Prognostic Impact of Ablative Therapy in Oligometastatic Non-Small-Cell Lung Cancer (NSCLC) with Unresectable Primary Tumor
Abstract
Purpose
Patients with Oligometastatic (OM) non-small-cell lung cancer (NSCLC) (≤ 3 metastases) might benefit from radical ablative treatment of both primary tumor and metastases. However, the role of ablative therapy in OM patients with locally advance primary tumor has not been evaluated.
The aim of our study was to analyze the efficacy and safety of ablative treatment in patients with unresectable primary tumor and synchronous Oligometastases, in terms of response rate, progression free survival (PFS) and overall survival (OS).
Methods/Patients
Retrospective study of patients with Oligometastatic NSCLC (≤ 3 lesions in a unique location) and unresectable primary tumor, treated with radical intent (chemo-radiotherapy for primary tumor and surgery, radiotherapy, Stereotactic Body Radiation Therapy (SBRT) or radiofrequency for all known metastases) between October 2011 and March 2015.
Results
Thirty patients met inclusion criteria. Median age was 58 years, 76.6% were male, and 96.6% had ECOG 0-1. Histology: Adenocarcinoma (63.6%), squamous carcinoma (20%), and other histology (13.4%). All patients had unresectable primary tumor and/or mediastinal lymph nodes. Site of metastases: brain (46.6%), lung (23.3%), bone (20%), other locations (9.9%). Sequential thoracic radiotherapy (40%) and concomitant radiotherapy (60%). Treatment of metastases: SBRT (53.3%), external radiotherapy (26.6%), surgery (13.6%), radiofrequency (3.3%), none (3.3%). Toxicity grade 3 (29.4%). Response rate was 69.9%, median PFS 11.1 months (IC 95%: 8.2-13.7), and median OS 20.2 months (IC: 15.6-20.3).
Conclusions
Radical treatment for oligometastatic and unresectable NSCLC patients is a safe therapeutic strategy and it could be contemplated as an effective therapeutic alternative in selected patients.
Keywords
Non-Small-Cell Lung carcinoma, Oligometastatic disease, Ablative treatment, Unresectable NSCLC, Survival
Introduction
Non-Small-Cell Lung carcinoma (NSCLC) is the leading cause of cancer death worldwide [1]. At diagnosis, half of patients present with disseminated disease (stage IV); they are considered incurable and treated with palliative intent. However, an intermediate state between locally-advanced and metastatic disease has been proposed: The Oligometastatic disease [2-4]. This concept refers to cases with limited number of metastatic lesions, which in many cases can be treated with radical therapies such as surgery or radiotherapy [5] and could be considered a differentiated subgroup of patients.
Clinical relevance of the Oligometastatic state is the identification of potentially curable patients with radical treatment of the primary tumor and the metastases such as surgery, stereotactic body radiation therapy (SBRT), or radiofrequency [6]. Several series have been published recently evaluating the evolution of patients with Oligometastatic NSCLC treated with radical intention. Although most of these studies are retrospective with small number of patients and heterogeneous populations, all of them coincide in demonstrating that these patients achieve median progression free survival (PFS), overall survival (OS) and survival rates at one, two and three years higher than expected for patients with advanced NSCLC [5,6]. Until very recently, the only prospective experience published was a phase II study in which 40 patients with Oligometastatic NSCLC were treated with surgery or radiotherapy of the primary tumor and metastases, obtaining a median PFS of 12.1 months, a median OS of 13.5 months, and a survivor rate at one, two and three years of 56.4%, 23.3% and 17.5% respectively [7]. A meta-analysis of different cohorts of patients with Oligometastatic NSCLC described different prognostic subgroups of patients regarding the timing of metastases (synchronous vs. metachronous) and the presence of mediastinal lymph node involvement; factors that were linked to worse outcome [8], adding controversy to the optimal management for these specific subgroup of patients. Recently, Gomez, et al. published the first multicenter randomized trial comparing aggressive local therapy with maintenance treatment or observation. PFS was statistically significant higher in patients who received aggressive therapy (11.7 vs. 3.9 months; p = 0.0054) [9].
The objectives of this study were to analyze the efficacy and safety of ablative treatment in patients diagnosed of NSCLC who present locally advance primary tumor and 1-3 synchronous metastases in terms of response rate (RR), PFS and OS, and determine predictors of survival, in order to better guide clinical care and the design of future clinical trials.
Materials and Methods
Retrospective study of patients diagnosed with unresectable advanced primary NSCLC with 3 or less metastases in a unique location, who were treated with radical intention including chemo-radiotherapy for primary tumor and ablative treatment for all known metastases.
Patients characteristics
Medical records from advanced NSCLC patients diagnosed between October 2011 and March 2015 in two different institutions of Valencia (Spain) were reviewed systematically to identify Oligometastatic patients. Institutional review boards of both institutions approved the protocol. For the purpose of this study, Oligometastatic was defined as three or fewer metastases in a single organ. Eligible patients were required to meet the following criteria: ECOG performance status (PS) 0-2, histological or cytological confirmed diagnosis of NSCLC; unresectable primary tumor at the time of diagnosis treated with first-line chemotherapy based in platinum-doublet and thoracic radiotherapy with radical doses; and ablative treatment for all known metastases, defined as conventional radiotherapy, SBRT, radiofrequency or surgery. Radiotherapy treatment was considered radical for primary tumors or metastases if radiotherapy (RT) was prescribed with a dose that was biologically equivalent to a treatment schedule of ≥ 13 fractions of 3 Gy.
Data regarding baseline radiologic measurements of the tumor prior to the start of the treatment; the extent of the disease at diagnosis and subsequent evaluation of the response to treatment using CT scans or PET-CT scans according to RECIST 1.1 criteria and blood laboratory tests prior to each cycle of chemotherapy assessing blood counts, kidney and liver function, were obtained by reviewing the medical records.
All patients' treatment plans had been discussed in a multidisciplinary tumor board, where a consensus was reached that radical treatment to all known sites of disease was clinically reasonable and feasible. To limit potential bias, including selection bias, patients were included based on the 'intention-to-treat' decision of the multidisciplinary tumor board. As such, patients who progressed or died prior to or during treatment were still included in the study database.
Primary endpoints were OS and PFS. OS was calculated from the first day of treatment (chemotherapy, radiotherapy or surgery) until the last date of follow-up or death. PFS was calculated from first day of treatment until any relapse, local or distant, or death (whichever occurred first). First progression was scored based on available clinical or imaging reports of any progression (local or distant). Toxicity was a secondary end-point and was scored based on the Common Terminology Criteria for Adverse Events version 4.0.
Statistical analysis
A descriptive statistical analysis was carried out, including central tendency and dispersion parameters for the quantitative variables, and absolute and relative frequencies for categorical variables. The Chi-squared test was used to compare two or more independent subject groups for categorical variables. The survival curve was estimated using the Kaplan-Meier method and compared using the log-rank test. All statistical analyses and the results were processed using the statistical software package SPSS 19.0.
Results
Between October 2011 and March 2015 a total of 30 patients met the eligible criteria. Baseline patient and tumor characteristics are summarized in (Table 1). The median age was 58 years (range 40-76), and 23 males (76.6%) and 7 females (23.4%) were included. Twenty-nine patients (96.6%) presented ECOG-PS 0-1, and 29 were smokers or former smokers. The most frequent histology was adenocarcinoma (63.3%) followed by squamous carcinoma (20%). All patients had stage III intra-thoracic disease; nine patients (33.1%) presented with stage T4 and 21 (66.9%) classified as N2 or N3.
Twenty-three patients (76.6%) had a solitary metastasis, five patients (16.6%) had two metastases and two (6.6%) had three metastases. The most common site for metastases was brain (14 patients, 46.6%), followed by lung (7 patients, 23.3%), bone (6 patients, 20%), adrenal (2 patients), and extra-thoracic lymphatic node (1 patient). Treatment to the primary site included concurrent chemo-radiation in 18 patients (60%) and sequential chemo-radiation in 12 patients (40%). All patients received at least one cycle of platinum-based doublet. Most metastases were treated by either SBRT (53.3%) or conventional radiotherapy alone (26.6%). Four patients were treated with surgical resection of the metastases (13.3%), one with radiofrequency of a lung metastasis. One patient did not receive the planned metastasis ablative treatment due to early progression during chemo-radiotherapy treatment.
The toxicity and safety profile is summarized in (Table 2). The most frequent grade 3-4 side effects were anemia (20%), asthenia (13.3%) and febrile neutropenia (10.3%). We also observed one case of grade 3 diarrhea, one case of grade 3 pneumonitis based on a temporary need for oxygen, and two cases of grade 3 vomiting. No toxic-deaths were observed. Ten patients required at least one delay of chemotherapy cycle (33.3%), and five (16.6%) required a chemotherapy dose reduction. Six patients (20%) needed to be hospitalized during treatment.
Overall response rate (Table 3) was 70% (21 patients), and the disease control rate was 86.6% (26 patients).
Four patients showed disease progression during or at the first evaluation after completing treatment (13.3%).
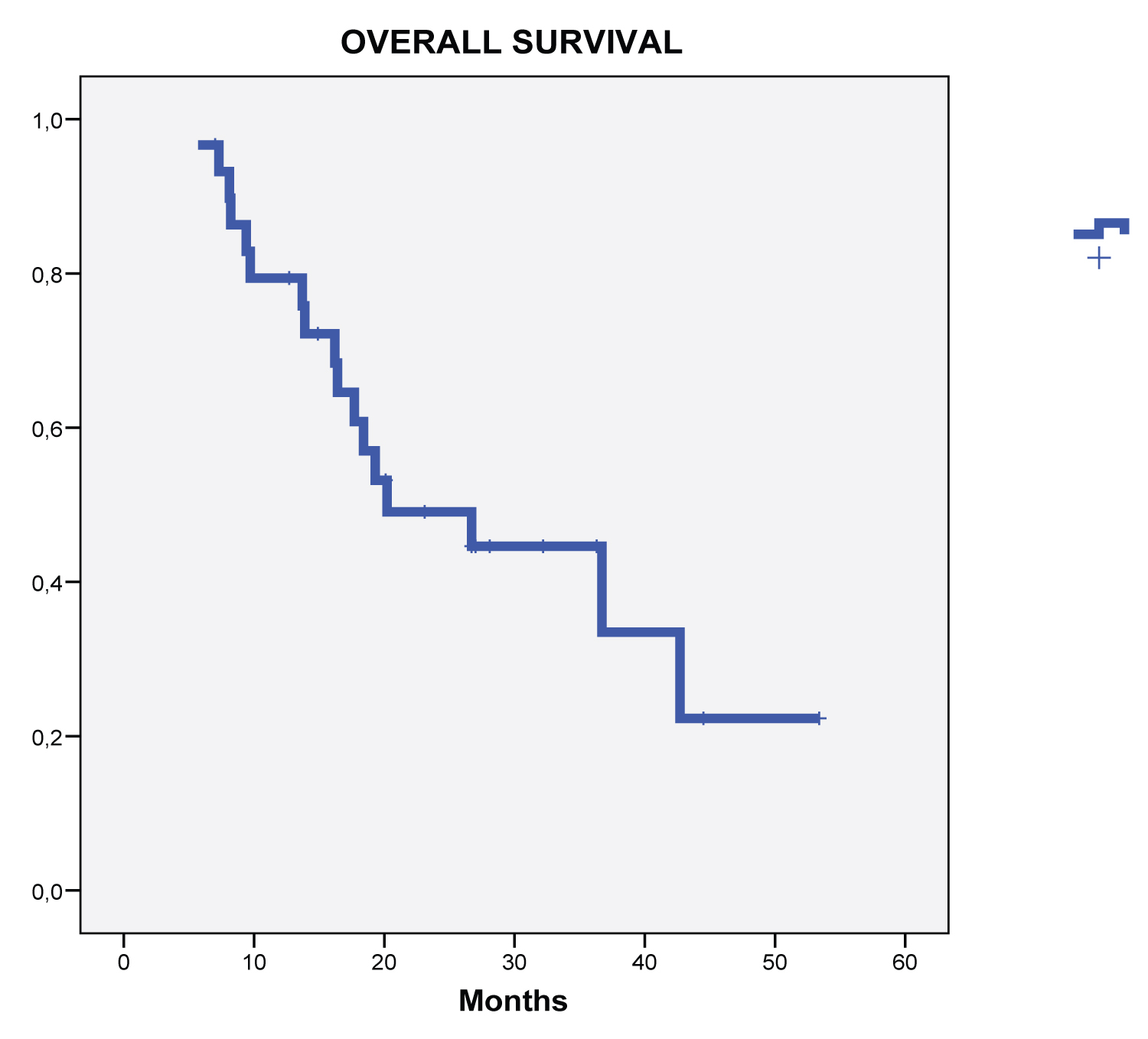
Median actuarial follow-up for the whole group was 26.1 months, and 17 death events occurred. Median OS was 20.2 months, with an actuarial 1 year OS of 76.6%, a 2 year OS of 36.6% and 3 year OS of 16.6% (Figure 1). Median PFS was 11.1 months, with actuarial 1 and 2-year PFS of 33.3 and 10%, respectively.
In the univariate analysis, factors associated with better outcome in terms of PFS and OS were: Age (< 70 vs. ≥ 70 years), sex, ECOG-PS (0 vs. 1-2), histology (squamous vs. non-squamous), mediastinal lymphnode infiltration (N2 vs. N0-1), brain metastases (yes vs. no) and maintenance treatment (yes vs. no). Results are summarized in (Table 4). Factors associated with longer OS were non-Squamous histology (36.7 vs. 13.9 months, p = 0.005), and lack of mediastinal lymphnode infiltration (42.7 vs. 17.7 months, p = 0.05). Factors associated with longer PFS were non-squamous histology (12.1 vs. 8.1 months, p = 0.041), and maintenance treatment after radical therapy (12.1 vs. 9.6 months, p = 0.041).
Discussion
This two-center pooled retrospective analysis of patients undergoing radical treatment for NSCLC with 1 to 3 synchronous metastases found that over 75% of the treated patients had a solitary metastasis, most often located in the brain. Radical treatment resulted in a median OS of 20.2 months and a median PFS of 11.1 months.
The data reported in the present study are consistent with previously published literature, suggesting that a radical treatment strategy for Oligometastatic NSCLC patients achieves high rates of survival, as more than one third of the patients survived longer than two years after treatment, with acceptable toxicity. Overall, our data are comparable with the results observed in a recent prospective phase II study of 39 patients who received radical treatment for NSCLC with synchronous oligometastases, in which the primary tumor was treated with chemo-radiotherapy [7]. Median OS in this trial was 13.5 months, and the reported survival rates at 1 and 2 years were 56 and 23%, respectively, compared with 76.6% and 36.6% in the present study. The 1- and 2-year PFS were 51% and 13% in the De Ruysscher study, versus 33.3% and 10% in the present study.
Patient selection plays a pivotal role in these outcome data: only fit patients will be eligible for radical treatment over the primary tumor and mediastinal lymph nodes and ablative therapy for all metastatic sites. These patients generally present with a more favorable ECOG-PS and have fewer co-morbidities, while those who are unfit will be not considered for such treatment. Therefore, the results of this study must be considered within the context of its limitations, particularly in light of its retrospective nature. Furthermore, patients in other published series are heterogeneous regarding intra-thoracic stage, radical treatment for primary tumor, number and location of metastases, making it difficult to compare their outcomes. Ashworth, et al. conducted a meta-analysis to assess prognostic factors for patients with oligometastatic NSCLC after treatment [8]. They found that independent prognostic factors for better outcome were synchronous vs. metachronous metastases, lack of mediastinal nodal involvement, and adenocarcinoma histology. They differentiated three different prognostic groups: 1) Low risk - If metachronous metastases (5-year OS, 47.8%), 2) Intermediate risk: Synchronous metastases and N0 (5-year OS, 36.2%), and 3) High-risk: Synchronous metastases and N1/N2 disease (5-year OS, 13.8%). It is important to note that following this classification, 90% of our patients belonged to the high-risk group (N1-2) and 10% to the intermediate group (N0) and our data can only be extrapolated to patients in this specific situation. We found significant differences in prognosis for patients with N2 vs. N0-1, which coincides with previous observations. We also observed differences in prognosis, both for OS and PFS, favoring non-squamous histology; this factor could be an important issue for patient selection in this setting. Despite most of our patients belonging to the high-risk group, survival outcomes observed in our study could be considered much more similar to patients with stage III disease than to unselected stage IV patients. Median OS observed for stage III patients treated with chemo and radiotherapy, followed or not with surgery, range between 22 and 23 months [10]. Median OS observed in our cohort of patients was 20.2 months, similar to the aforementioned stage III populations. Importantly, our study confirms that, although some patients achieve long-term survivorship beyond two years, most patients' progress within a relatively short period, suggesting that undetectable micro-metastases were likely present at the time of diagnosis in many patients.
Knowledge about the molecular bases that govern the development of metastases is still scarce, especially in regard to the differentiation between oligometastatic vs. polymetastatic disease. Recent studies have shown patterns of expression of micro RNAs capable of differentiating patients in both situations [11,12]. Micro RNAs are small molecules of non-coding RNA, whose function is the post-transcriptional regulation of gene expression [13]. Its role in the regulation of the process of metastases has been widely recognized, and different micro RNAs have been associated with both oncogenic and tumor suppressor pathways in the different stages of this process [14,15]. A constant in studies with micro RNAs is the evidence that different micro RNAs are able to regulate the same target gene, as well as that each of the micro RNAs can participate in the control of hundreds of different genes. These findings allow us to hypothesize that some groups of micro RNAs could play a role in the regulation of the oligometastatic phenotype. Recently Uppal, et al. described a group of four micro RNAs encoded at locus 32 of the long arm of chromosome 14, (mir-127-5p, miR-369-3p, miR-544a, and miR-655-3p) whose over expression seems to be associated with the oligometastatic phenotype [16]. They found that in several series of oligometastatic patients the over expression of this family of micro RNAs was associated with a better prognosis and a lower risk of developing new metastases. Likewise, they demonstrated that the expression of these micro RNAs reduces the metastatic properties of the cells in vitro, and inhibits the development of pulmonary metastases in vivo in animal models through the reduction of cell mobility, repression of the cellular cytoskeleton organization and of the signals of the molecular cascade mediated by TGF-beta, all of which are involved in processes essential for the process of metastatic tumor dissemination. Whether micro RNAs could play a role as biomarkers of survival outcomes in oligometastatic disease remains controversial.
An important bias of the present study is the fact that follow-up was not standardized and imaging protocols varied widely. This makes the identification and differentiation of local and distant recurrences less reliable. Data on some variables of interest, such as quality of life, were not available. Without a control group of patients with oligometastatic disease who did not receive aggressive treatment, it is difficult to ascertain whether such aggressive treatment to metastases actually improves OS. For patients with synchronous NSCLC oligometastases, a prospective multi-center trial would be necessary to detect any potential survival benefit of radical treatment over chemotherapy alone.
Conclusion
Radical treatment of selected NSCLC patients presenting with 1-3 synchronous metastases is safe and can result in favorable 2-year survival. Prospective clinical trials, ideally randomized, should evaluate the role of radical treatment strategies in patients with oligometastatic NSCLC.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors thank Dr. Ingrid de Ruiter for improving the use of English in the manuscript.
Compliance with Ethical Standards
Due to the characteristics of the study, the ethics committee approved the conduction of the study without obtaining the informed consent from the participating patients since a previous consent for prior analysis had been obtained and most of the patients had died at the moment of the current analysis.
References
- Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62: 10-29.
- Hellman S, Weichselbaum RR (1995) Oligometastases. J Clin Oncol 13: 8-10.
- Weichselbaum RR, Hellman S (2011) Oligometastases revisited. Nat Rev Clin Oncol 8: 378-382.
- Oh Y, Taylor S, Bekele BN, et al. (2009) Number of metastatic sites is a strong predictor of survival in patients with non-small cell lung cancer with or without brain metastases. Cancer 115: 2930-2938.
- Khan AJ, Mehta PS, Zusag TW, et al. (2006) Long term disease-free survival resulting from combined modality management of patients presenting with oligometastatic, non-small cell lung carcinoma (NSCLC). Radiother Oncol 81: 163-167.
- Siva S, Mac Manus M, Ball D (2010) Stereotactic radiotherapy for pulmonary oligometastases: A systematic review. J Thorac Oncol 5: 1091-1099.
- Ruysscher D, Wanders R, van Baardwijk A, et al. (2012) Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: Long-term results of a prospective phase II trial (Nct01282450). J Thorac Oncol 7: 1547-1555.
- Ashworth AB, Senan S, Palma DA, et al. (2014) An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic Non-Small-Cell lung cáncer. Clinical Lung Cancer 15: 346-355.
- Gomez DR, Blumenshein GR Jr, Lee JJ, et al. (2016) Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: A multicentre, randomixed, controlled, phase 2 study. Lancet Oncol 17: 1672-1682.
- Albain KS, Swann RS, Rusch VR, et al. (2009) Radiotherapy plus chemotherapy with or without surgical resection for stage III Non-Small Cell Lung Cacner. Lancet 374: 379-386.
- Lussier YA, Xing HR, Salama JK, et al. (2011) MicroRNA expression characterizes oligometastasis (es). PLoS One 6: e28650.
- Lussier YA, Khodarev NN, Regan K, et al. (2012) Oligo-and polymetastatic progression in lung metastasis (es) patients is associated with specific MicroRNAs. PLoS One 7: e50141.
- Ambros V (2004) The functions of animal micro RNAs. Nature 431: 350-355.
- Iorio MV, Croce CM (2012) Causes and consequences of micro RNA dysregulation. Cancer J 18: 215-222.
- Zhang J, Ma L (2012) Micro RNA control of epithelial-mesenchymal transition and metastasis. Cancer Metastasis Rev 31: 653-662.
- Uppal A, Wightman SC, Mallon S, et al. (2014) 14q-32-encoded micro RNAs mediate an oligometastatic phenotpe. Oncotarget 6: 3540-3552.
Corresponding Author
Dr. Oscar Juan-Vidal, Hospital Universitario y Politecnic de La Fe, Av. Fernando Abril Martorell 106, Valencia, Spain, Tel/Fax: +34-96-638-44-35.
Copyright
© 2020 Garde-Noguera J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.