of kinema. Food Microbiol 11: 317325.
- Tamang JP (2003) Native microorganisms in the fermentation of kinema. Indian J Microbiol 2: 127-130.
- Singh TA, Devi KR, Jeyaram K, et al. (2014) Microbial and endogenous origin of fibrinolytic activity in traditional fermented foods of Northeast India. Food Res Int 55: 356-362.
- Tamang JP, Sarkar PK, Hesseltine CW (1988) Traditional fermented foods and beverages of Darjeeling and Sikkim - A Review. J Sci Food Agric 44: 375-385.
- Tamang B, Tamang JP (2009) Traditional knowledge of bio-preservation of perishable vegetable and bamboo shoots in Northeast India as food resources. Indian J of Trad Knowl 81: 89-95.
- Keisam S, Tuikhar, N, Jeyaram K, et al. (2019) Toxigenic and pathogenic potential of enteric bacterial pathogens prevalent in the traditional fermented foods marketed in the Northeast region of India. Int J Food Microbiol 296: 21-30.
- Sha SP, Suryavanshi MV, Tamang JP, et al. (2018) Diversity of yeasts and moulds by culture-dependent and culture-independent methods for mycobiome surveillance of traditionally prepared dried starters for the production of Indian alcoholic beverages. Front Microbiol 9: 2237.
- Ray M, Ghosh K, Singh, S, et al. (2016) Folk to functional: An explorative overview of rice-based fermented foods and beverages in India. J Ethnic Foods 3: 5-18.
- Acuña L, Morero RD, Bellomio A (2011) Development of wide-spectrum hybrid bacteriocins for food bio-preservation. Food Bioproc Tech 4: 1029-1049.
- Fontana L, Bermudez-Brito M, Plaza-Diaz J, et al. (2013) Sources, isolation, characterisation and evaluation of probiotics. Br J Nutr 109: S35-S50.
- Gibson GR, Roberfroid MB (1995) Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J Nutr 125: 1401-1412.
- Ouwehand AC, Salminen, S, Isolauri E (2002) Probiotics: An overview of beneficial effects. Lactic acid bacteria: genetics, metabolism and applications, Antonie Van Leeuwenhoek 82: 279-289.
- Khan MT, Nieuwdorp M, Bäckhed F (2014) Microbial modulation of insulin sensitivity. Cell Metab 20: 753-760.
- Jeong Y, Kim H, Lee JY, et al. (2021) The antioxidant, anti-diabetic, and anti-adipogenesis potential and probiotic properties of lactic acid bacteria isolated from human and fermented foods. Fermentation 7: 123.
- Kumar M, Karthika S, Anjitha N, et al. (2022) Screening for probiotic attributes of lactic acid bacteria isolated from human milk and evaluation of their anti-diabetic potentials. Food Biotechnol 36: 234-265.
- Yadav H, Jai S, Sinha, PR (2007) Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition 23: 62-68.
- Panwar H, Calderwood D, Grant IR, et al. (2016) Lactobacilli possess inhibitory activity against dipeptidyl peptidase-4 (DPP-4). Ann Microbiol 66: 505-509.
- Marella S, Hema K, Shameer S, et al. (2020) Nano-ellagic acid: Inhibitory actions on aldose reductase and α-glucosidase in secondary complications of diabetes, strengthened by in silico docking studies. 3 Biotech 10: 1-15.
- Yadav H, Lee JH, Lloyd J, et al. (2013) Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem 288: 25088-25097.
- Yoo JY, Kim SS (2016) Probiotics and prebiotics: Present status and future perspectives on metabolic disorders. Nutrients 8: 173.
- Herich R, Levkut M (2002) Lactic acid bacteria, probiotics and immune system. Veterinarni Medicina-Praha 47: 169-180.
- Yadav R, Shukla P (2017) An overview of advanced technologies for selection of probiotics and their expediency: a review. Crit Rev Food Sci Nutr 57: 3233-3242.
- Zhang H, Sun J, Liu X, et al. (2013) Lactobacillus paracasei subsp. paracasei LC01 positively modulates intestinal microflora in healthy young adults. J Microbiol 51: 777-782.
- Jacobsen CN, Rosenfeldt Nielsen V, Hayford AE, et al. (1999) Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl Environ Microbiol 65: 4949-4956.
- Pession E (2012) Lactic acid bacteria contribution to gut microbiota complexity: lights and shadows. Front Cell Infect Microbiol 2: 86.
- Kailasapathy K, Chin J (2000) Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol Cell Biol 78: 80-88.
- Preedy VR (2010) Bioactive foods in promoting health: Probiotics and prebiotics. Academic Press, United States.
- Tassell MLV, Miller MJ (2011) Lactobacillus adhesion to mucus. Nutrients 3: 613-636.
- Buck BL, Altermann E, Svingerud T, et al. (2005) Functional analysis of putative adhesion factors in Lactobacillus acidophilus NCFM. Appl Environ Microbiol 71: 8344-8351.
- Sudershan RV, Rao P, Polasa K (2009) Food safety research in India. Asian J Food AgInd 2: 412-433.
- Swaminathan BB, Gerner-Smidt P (2007) The epidemiology of human listeriosis. Microbes Infect 9: 1236-1243.
- Jaradat ZW, Schutze GE, Bhunia AK (2002) Genetic homogeneity among Listeria monocytogenes strains from infected patients and meat products from two geographic locations determined by phenotyping, ribotyping and PCR analysis of virulence genes. Int J Food Microbiol 76: 1-10.
- Ryser ET, Marth EH (2007) Listeria, listeriosis, and food safety. (3rd edn), CRC press.
- Ikeh MAC, Obi SKC, Ezeasor DN, et al. (2010) Incidence and pathogenicity profile of Listeria sp. isolated from food and environmental samples in Nsukka, Nigeria. Afr J Biotechnol 9: 4776-4782.
- Ama El-Malek, Ali SFH, Hassanein R, et al. (2010) Occurrence of Listeria species in meat, chicken products and human stools in Assiut city, Egypt with PCR use for rapid identification of Listeria monocytogenes. Vet World 3: 353-359.
- Farber JM, Peterkin PI (1991) Listeria monocytogenes, A food-borne pathogen. Microbiol Rev 55: 476-511.
- Bajard S, Rosso L, Fardel G, et al. (1996) Particular behaviour of Listeria monocytogenes under sub-optimal conditions. Int J Food Microbiol 29: 201-211.
- Le Monnier A, Autret N, Join-Lambert OF (2007) ActA is required for crossing of the feto-placental barrier by Listeria monocytogenes. Infect Immun 75: 950-957.
- Disson O, Grayo S, Huillet E, et al. (2008) Conjugated action of two species-specific invasion proteins for feto-placental listeriosis. Nature 455: 1114-1118.
- Robbins JR, Skrzypczynska KM, Zeldovich, VB, et al. (2010) Placental syncytio-trophoblast constitutes a major barrier to vertical transmission of Listeria monocytogenes. PLoS Pathog 6: e1000732.
- Goulet V, King LA, Vaillant V, et al. (2013) What is the incubation period for listeriosis? BMC Infect Dis 13: 1-7.
- Gomez-Mampaso, E, Mochales Baquero F, de Rafael Nerpel L, et al. (1980) Listeriosis and fertility (author's transl). Reproduccion 4: 309-314.
- Lorentzen U, Nyholm HC, Moller-Hansen K.J (1997) Listeriosis igraviditet Tredje trimester. Ugeskr-Laeger 159:
- Tridente V, Cataldi UM, Mossa B, et al. (1998) Caso d'infezione MATERNA e neonatale provocata da Listeria monocytogenes. Clinica Terapeutica 149: 307-311.
- Kaur S, Malik SV, Vaidya VM, et al. (2007) Listeria monocytogenes in spontaneous abortions in humans and its detection by multiplex PCR. J Appl Microbiol 103: 1889-1896.
- Gouws PA, Liedemann I (2005) Evaluation of diagnostic PCR for the Detection of Listeria monocytogenes in food products. Food Tech Biotech 43: 201-205.
- Parihar VS, Barbuddhe SB, Danielsson-Tham ML, et al. (2008) Isolation and characterization of Listeria species from tropical seafoods. Food Control 19: 566-569.
- Bracegirdle P, West AA, Lever MS, et al. (1994) A comparison of aerosol and intra-gastric routes of infection with Listeria spp. Epidemiol Infect 112: 69-79.
- Bakardjiev AI, Stacy BA, Portnoy DA (2005) Growth of Listeria monocytogenes in the guinea pig placenta and role of cell-to-cell spread in fetal inf. J Infect Dis 191: 1889-1897.
- Abram M, Schluter D, Vuckovic D, et al. (2003) Murine model of pregnancy-associated Listeria monocytogenes infection. FEMS Immunol Med Microbiol 35: 177-182.
- Winkhaus-Schindl I, Seeliger HP, Andries L (1966) Listeriosis as a confirmed cause in several patients with habitual abortions. Geburtshilfe Frauenheilkd 26: 1377-1379.
- Romana C, Salleras L, Sage M (1989) Latent listeriosis may cause habitual abortion intrauterine deaths, fetal malformations. When diagnosed and treated adequately normal children will be born. Acta Microbiol Hung 36: 171-172.
- Southwick F, Purich D (1996) Intracellular pathogenesis of listeriosis. N Eng J Med 334: 770-776.
- Klatt EC, Pavlova Z, Teberg AJ, et al. (1986) Epidemic neonatal listeriosis at autopsy. Hum Pathol 17: 1278-1281.
- Gogate AA, Deodhar LP (1981) Meningitis due to Listeria monocytogenes: (A case report). J Postgrad Med 27: 240-242.
- Aljicevic M, Beslagic E, Zvizdic S, et al. (2005) Listeria monocytogenes in women of reproductive age. Med Arh 59: 297-298.
- Bhujwala RA, Hingorani V (1975) Perinatal listeriosis: A bacteriological and serological study. Indian J Med Res 63: 1503-1508.
- Gupta V, Gautam V, Mehta N, et al. (2003) Listeriosis in second trimester of pregnancy: Case report from India. Jpn J Infect Dis 56: 60-61.
- McLauchlin J, Mitchell RT, Smerdon WJ, et al. (2004) Listeria monocytogenes and listeriosis: A review of hazard characterisation for use in microbiological risk assessment of foods. Int J Food Microbiol 92: 15-33.
- Chugh TD (2008) Emerging and re-emerging bacteria diseases in India. J Biosci 33: 549-555.
- Tirumalai PS (2013) Listeriosis and Listeria monocytogenes in India. Wudpecker J Food Technol 1: 98-103.
- Usha K, Desai MW, Daftary VG (1966) Listeriosis - A clinical and bacteriological study. J Obst Fr Gynec 16: 304306.
- Saha M, Debnath C, Pramanik AK (2015) Listeria monocytogenes: An emerging food borne pathogen. Int J Curr Microbiol App Sci 4: 52-72.
- Bhujwala RA, Hingorani V, Chandra RK (1973) Genital listeriosis in Delhi (India): A pilot study. The Indian J Med Res 61: 1284-1288.
- Stephen S, Indrani R, Achyutha Rao, et al. (1978) Listeriosis and human abortions including A brief review of literature. J Obstet Gynecol India 28: 497-501.
- Barbuddhe SB, Malik SVS, Bhatnagar S, et al. (1999) Cytotoxic T-cell, delayed type hypersensitive and listeriolysin O responses in experimental bovine listeriosis. Vet Microbiol 64: 333-341.
- Barbuddhe SB, Malik SVS, Kumar P (1999) High seropositivity against listeriolysin O in humans. Ann Trop Med Parasitol 93: 537-539.
- Barbuddhe SB, Malik, SVS, Kumar JA, et al. (2012) Epidemiology and risk management of listeriosis in India. Int J food Microbiol 154: 113-118.
- Thomas A, Verma IC, Singh M, et al. (1981) Study of neonatal listeriosis in North India. Indian J Med Res 73: 28-32.
- Revathi G, Suneja, A, Talwar V, et al. (1995) Fatal pericarditis due to Listeria monocytogenes. Eur J Clin Microbiol Infect Dis 14: 254-255.
- Gomber S, Revathi G, Gupta, et al. (1998) Perinephric abscess (presenting asabdominal pain) due to Listeria monocytogenes. Ann Trop Paediatr 18: 61-62.
- Dhanashree B, Otta SK, Karunasagar I, et al. (2003) Incidence of Listeria spp. in clinical and food samples in Mangalore, India. Food Microbiol 20: 447-453.
- Srivastava S, Sen MR, Kumar A, et al. (2005) Neonatal listeriosis. Indian J Pediatr 72: 1059-1060.
- Peer MA, Nasir RA, Kakru DK, et al. (2010) Listeria monocytogenes meningoencephalitis in an immunocompetent, previously healthy 20-month old female child. Indian J Med Microbiol 28: 169-171.
- Mokta KK, Kanga AK, Kaushal RK (2010) Neonatal listeriosis: A case report from sub-Himalayas. Indian J Med Microbiol 28: 385-387.
- Dias M, Sukumar T, Tina D (2014) Listeria monocytogenes meningitis in an elderly, alcoholic male. International Journal of Health & Allied Sciences 3: 197197.
- Nirhale SP, Ostwal P, Rao P, et al. (2016) An uncommon meningitis in an immunocompetent individual. Neurol India 64: 168-171.
- Miraclin AT, Perumalla SK, Prasad JD, et al. (2018) Septicemic listeriosis: An emerging food-borne illness in India? Indian J Med Microbiol 36: 145-146.
- Mahadevaiah T, Rangappa P, Jacob I, et al. (2018) A rare case of Listeria monocytogenes meningitis in an Immunocompetent adult. Indian J Crit Care Med 22: 892-893.
- Gulla KM, Gupta D, Sachdev A (2019) Listeria meningitis in an immunocompetent child. Trop Doctor 49: 243-245.
- Ajimsha A, Viswamohanan I, Krishna GR, et al. (2020) A fatal case of meningitis and sepsis in an immune-compromised female. J Acad Clin Microbiol 22: 88-91.
- Barbuddhe SB, Chaudhari SP, Malik SVS (2002) The occurrence of pathogenic Listeria monocytogenes and antibodies against listeriolysin O in buffaloes. J Vet Med B Infect Dis Vet Public Health 49: 181-184.
- Shakuntala I, Malik SVS, Barbuddhe SB, et al. (2006) Isolation of Listeria monocytogenes from buffaloes with reproductive disorders and its confirmation by polymerase chain reaction. Vet Microbiol 117: 229-234.
- Suriyapriya S, Selvan P, Porteen K, et al. (2016) Prevalence of Listeria spp. in Traditional Indian Dairy Products from Chennai Metropolis, Tamil Nadu. Procedia Food Sci 6: 230-234.
- Davies J (1990) What are antibiotics? Archaic functions for modern activities. Mol Microbiol 4: 1227-1232.
- Adzitey F (2015) Antibiotic classes and antibiotic susceptibility of bacterial isolates from selected poultry; A mini review. World Vet J 5: 36-41.
- Ullah H, Ali S (2017) Classification of anti-bacterial agents and their functions. Antibacterial agents.
- Nemeth J, Oesch G, Kuster SP (2015) Bacteriostatic versus bactericidal antibiotics for patients with serious bacterial infections: Systematic review and metaanalysis. J Antimicrob Chemother 70: 382-395.
- Noll M, Kleta S, Al Dahouk S (2018) Antibiotic susceptibility of 259 Listeria monocytogenes strains isolated from food, food-processing plants and human samples in Germany. J Infect Public Health 11: 572-577.
- Cleveland J, Montville TJ, Nes IF, et al. (2001) Bacteriocins: Safe, natural antimicrobials for food preservation. Int J Food Microbiol 71: 1-20.
- He J, Eckert R, Pharm T, et al. (2007) Novel synthetic antimicrobial peptides against Streptococcus mutans. Antimicrob Agents Chemother 51: 1351-1358.
- Santos-Filho NA, Fernandes RS, Sgardioli BF, et al. (2017) Antibacterial activity of the non-cytotoxic peptide (p-BthTX-I) 2 and its serum degradation product against multidrug-resistant bacteria. Molecules 22: 1898.
- Wei XB, Wu RJ, Si DY, et al. (2016) Novel hybrid peptide cecropin A (1-8)-LL37 (17–30) with potential antibacterial activity. Int J Mol Sci 17: 983.
- Güllüce M, Karadayı M, Barış Ö (2013) Bacteriocins: Promising natural antimicrobials. Local Environ 3: 6-10.
- Arbulu S, Jiménez JJ, Gútiez L, et al. (2015) Cloning and expression of synthetic genes encoding the broad antimicrobial spectrum bacteriocins SRCAM 602, OR-7, E-760, and L-1077, by recombinant Pichia pastoris. Biomed Res Int 2015: 767183.
- Silva CC, Silva SP, Ribeiro SC (2018) Application of bacteriocins and protective cultures in dairy food preservation. Front Microbiol 9: 594.
- Klocke M, Mundt K, Idler F, et al. (2005) Heterologous expression of enterocin A, A bacteriocin from Enterococcus faecium, fused to a cellulose-binding domain in Escherichia coli results in a functional protein with inhibitory activity against Listeria. Appl Microbiol Biotechnol 67: 532-538.
- Chalasani AG, Dhanarajan G, Nema S, et al. (2015) An antimicrobial metabolite from Bacillus spp.: Significant activity against pathogenic bacteria including multidrug-resistant clinical strains. Front Microbiol 6: 1335.
- Mills S, Serrano L, Griffin C, et al. (2011) Inhibitory activity of Lactobacillus plantarum LMG P-26358 against Listeria innocua when used as an adjunct starter in the manufacture of cheese. Microb Cell Fact 10: S1-S11.
- Ennahar S, Sashihara T, Sonomoto K, et al. (2000) Class IIa bacteriocins: biosynthesis, structure and activity. FEMS Microbiol Rev 24: 85-106.
- Abanoz HS, Kunduhoglu B (2018) Antimicrobial activity of a bacteriocin produced by Enterococcus faecalis KT11 against some pathogens and antibiotic resistant bacteria. Korean J Food Sci Anim Resour 38: 1064-1079.
- Klaenhammer TR (1993) Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev 12: 39-85.
- Deegan LH, Cotter PD, Hill C, et al. (2006) Bacteriocins: biological tools for bio-preservation and shelf-life extension. Int Dairy Technol 16: 10581071.
- Zhang J, Yang Y, Yang H, et al. (2018) Purification and partial characterization of bacteriocin Lac-B23, a novel bacteriocin production by Lactobacillus plantarum J23, isolated from Chinese traditional fermented milk. Front Microbiol 9: 2165.
- Elayaraja S, Annamalai N, Mayavu P, et al. (2014) Production, purification and characterization of bacteriocin from Lactobacillus murinus AU06 and its broad antibacterial spectrum. Asian Pac J Trop Biomed 4: S305-S311.
- Kumariya R, Garsa AK, Rajput YS, et al. (2019) Bacteriocins: Classification, synthesis, mechanism of action and resistance development in food spoilage causing bacteria. Microb Pathog 128: 171-177.
- Abriouel H, Franz CM, Omar NB, et al. (2011) Diversity and applications of Bacillus bacteriocins. FEMS Microbiol Rev 35: 201-232.
- Gillor O, Etzion A, Riley MA (2008) The dual role of bacteriocins as anti-and probiotics. App Microbiol Biotechnol 81: 591-606.
- Dicks L, Botes M (2010) Probiotic lactic acid bacteria in the gastro-intestinal tract: Health benefits, safety and mode of action. Benef Microbes 1: 11-29.
- Hegarty JW, Guinane CM, Ross RP, et al. (2016) Bacteriocin production: A relatively unharnessed probiotic trait? F1000Res 5: 2587.
- Dreyer L, Smith C, Deane SM, et al. (2019) Migration of bacteriocins across gastrointestinal epithelial and vascular endothelial cells, as determined using in vitro simulations. Sci Rep 9: 1-11.
- Abdel-Shafi S, Al-Mohammadi AR, Negm S, et al. (2014) Partial purification of two novel variants of bacteriocins produced by lactic acid bacteria. Glob Vet 13: 583-589.
- Valdes-Stauber N, Scherer S (1996) Nucleotide sequence and taxonomical distribution of the bacteriocin gene Lin cloned from Brevibacterium linens M18. Appl Environ Microbiol 62: 1283-1286.
- Qin Y, Wang Y, He Y, et al. (2019) Characterization of subtilin L-Q11, a novel class I bacteriocin synthesized by Bacillus subtilis L-Q11 isolated from orchard soil. Front Microbiol 10: 484.
- Huang T, Geng, H, Miyyapuram VR, et al. (2009) Isolation of a variant of subtilosin A with hemolytic activity. J Bacteriol 191: 5690-5696.
- Dhama K, Karthik K, Tiwari R, et al. (2015) Listeriosis in animals, its public health significance (food-borne zoonosis) and advances in diagnosis and control: A comprehensive review. Vet Q 35: 211-235.
- Loo KY, Letchumanan V, Dhanoa A, et al. (2020) Exploring the pathogenesis, clinical characteristics and therapeutic regimens of Listeria monocytogenes. Microbiol 3: 01-13.
- Vivant AL, Garmyn D, Piveteau P (2013) Listeria monocytogenes, A downto-earth pathogen. Front Cell Infect Microbiol 3: 87.
- Vázquez-Boland JA, Kuhn M, Berche P, et al. (2001) Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev 14: 584-640.
- Cossart P, Mengaud J (1989) Listeria monocytogenes, A model system for the molecular study of intracellular parasitism. Mol Biol Med 6: 463-474.
- Pinner RW, Schuchat A, Swaminathan B, et al. (1992) Role of foods in sporadic Listeriosis: II. Microbilogic and epidemiologic investigation. JAMA 267: 2046-2050.
- Gregory SH, Wing EJ (1990) Accessory function of Kupffer cells in the antigen-specific blastogenic response of an L3T4+ T-lymphocyte clone to Listeria monocytogenes. Inf Immun 58: 2313-2319.
- Havell EA (1987) Production of tumor necrosis factor during murine listeriosis. J Immunol 139: 4225-4231.
- Tripp CS, Wolf SF, Unanue ER (1993) Interleukin 12 and tumor necrosis factor alpha are co-stimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Pro Nat Acad Sci 90: 3725-3729.
- Hsieh CS, Macatonia SE, Tripp CS, et al. (1993) Development of Th1 CD4+T cells through IL-12 produced by Listeria-induced macrophages. Science 260: 547-549.
- Huang S, Hendriks W, Althage A, et al. (1993) Immune response in mice that lack the interferon-gamma receptor. Science 259: 1742-1745.
- Rothe J, Werner L, Lotscher H, et al. (1993) Mice lacking the tumor necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature 364: 798-802.
- Pfeffer K, Matsuyama T, Kundig TM, et al. (1993) Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock: yet succumb to L. monocytogenes infection. Cell 73: 457-467.
- Conlan JC, North RJ (1991) Neutrophil-mediated dissolution of infected host cells as a defence strategy against a facultative intracellular bacterium. J Exp Med 174: 741-744.
- Rogers HW, Unanue ER (1993) Neutrophils are involved in acute, nonspecific resistance to Listeria monocytogenes in mice. Infect Immun 61: 5090-5096.
- Guleria I, Pollard JW (2001) Aberrant macrophage and neutrophil population dynamics and impaired Th1 response to Listeria monocytogenes in colony-stimulating factor 1-deficient mice. Infect Immun 69: 1795-1807.
- Cossart P, Lecuit M (1998) Interactions of Listeria monocytogenes with mammalian cells during entry and actin-based movement: Bacterial factors, cellular ligands and signalling. EMBO J 17: 3797-3806.
- Niederkorn JY (2006) See no evil, hear no evil, do no evil: The lessons of immune privilege. Nat Immunol 7: 354-359.
- Thellin O, Heinen E (2003) Pregnancy and the immune system: Between tolerance and rejection. Toxicology 185: 179-184.
- Petroff MG (2005) Immune interactions at the maternal fetal interface. J Reprod Immunol 68: 1-13.
- Krishnan L, Pejcic-Karapetrovic B, Gurnani K, et al. (2010) Pregnancy does not deter the development of a potent maternal protective CD8+ T-cell acquired immune response against Listeria monocytogenes despite preferential placental colonization. Am J Reprod Immunol 63: 54-65.
- Espaze EP, Reynaud AE (1988) Antibiotic susceptibilities of Listeria: In vitro studies. Inf 16: S160-S164.
- Vieco-Saiz N, Belguesmia Y, Raspoet R, et al. (2019) Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front Microbiol 10: 57.
- Hof H, Nichterlein T, Kretschmar M (1997) Management of listeriosis. Clin Microbiol Rev 10: 345-357.
- Altuntas EG, Kocan D, Cosansu S, et al. (2012) Antibiotic and bacteriocin sensitivity of Listeria monocytogenes strains isolated from different foods. Food Nutr Sci 3: 363-368.
- Griffiths MW (2003) Listeria Properties and Occurrence. “Listeria: Properties and Occurrence,” In: B. Caballero, L. Trugo and P. M. Finglas, Eds., Encyclopedia of Food Science, Food Technology and Nutrition. (2nd edn), London Academic Press, London, 3562-3573.
- Bourlioux P, Koletzko B, Guarner F, et al. (2003) The intestine and its microflora are partners for the protection of the host: report on the Danone Symposium “The Intelligent Intestine,” held in Paris, June 14, 2002. Am J Clin Nutr 78: 675-683.
- Guerin-Danan C, Chabanet C, Pedone C, et al. (1998) Milk fermented with yogurt cultures and Lactobacillus casei compared with yogurt and gelled milk: Influence on intestinal microflora in healthy infants. Am J Clin Nutr 67: 111-117.
- Salminen S, Isolauri E, Salminen E (1996) Clinical uses of probiotics for stabilizing the gut mucosal barrier: successful strains and future challenges. Antonie Van Leeuwenhoek 70: 347-358.
- Mattar A, Teitelbaum DH, Drongowski R, et al. (2002) Probiotics up-regulate MUC-2 mucin gene expression in a Caco-2 cellculture model. Pediatr Surg Int 18: 586-590.
- Corr SC, Gahan CG, Hill C (2007) Impact of selected Lactobacillus and Bifidobacterium species on Listeria monocytogenes infection and the mucosal immune response. FEMS Immunol Med Microbiol 50: 380-388.
- Mack DR, Michail S, Wei S, et al. (1999) Probiotics inhibit entero-pathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am J Physiol 276: G941-G950.
- Shen TY, Qin HL, Gao ZG, et al. (2006) Influences of enteral nutrition combined with probiotics on gut microflora and barrier function of rats with abdominal infection. World J Gastroenterol 12: 4352-4358.
- Qin HL, Shen TY, Gao ZG, et al. (2005) Effect of lactobacillus on the gut microflora and barrier function of the rats with abdominal infection. World J Gastroenterol 11: 2591-2596.
- Cook SI, Sellin JH (1998) Short chain fatty acids in health and disease. Aliment Pharmacol Ther 12: 499-507.
- Fukushima Y, Kawata Y, Hara H, et al. (1998) Effect of a probiotic formula on intestinal immunoglobulin A production in healthy children. Int J Food Microbiol 42: 39-44.
- Silva AM, Barbosa FHF, Duarte R, et al. (2004) Effect of Bifidobacterium longum ingestion on experimental salmonellosis in mice. J Appl Microbiol 97: 29-37.
- Riera Romo M, Pérez-Martínez D, Castillo Ferrer C (2016) Innate immunity in vertebrates: An overview. Immunol 148: 125-139.
- Medellin-Pena MJ, Griffiths MW (2009) Effect of molecules secreted by Lactobacillus acidophilus strain La-5 on Escherichia coli O157: H7 colonization. Appl Environ Microbiol 75: 1165-1172.
- Schlee M, Harder J, Koten B, et al. (2008) Probiotic lactobacilli and VSL#3 induce enterocyte b-defensin 2. Clin Exp Immunol 151: 528-535.
- Dabour N, Zihler A, Kheadr E, et al. (2009) In vivo study on the effectiveness of pediocin PA-1 and Pediococcus acidilactici UL5 at inhibiting Listeria monocytogenes. Int J Food Microbiol 133: 225-233.
- Ryan VE, Bailey TW, Liu D, et al. (2021) Listeria adhesion protein-expressing bioengineered probiotics prevent feto-placental transmission of Listeria monocytogenes in a pregnant Guinea pig model. Microb Pathog 151: 104752.
Corresponding Author
Rajagopal Kammara, Chief Scientist, Faculty Department of Microbiology and Fermentation Technology, CSIR-CFTRI, Mysore, India
Copyright
© 2023 Jain PM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.