Advances in Microbial Leaching as a Non-Conventional Technique for Metal Recovery from Ores: A Review
Abstract
The need for heavy metal recovery, together with the profitability of precious and base metals are strong incentives for researchers to find a sustainable method for metal recovery from ore and waste. The scientific community is trying to improve the efficiency of non-conventional methods for metal recovery from ores and wastes using bioleaching, a more sustainable method in comparison to conventional metallurgical traditional methods. The main objective of this review is describes how to use microbes for bio-dissolution and recovery of strategic elements as uranium, thorium and rare earth elements from ore especially low grade and the kinetic models that describe the bioleaching processes. In addition, the application of novel approaches to understand how the contribution of microorganisms and their genetic modification can affect the processes, are reviewed.
Keywords
Bioleaching, Microorganisms, mechanisms, Low grade ores, Strategic elements
Introduction
Bioleaching is an emerging technology with significant potentials to add value to the mining industries so as to deliver attractive environmental and social benefits to all the associates [1]. Bioprocessing of ores and concentrates to recover rare earth elements (lanthanides) and other metals, is established as well as an evolving area of biotechnology [2]. The bioleaching process presents two advantages as compared to the conventional chemical leaching processes: (a) Very low concentrations of organic compounds present in such a situation represent a lower ecological risk; and (b) Even with a lower final yield, the economic cost of a such process is lower. Both characteristics could facilitate its industrial application [3]. One possible solution is to develop other leaching processes, such as bioleaching. Several mechanisms may be involved in bioleaching these include, acidolysis, complexolysis, redoxolysis and bioaccumulation. Acidolysis is the main principal mechanism in bioleaching of metals where, the fungus and bacterium produce varieties of organic acids as citric acid, oxalic and gluconic acids during the bioleaching [4]. The ability of a variety of microorganisms to mobilize and leach metals from solid materials is based on three principles, namely (i) The transformation of organic or inorganic acids (protons); (ii) Oxidation and reduction reactions and (iii) The excretion of complexing agents. Metals can be leached either directly (i.e. physical contact between microorganisms and solid material) or indirectly (e.g. bacterial oxidation of Fe 2+ to Fe 3+ which catalyses metal solubilization as an electron carrier [5]. Acidothiobacillus ferrooxidansis [6] well known iron and sulphur oxidizing bacterium and its role in the mining industry has been extensively reviewed.
Siderophores are low molecular weight Fe 3+ coordination compounds excreted by microorganisms, particularly bacteria and fungi, to enable accumulation of iron from the environment. Although virtually specific for Fe 3+ , siderophores and analogous compounds can complex certain other metals, e.g. Ga 3+ , Cr 3+ , Sc 3+ , Ni 2+ , U 6+ and Th 4+ . Microbial transformations of toxic metals have been the subject of numerous investigations over the past several years. Siderophores are low molecular weight (< 14 kDa) iron chelating compounds synthesized by microbes in large quantity under iron limitation conditions. Pseudomonas spp. have been known for their siderophores production for many years and therefore many reports on the isolation and characterization of their siderophores have been published [7].
Microorganisms can synthesis different type of polymeric substances (PSs) according to their location in the cell, structure, sugar composition, type of bonds between monomers, and systematic affinity. The extracellular polymeric substances (EPSs) are a complex mixture of macromolecular polyelectrolytes including polysaccharides, proteins, nucleic acids [8] amino acids, lipids or humic substances [9] . Extracellular polysaccharides (EPSs) are characteristically highly abundant in nature, renewable, nontoxic, intrinsically biodegradable and relatively cheap. Besides the ability of binding with heavy metals, EPSs also possess advantage of aggregation of pollutant particles, stabilization of the floc structure, formation of a protective barrier, and retention of water [10]. The activities of fungal EPSs in such processes as mineral solubilization and heavy metal sorption. This review focuses on the process of bioleaching, a conventional and eco-friendly method for the recovery of metals by application of microorganisms. The bio-dissoluion of metals from their ores produces concentrated solutions of metal such as copper, gold, uranium etc., which can be recovered by hydrometallurgical processes. The microbial extraction of metals is done through leaching by organic acids fungal producer, acidophilic sulfur oxidizing and iron-oxidizing bacteria.
Aspects of Microbial Leaching
Generally, bioleaching is a process described as “the dissolution of metals from their mineral sources by certain naturally occur microorganisms” or the use of microorganisms to transform elements so that the elements can be extracted from a material when water is filtered through it [11]. Microbial technology helps in case of recovery of ores which cannot be economically processed with chemical methods, because they contain low grade elements. Therefore, large quantity of low grade ores are produced during the separation of high grade ores. The metal solubilization process is due to a combination of chemistry and microbiology: chemistry, because the solubilization of the metal is considered to be mainly a result of the action of ferric iron and/or acid on the mineral, and microbiology, because microorganisms are responsible for producing the ferric iron and acid. Microbially metal-extraction processes are usually more economical and eco-friendly than physicochemical processes [12]. Currently, microbial-based processes are used for leaching strategic elements as copper, uranium, thorium and rare earth elements (REEs) enhancing the recovery of gold from refractory ores, and treating industrial wastewater to recover metal values.
Characterization of Bioleached Microbes
The bioleaching process presents two advantages, as compared to the conventional chemical leaching processes: (a) The very low concentrations of organic compounds present in such a situation represent a lower ecological risk; and (b) Even with a lower final yield, the economic cost of a such process is lower. Both characteristics could facilitate its industrial application [3]. The ability of a variety of microorganisms to mobilize and leach metals from solid materials is based on three principles, namely (i) The transformation of organic or inorganic acids (protons); (ii) Oxidation and reduction reactions and (iii) The excretion of complexing agents. Metals can be leached either directly (i.e. physical contact between microorganisms and solid material) or indirectly (e.g. bacterial oxidation of Fe 2+ to Fe 3+ which catalyses metal solubilization as an electron carrier) [5]. There are two different kinds of microbes used in bioleaching: autotrophic and heterotrophic. Autotrophic microbes are not suitable for bioleaching of metals as it greatly increases the pH of the medium and contains no energy sources (sulfur or reduced iron) for the growth of chemolithoautotrophic bacteria (e.g., acidophilic Thiobacillus sp.) as previously mentioned by [13]. In contrast, heterotrophic microbes as fungi have several advantages as far as leaching metals from theores. Firstly, chemoorganoheterotrophic fungi can use utilize organic carbon source as substrate and can dissolve uranium at high pH. And the secreted organic acids can react with calcium, aluminium, iron, and other elements in gangue to form complexes with higher solubility. Secondly, in addition to acidic ore, fungi can transform uranium oxides, carbonates, and phosphates to form uranium complexes with carboxylic acids. Thirdly, fungi are the heterotrophic microorganisms characterized by rapid growth rate, large biomass and short extraction cycle [14]. Fourthly, fungal leaching has low anti-corrosion requirement for equipment, since the organic acids produced by fungi are mainly weak acids, which show less environmental hazards than H 2 SO 4 and other strong acids and can be degraded by environmental microorganisms. As an environment-friendly method, fungal leaching is of huge development potential and wide application prospect and has been extensively studied [15].
Mechanisms of Microbial Leaching
Indeed, there are proposed mechanisms for bio-dissolution of minerals by lichens, fungi, and bacteria include the use of microorganisms for oxidation or reduction of metals in the mineral [16], production of organic acids [17], chelates [18] and polysaccharide slimes [19].
Oxidation of metals
a. Oxidation of sulphur ions to sulphuric acid
Bioleaching using sulphur-oxidizing bacteria does require the initial preacidification process. The pH of the sludge decreased from an initial value of 7.0 to 2.0 owing to the production of sulphuric acid through the sulphur- oxidizing-microorganisms [20].
The bioleaching process is primarily achieved by two mechanisms: (I) direct (Eq. (1)) the bacteria involved in bioleaching process can enhance the oxidation of insoluble metal sulphides to soluble metal sulphates; and (ІI) indirect (Eqs. (2) and (3)) elemental sulphur or reduced sulphur compounds are oxidized to sulphuric acid by these bacteria, thereby lowering the pH and subsequently increasing the metal solubilization [21].
Where, M is a bivalent metal.
Oxidation of ferrous ions to ferric ions
Bioleaching microbes have number of features in common that make their role especially suitable for mineral solubilization. The most important microbes involved in the bio oxidation of minerals are those responsible for producing ferric ion (Eq. 4) and sulphuric acid (Eq. 5) required for the bioleaching reactions. Ferric sulphate, a powerful oxidizing agent, oxidizes the copper sulphide minerals leading to the in situ leaching of copper by the sulphuric acid generated therein.
Acidothiobacillus ferrooxidans was used for bioleaching of REEs a phosphate ore (fluorapatite/clays as main minerals). We studied the leaching kinetics, simplistically described as: Rt = [(k1 A p )/(k2 C t )],where, Rt is the leaching rate, Ap is the total particle surface area, and, Ct, the bacterial count at time t; k1is the leaching rate constant for the surface area term (Ap), and k2 is the coefficient for the bacterial activity-H 2 SO 4 producing ability of bacteria [22].
Microorganisms are important in metal recovery from ores, particularly sulphide ores. Copper, zinc, gold, etc. can be recovered from sulphide ores by microbial leaching. Thiobacillus ferrooxidans is the most studied organism in microbial leaching, but other iron or sulphide/sulphur-oxidizing bacteria as well as archea are potential microbial agents for metal leaching at high temperature or low pH environment. Oxidation of iron or sulphur can be selectively controlled for leaching of desired metals leaving undesired metals, (e.g. Fe) [23].
Scheme (1) shows a brief summary of classical understanding of oxidation reactions carried out by T. ferrooxidans and metal leaching reactions achieved as the result. T. ferrooxidans was the principal organism considered responsible for the microbial leaching.
Sulphide oxidation
(A) Metal leaching
Ferrous ion oxidation
(A+B) Metal leaching
C. Ferric ion as an oxidizing agent
Indirect method
Scheme (1) Classical understanding of reactions carried out by T. ferrooxidans and metal leaching.
c. Advantages of mineral biooxidation process
A further advantageous characteristic of mineral biooxidation operations is that they are not usually subject to contamination by the generated unwanted microorganisms. Also, one of the most important characteristic of the acidophilic chemolithotrophs is (i) Their general tolerance to higher concentrations of metal and other ions. (ii) The levels of resistance show a considerable strain variation. (iii) The nutritional requirements of these organisms are provided by the aeration of an iron and/or sulphur containing mineral suspension in water or irrigation of a heap in a higher scale of operation. These microorganisms are employed for the leaching of metals from ores because of their rapid growth on the ore or concentrate [1]. The diversity of acidophilic micro-organisms that have direct and indirect roles in the oxidation of sulfidic ores and concentrates is considerable [24].
Production of organic acids
Bioleaching processes are mediated due to the chemical attack by the extracted organic acids on the ores. The acids usually have dual effect of increasing metal dissolution by lowering the pH and increasing the load of soluble metals by complexion/chelating into soluble organic-metallic complexes [25]. Acidolysis is the principal mechanism in bioleaching of metals by microbes which produced organic acids, such as citric, oxalic, malic and gluconic acids during bioleaching [26]. The metabolic process of fungi is similar to a great extent to those of higher plants with the exception of carbohydrate synthesis. The glycoltic pathway converts the carbon sources as (sucrose, glucose,…….., etc .) into variety of products including organic acids [27]. The metabolites contained organic acids dissolve metals from minerals by displacement of metal ion from the ore or soil matrix by hydrogen ions, or by the formation of metal complexes and chelates [28]. It was observed that Penicillium purpurogenium and Pseudomonas fluorescens SHA 281 have the ability to produce organic acids which have the ability to bioleach uranium from Gattar low grade ore according to [29].
During the growth studies of Penicillium purpurogenium and Pseudomonas uorescens SHA 281 , the substrates undergo microbial oxidation which resulted in the production of organic acids, citric, oxalic, tartaric and gluconic acids, that play a fundamental role in the environmental mobility of metal ions. Concerning P. purpurogenium MCDB contained sucrose as a carbon source and energy source. The decrease in pH was observed due to the organic acid production via incomplete oxidation by invertase enzyme to citric and oxalic acids [30] as Eq. 1 and 2:
Citric acid is a tricarboxylic acid which contains three carboxylic moities and one hydroxyle group ( pka = 6.39 ) as possible donor of proton (H + ) at 30 °C.
In doing so, P. fluorescens SHA 281 NB medium contained glucose as a carbon source and energy source. Glucose oxidase enzyme was used for oxidation of glucose this produced gluconic acid and H 2 O 2 (Eq. 5) [31] as follow:
The H 2 O 2 released during this process reacted with Fe(II) and oxidized Fe(II) to Fe(III) according to Eq. (6) [32]:
Similarly, gluconic acid contains one carboxylic moeity ( pka = 3.66 ) at 30 °C. So, the possible complex of uranium with gluconate anion is:
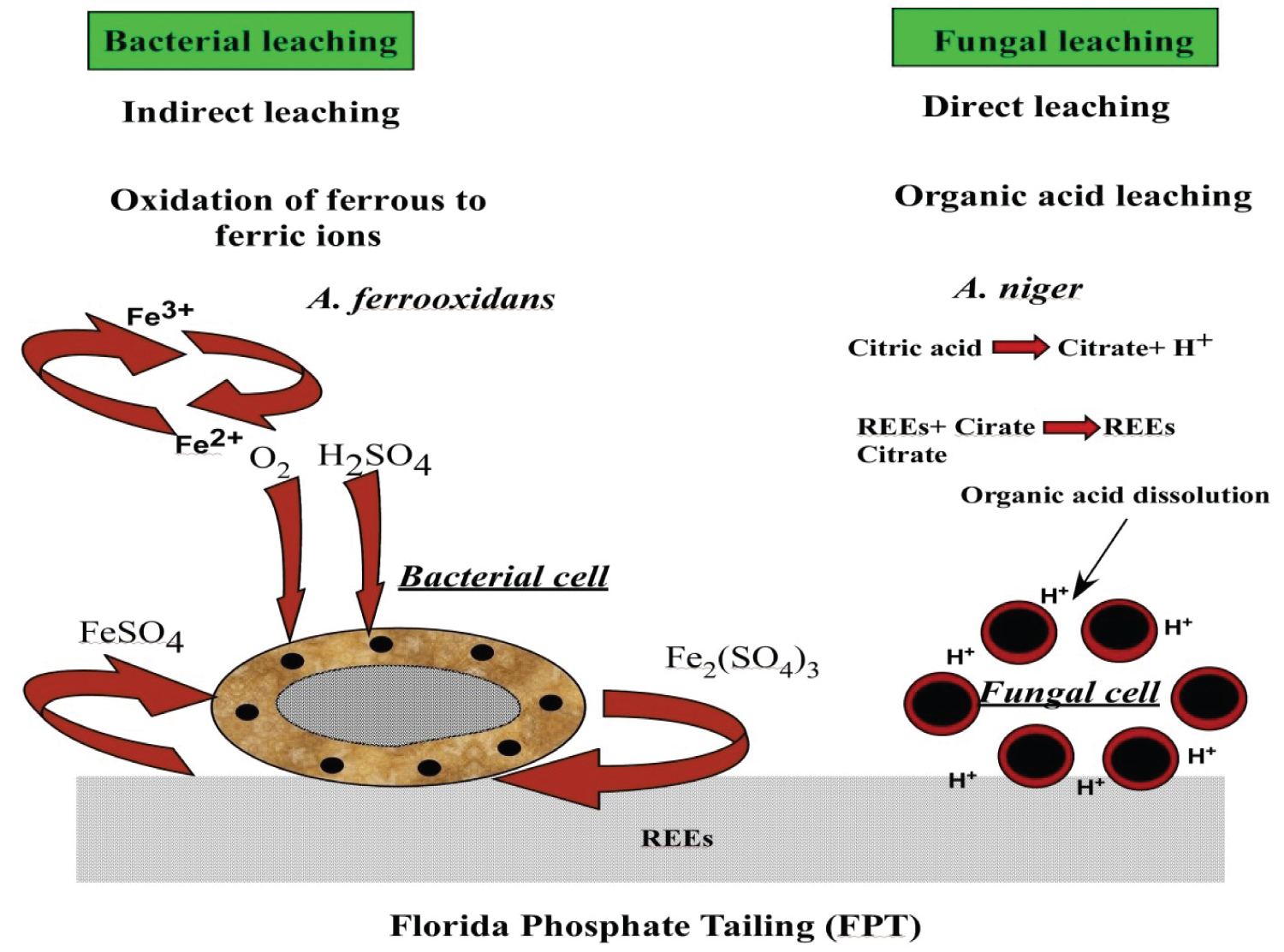
In this context, [33] investigated the role of Aspergillus ficuum and Pseudomonas aeruginosa exhibit good potential in generating varieties of organic acids effective for bioleaching some rare earth elements (REEs) from Egyptian monazite (purity 97%) and (thorium-uranium) concentrate. [34] mentioned that the bacterial Acidothiobacillus ferrooxidans (Af) and the fungal Aspergillus niger (An) for bio-leaching of REEs from a phosphate ore. Figure 1 appeared the scheme visualizing the mechanism of indirect, direct bio-leaching of A. ferrooxidans and A. niger for REEs from Florida phosphate tailings.
Production of chelating agents
There are some species of fungi and bacteria able to produce element-specific ligands (siderophores) that are able to change pH and enhance the chelation, which resulted in increased the mobilization of many elements as uranium (U), Thorium (Th) and traces elements. Other elements, such as thallium (Ti), lanthanides and actinides may be mobilized as a result of such microorganism's action. The most common siderophores-producing bacteria were P. aeruginosa , P. fluorescence and P. stutzeri . The most common producing fungi were Aspergillus flavus , A. niger and Rhizopus sp. in case of fungi. The release of uranium from its ore incubated with Pseudomonas sp. is attributed to the production of pyoverdine chelators (siderophores) as mentioned by [35]. Pseudomonas fluorescens is one of the fluorescent pseudomonads that secrete pyoverdines for its essential requirement for iron [36]. Pyoverdine is a water soluble, yellow-greenish fluorescent siderophore involved in high affinity transport of iron into the cell due to presence of a chromophore [37].
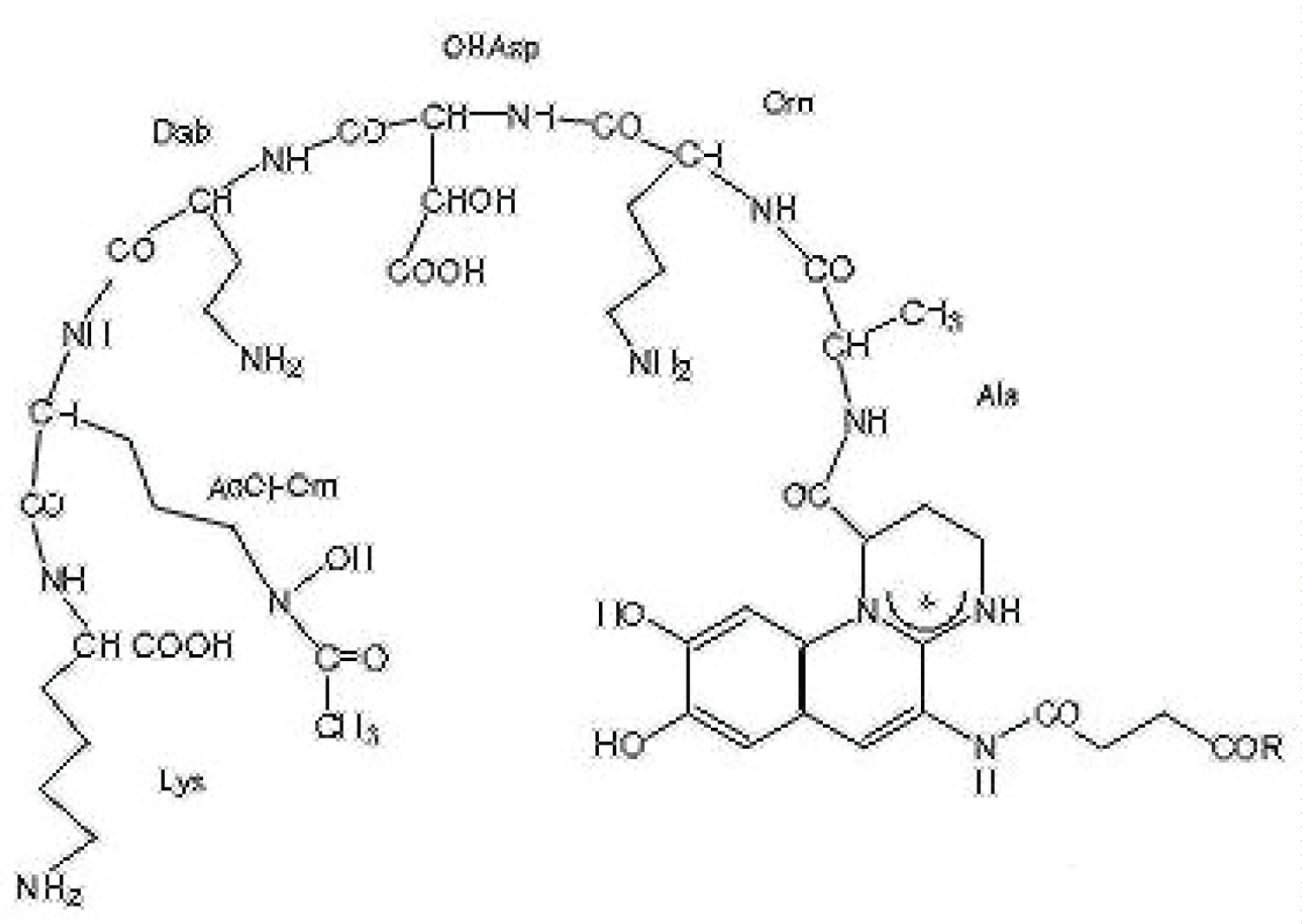
Siderophores are relatively low-molecular-mass (< 14 kDa). Siderophores are divided into classes based on their functional groups or on such special characteristics as pigmentation. Those having hydroxamic acid as their functional group constitute the hydroxamate class, while those using catechol to chelate Fe 3+ are called catecholates or catechol amides. Pyoverdine class siderophores have different chelation moieties, including hydroxamic acid, catechol and carboxyl group in the same molecule. Several authors have suggested that some bacteria produce different siderophores with varying affinity for Fe 3+ and other transition metals in order to supply the dells with essential trace elements [37,38]. Pyoverdine consist of three distinct structural parts, viz. a dihydroxyquinoline chromophore responsible for their fluorescence, a peptide chain comprising 6 to 12 amino acids bound to the chromophore carboxyl group and a small acid (or its monoamide) connected amidically to its NH 2 group. The peptide chain has a twofold function. It provides two of the ligand sites for Fe 3+ and it is for the recognition of the ferri-pyoverdine by specific receptors located at the surface of the producing cell [36]. [39] investigated that Pseudomonas aeruginosa SHA 282 was identified by 16S rDNA sequencing. It was found to produce catechol and hydroxamate derivates of pyoverdine. The gel electrophoresis of pyoverdine appeared that it contained two protein bands at ~12.5 and 9.5 kDa. The gene responsible for pyoverdine production was identified. This compound was able to complex and chel at with 87.43% and 90.16% of thorium (IV) from monazite and (Th-U) concentrate, respectively. The proposed structure of pyoverdine produced by Pseudomonas aeruginosa SHA 282 in Figure 2.
Under the above previous studied conditions, the complexation percentages of thorium from bioleached liquors of monazite and (Th-U) concentrate were found to be 87.43% and 90.16%, respectively. Avery simple model of complexation was previously mentioned by [40].
Where PYH 4 , was pyoverdine and n (possible ranging from 0 to 4) is the number of labile hydrogens liberated in the complexation process.
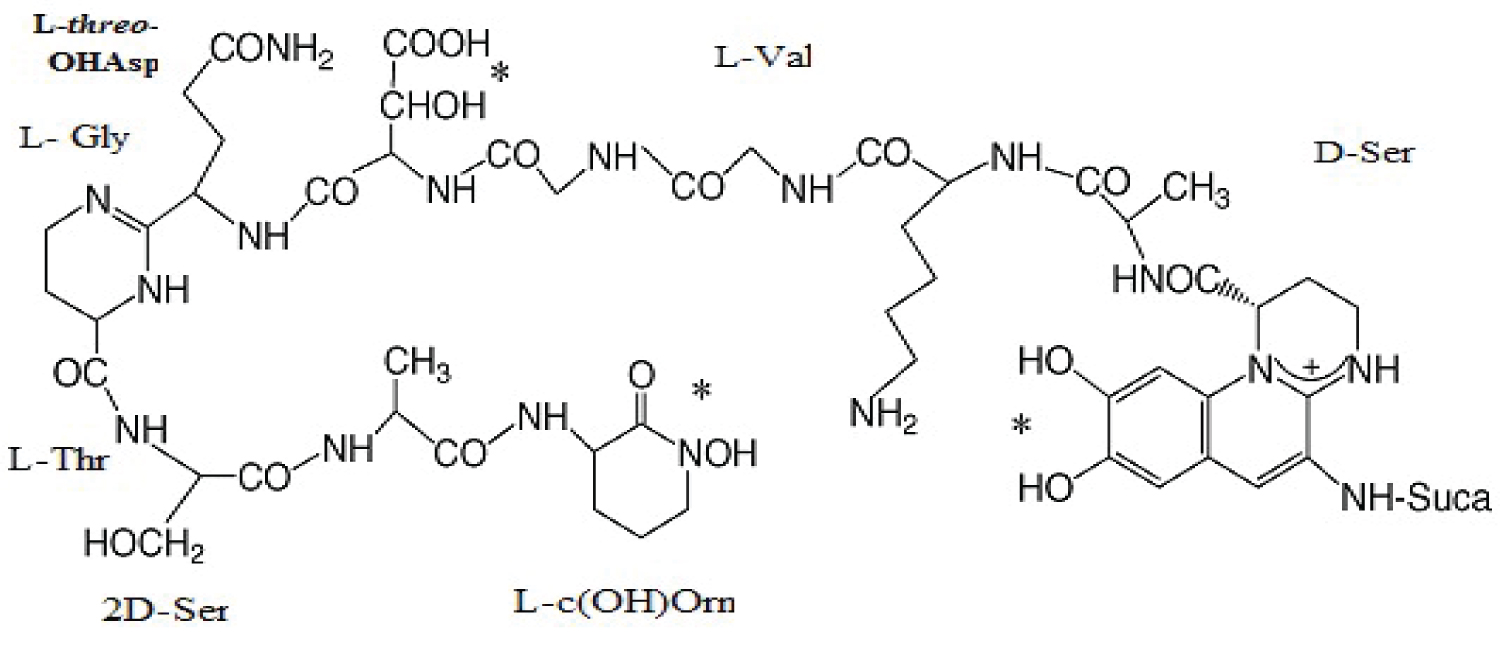
On the other hand, proposed Structure for pyoverdine produced by P. fluorescens SHA 281 was shown in Figure 3 as previously mentioned by [39].
Production of extracellular polymeric substances (EPSs)
Microorganisms can synthesis different type of polymeric substances (PSs) according to their location in the cell, structure, sugar composition, type of bonds between monomers, and systematic affinity. The extracellular polymeric substances (EPSs) are a complex mixture of macromolecular polyelectrolytes including polysaccharides, proteins, nucleic acids [41], amino acids, lipids or humic substances [42]. It is known that the formation of exopolymeric polysaccharides plays an important role in the attachment of microorganism on metal surfaces such as covellite, pyrite, or sphalerite. Extraction or loss of formation of these materials prevents the attachment of fungal and bacteria strains to the metal surface and also decreases the leaching efficiencies [43]. Ascomycota and Basidiomycota EPSs are mainly heteropolysaccharides but in the case of homopolysaccharides glucose is their only monomer. Exopolysaccharides are most frequently studied fungal PSs but their definition, classification, and origin are still not clear and should be explained. EPSs are rather defined by the separation/extraction method used than by theoretical consideration of the composition of the cell wall and macromolecules outside the cell wall. Bound or soluble EPSs contain not only high-molecular-weight polymeric compound exudates from microorganisms, but also products of cellular lysis and hydrolysis of macromolecules [44]. [45] mentioned the biosynthesis, extraction of extracellular polymeric substances (EPSs) from Aspergillus clavatus . The structure of exopolysaccharides was confirmed by FT-IR, 1 H, 13 C NMR, HPLC and mass spectroscopy. Based on this spectroscopy result, the exopolysaccharides produced by A. clavatus was α-D-glucopyranosyl units. The HPLC chromatography showed that the EPSs consist of one peak; glucose. Interaction between microorganisms and mineral surfaces occurs on two levels, physical sorption and chemical sorption [46]. In physical sorption, due to the low pH of the leaching environment, microbial cell envelopes are positively charged, leading to electrostatic interaction with the mineral surface. In chemical sorption, there a chemical bond develops between cell and minerals (disulphide bridges). In addition to this, extracellular metabolites are also formed during this phase near the attachment [47]. Extracellular polysaccharides (EPSs) are characteristically highly abundant in nature, renewable, nontoxic, intrinsically biodegradable and relatively cheap. Besides the ability of binding with heavy metals, EPSs also possess advantage of aggregation of pollutant particles, stabilization of the floc structure, formation of a protective barrier, and retention of water [48].
Microorganisms Attachment on the Surface of Ore
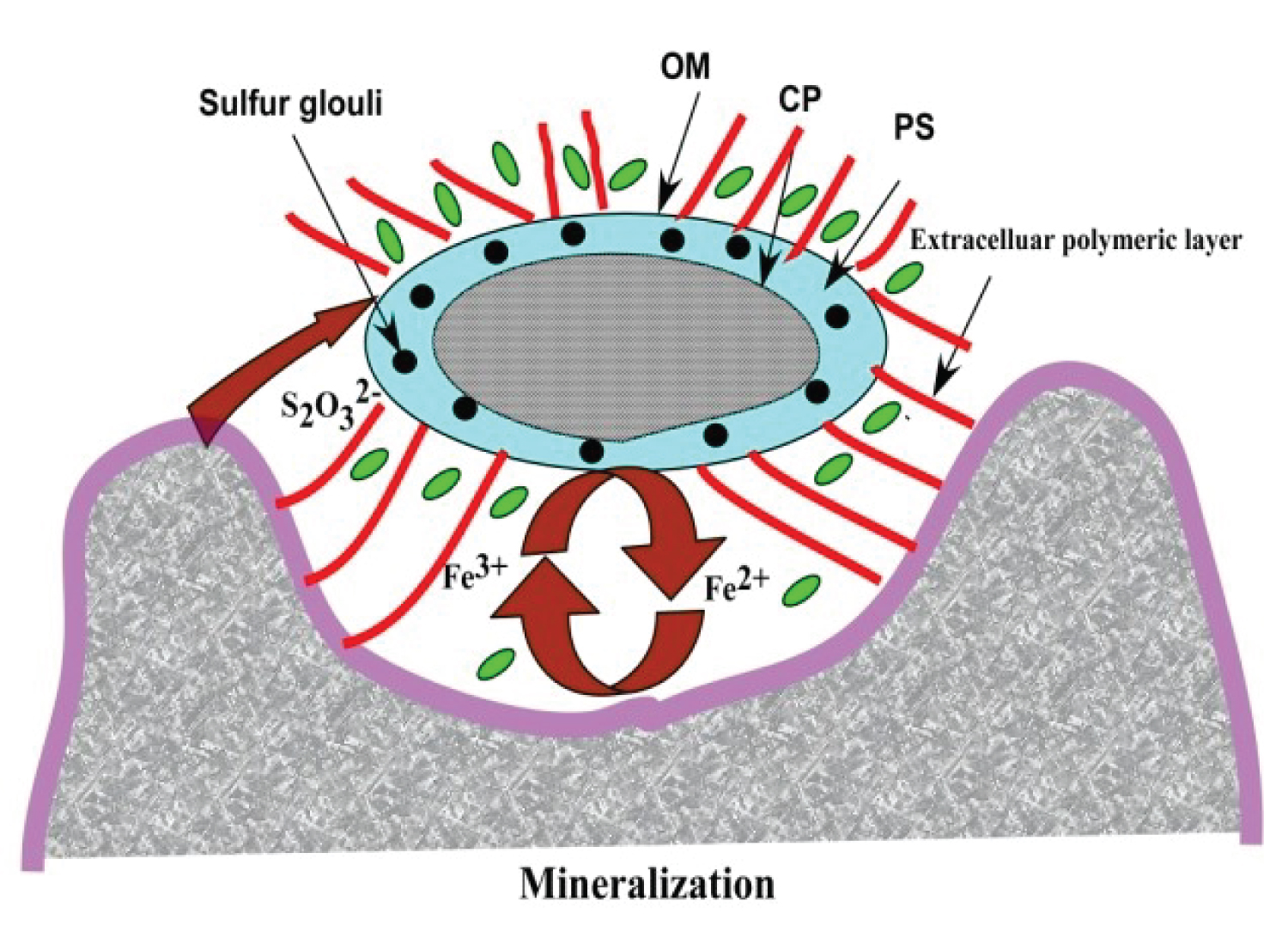
Bioleached microorganisms have the ability to degrade mineral substrates through biomechanical and biochemical processes [49]. At the fungal-mineral interface, three categories of phenomenon levels are of importance in understanding biotic weathering: (i) Mineral colonization; (ii) Microorganism’s exudation (iii) Cell wall-mineral surface interactions. A schematic representation of these interactions is given in Figure 4 [50]. Fungal hyphae grow in intimate contact with mineral grains [51] and they may exert mechanical forces by osmotically applied turgor pressure [52] resulting in biomechanical weathering of the minerals. [51] Mineral penetration is supported by mucilaginous slime produced by fungi which may contain acidic and metal-chelating metabolites [53]. Fungal or bacterial mineral colonization is highly heterogeneous and some minerals are preferentially colonized over others [50,54] furthermore individual hyphae show a tendency to grow on areas with a high concentration of active sites, such as crystal planes, cleavage, cracks and etch pits [55-57]. Exudation and uptake processes can dramatically alter the solution chemistry in the direct vicinity of the hyphae. Exudates can include a wide range of molecules, including protons, organic chelators, siderophores, and organic polymers (mucilage) [58] which can all enhance dissolution reactions either by direct adsorption and destabilisation of the mineral surface or by inducing changes in the chemical parameters driving dissolution reactions (i.e., pH and redox conditions).
Metal is released from sulfide minerals by Fe 3+ oxidation of the metal sulfide bond and is catalyzed by acidophilic iron oxidizing microorganisms that convert the resulting Fe 2+ back to Fe 3+ . Mineral oxidation occurs both at the point at which the microorganisms are attached to the mineral (biofilm growth) or planktonic growth [59]. Mineral oxidation has been exploited in the bio-hydrometallurgical technique of bioleaching [60]. Biofilms are structured groups of one or more microbial species encased in an extracellular polysaccharide (EPS) matrix and attached to a solid surface. Biofilms protect microorganisms from antimicrobial agents such as metals, thereby conferring an advantage during metal leaching as well as concentrating Fe 3+ that oxidizes the metal sulfide bond. Surface attachment, the pre-cursor to biofilm formation, is very important for mineral oxidation as previously shown in Figure 5. Acidophilic species that have been shown to bind and form biofilms on the surface of sulfide minerals include Acidithiobacillus ferrooxidans, At. caldus and Ferroplasma acidarmanus [61,62]. Surface attachment is not random, and occurs at dislocation sites such as cracks and the boundaries of mineral grains [63]. Numerous structural and genetically encoded regulatory determinants of biofilm development have been revealed. In some bacteria biofilm development is mediated by the quorum sensing molecules, N-acyl homoserine lactones (HSLs) that allow cell-cell communication [64] and At. ferrooxidans HSL production has been characterized. Physical attachment to the mineral surface is aided by EPS and in At. ferrooxidans the exoploymer is complexed with Fe 3+ and attaches to the mineral by electrostatic interactions [63]. At. ferro-oxidans EPS consists mainly of neutral sugars and lipids produced from UDP-glucose, UDP galactose, and dTDP-rhamnose precursors [65].
Importance and Cost of Bioleaching
Nowadays, the economics of any new process technology must be assessed relative to those of existing technologies. Hence, bioleaching is now applied on a commercial scale for leaching of copper and refractory gold ores and concentrates. The utility of bioleaching for uranium has been demonstrated in large scale [66]. However, the capital costs of bioleaching as non-conventional methods can be divided into association of construction and provision services, operating costs, and supplement of reagents and services. Generally, the capital costs of bioleaching are less than those of conventional chemical methods and smelting and roasting.
Bioleaching is a relatively simple technology that does not require significant instrumentation or sampling to provide high metal recovery. The operating costs for a bacterial oxidation process include the major categories of power, reagents, services, and labor. Except for the extraction of metal, no other products can be obtained in this process. However, the generation of low grade acid contaminated with dissolved metals and salts can be observed. The lower levels of these services required by the bioleaching process reduce the operating costs. Minimal process instrumentation is needed; only pH, dissolved oxygen, and temperature measurement are required. Furthermore, the adjustments of the conditions can be made manually by the operators. Since the process operates at temperature and pressure close to ambient, the maintenance costs of the conventional design are low compared to those of alternative technologies such as pressure oxidation and roasting. Lastly, since microorganisms are applied in the metal extraction process, the costs can be reduced in terms of handling environmental problems associated with hydrometallurgical processes as previously mentioned by [67].
Future Evaluations
Worldwide reserves of high-grade ores are decreasing at alarming rate due to a rapid increase in the demand for metals. However, there exist large stockpiles of the low grade ores. Bioleaching a ‘green technology’ is an emerging technology refers to the conversion of metals into their water soluble forms by microorganisms [29]. uHu The bioleaching indicate the challenges but also the promising process of biological leaching as alternative process for recovering metals from ores [32]. Bioleaching of “dirty” concentrates, which have high smelter penalty costs, represents some of the most attractive new applications for stirred reactor bioleaching. In addition to stirred reactors, bioleaching of copper from chalcopyrite ore in heaps using thermophiles will likely become a reality within the next few years. Electrochemical interactions in the bioleaching of complex sulfides could be taken an advance seat in the metal extraction process. Mobilization of metals from electronic waste materials through the bioleaching process robustly helps waste management in the electronic and galvanic industry [67]. Bioleaching process is the recovery of metals from different wastes such as industrial sludge, galvanic wastes, and electronics wastes. In fact, the future evaluations will be focused on the rule of bioleaching in the extraction and recovery of many metals.
References
- Pradhan N, Nathasarma KC, Srinivasa Rao K, et al. (2008) Heap bioleaching of chalopyrite: A review. Min Engineer 21: 355-365.
- Rawlings DE, Johnson DB (2007) The microbiology of biomining: Development and optimization of mineral-oxidizing microbial consortia, Microbiol 153: 315-324.
- Castro MI, Fietto JLR, Viera RX, et al. (2000) Bioleaching of zinc and nickel from silicates using Aspergillus niger cultures. Hydrometal 57: 39-49.
- Johnson DB (2006) Biohydrometallurgy and the environment: Intimate and important interplay. Hydrometall 83: 153-166.
- Aung KMM, Ting YP (2005) Bioleaching of spent fluid catalytic cracking catalyst using Aspergillus niger. J biotechnol 116: 159-170.
- Kelly DP, Wood AP (2000) Reclassification of some species of Thiobacillus to the newly designated genera Acidithiobacillus Halothiobacillus and Thermithiobacillus. Int J Syst Evol Bacteriol 50: 511-516.
- Ali SS, Vidhale NN (2011) Evaluation of siderophores produced by different clinical isolate Pseudomonas aeruginosa. Inter J Microbiol Res 3: 131-135.
- Omoike AO, Chorover J (2004) Spectroscopic study of extracellularpolymeric substances from Bacillus subtilis: Aqueous chemistryand adsorption effects. Biomacromolecule 5: 1219-1230.
- Van Hullebusch ED, Zandvoort MH, Lens PLN (2003) Metal immobilization by biofilms: Mechanisms and analytical tools. Rev Environ Sci Bio/Technol 2: 9-33.
- Comte S, Guibaud G, Baudu M (2006) Biosorption properties of extracellular polymeric substances (EPS) resulting from activated sludge according to their type: Soluble or bound. Proc Biochem 41: 815-823.
- Brierley J (1990) Biotechnology for the extractive metals industries. The Journal of The Minerals, Metals & Materials Society (TMS) 42: 28-30.
- Rawlings DE (2004) Microbially assisted dissolution of minerals and its use in the mining industry. Pur Appl Chemist 76: 847-859.
- Burgstaller W, Schinner F (1993) Leaching of metals with fungi. J of Biotechnol 27: 91-116.
- Desouky OA, El-Mougith AA, Hassanien WA, et al. (2016) Extraction of some strategic elements from thorium-uranium concentrates using bioproducts of Aspergillus ficuum and Pseudomonas aeruginosa. Ara J of Chemist 9: S795-S805.
- Ilyas S, Sarwar S, Bhatti HN (2012) Effect of organic acids produced by Penicillium notatum on the extraction of metals ions from Brown Shale. J Chem Soc Pakist 34: 1040-1047.
- Ivarson KC, Ross GJ, Miles NM (1980) The microbiological formaton of basic ferric sulfates. 3 Influence of clay minerals on crystallization. Can J Soil Sci 60: 137-140.
- Bosecker K (1993) Bioleaching of silicate manganese ores. Geomicrobiol J 11: 195-203.
- Holmen BA, Casey WH (1996) Hydroxamate ligands, surface chemistry, and the mechanism of ligand-promoted dissolution of goethite [α-FeOOH (s)]. Geochimica et Cosmochimica Acta 60: 4403-4416.
- Malinovaskaya IM, Kosenko LV, Votselko SK, et al. (1990) Role of Bacillus Muilaginosus polysaccharide in degredation of silicate minerals. Microbiol 59: 702-778.
- Tyagi RD, Meunier N, Blais IF (1996) Simultaneous sewage sludge digestion and metal leaching, effect of temperature. Appl Microbiol Biotechnol 46: 422-431.
- Rulkens WH, Grotenhuis JTC, Tichy R (1995) Methods of cleaning contaminated soils and sediments. In: Salomons W, Fo¨ rstner U, Mader P, Heavy Metals. Springer, Berlin.
- Hussien S, Patra P, Somasundaran P, et al. (2018) Assessment of ‘Bacterial (Acidic)-Leaching’ of Rare Earth Elements from a Phosphate Ore. Advances Environ Stud 2: 91-97.
- Suzuki I (2001) Microbial leaching of metals from sulfide minerals. Biotechnol Adv 19: 119-132.
- Schippers A (2007) Microorganisms involved in bioleaching and nucleic acid-based molecular methods for their identification and quantification. In: ER Donati, W Sand, Microbial Processing of Metal Sulfides. Springer, Dordrecht, The Netherlands.
- Vasan SS, Jayant modak M, Natarajan KA (2001) Some recent advances in the bioprocess of Bauxite. Inter J Miner Pro 62: 173-186.
- Hussien SS, Mosbah AS, El-mougith AA, et al. (2021) Bio-dissolution process as environmental technology for uranium leaching from El-Sella ore material by aspergillus sulphurous. Geomicrobiology Journal 38: 540-548.
- Nalini J, Sharma DK (2004) Biohydrometallurgy for nansulfidic minerals-A Review. Geomicrobiol J 21: 135-144.
- Ren XW, Li JP, Li JX, et al. (2009) Biological leaching of heavy metals from a contaminated soil by Aspergillus niger. J Hazard Mater 167: 164-169.
- Hussien SS, Desouky OA, Mohamadey SE (2015) Microbial Leaching of Uranium from Low Grade Ore and Waste Sample of Northern Part of Gabal Gatter, Egypt Using Penicillium Purpurogenium and Pseudomonas fluorescens SHA 281. Journal of Progressive Research in Biology 3: 127-141.
- Ruijter GJG, Kubicek CP, Visser J (2002) Production of organic acids by fungi. In: Osie wacz HD, The mycota: A comprehensive treatise on fungi as experimental systems for basic and applied research. Ind Appl Springer-Verlag, Berlin.
- Eskandarian M, Karimi A, Shabgard M (2013) Studies on enzymatic iomachining of copper by glucose oxidase. J Tai Instit of Chem Eng 44: 331-335.
- Karimi A, Aghbolaghy M, Khataee A, et al. (2012) Use of enzymatic bio-Fenton as a new approach in decolorization of malachite green. Sci Wor J 2012: 1-6.
- Hassanien WA, Desouky OA, Hussien SS (2014) Bioleaching of some Rare Earth Elements from Egyptian Monazite using Aspergillus ficuum and Pseudomonas aeruginosa. Walailak J Sci Tech 11: 809-823.
- Somasundaran P, Hussien S, Patra P, et al. (2018) Environmentally Benign Bio-Leaching Extraction of Rare Earth Elements from Non-Conventional Resources. Ann Microbiol Res 2: 54-60.
- Kalinowski EB, Oskarssona A, Albinssonb Y, et al. (2004) Microbial leaching of uranium and other trace elements from shale mine tailings at Ranstad. Geoderm 122: 177-194.
- Meyer JM (2000) Pyoverdines: Pigments, siderophores and potential taxonomic markers of fluorescent pseudomonas species. Arch Microbiol 174: 135-142.
- Budzikiewicz H (1993) Secondary metabolites from fluorescent Pseudomonas. FEMS Microbiol Rev 10: 209-228.
- Crichton RR (1991) Iron assimilation in plants and fungi. In: RR Crichton, Inorganic biochemistry of iron metabolism. Ellis Horwood, New York, 77-89.
- Hussien S, Desouky OA, Abdel-Haliem MF, et al. (2013) Enhancement the production of siderophores-pyoverdineby pseudomonas aeruginosa SHA 282 and its chelation with thorium (IV). Wor Res J Biotechol 1: 17-23.
- Bouby M, Billard I, MacCordick J (1998) Complexation of Th (IV) with the siderophores pyoverdine A. J Alloys and Comp 273: 206-210.
- Hussien SS (2020) Microbial leaching of El-Sella mineralization by Aspergillus clavatus a fact of fungal uranium interface. International Journal of Environmental Studies 77: 275-296.
- Hussien SS (2021) New studies of uranium biosorption by extracellular polymeric substances (EPSs) of Aspergillus clavatus, using isotherms, thermodynamics and kinetics. International Journal of Environmental Studies 78: 228-246.
- Edwards KJ, Bond PL, Banfield JF (2000) Characteristics of attachment and growth of Thiobacillus caldus on sulphide minerals: A chemotactic response to sulphur minerals? Environ Microbiol 2: 324-332.
- Jaroszuk MO, Wilkolazka AJ, Scisel JJ, et al. (2015) Extracellular polysaccharides from Ascomycota and Basidiomycota: Production conditions, biochemical characteristics, and biological properties. World J Microbiol Biotechnol 31: 1823-1844.
- Hussien SS (2019) Biosynthesis, Extraction and Characterization of Extracellular Polymeric Substances (EPSs) from Aspergillus clavatus. Adv Environ Stud 3: 216-228.
- Norris PR, NP Burton, NAM Foulis (2000) Acidophiles in bioreactor mineral processing. Extremophiles 4: 71-76.
- Ewart DK, Hughes MH (1991) The Extraction of Metals from Ores Using Bacteria. Adv Inorg Chem 36: 103-135.
- Pezzoni M, Lemos M, Pizarro RA, et al. (2022) Environmental signal for alginate production in pseudomonas aeruginosa: Role of this polysaccharide in the protection of planktonic cells and biofilms against lethal UVA doses. Photochem Photobiol Sci 21: 1459-1472.
- Bonneville S, Smits MM, Brown A, et al. (2009) Plant-driven fungal weathering: early stages of mineral alteration at the nanometer scale. Geology 37: 615-618.
- Smits MM, Herrmannb AM, Duanec M, et al. (2009) The fungal-mineral interface: Challenges and considerations of micro-analytical developments. Fungal biology reviews 23: 122-131.
- Van Breemen N, Finlay RD, Lundstrom US, et al. (2000) Mycorrhizal weathering: A true case of mineral plant nutrition? Biogeochemistry 49: 53-67.
- Money MP (2004) The fungal dining habit-a biomechanical perspective. Mycologist 18: 71-76.
- Burford EP, Fomina M, Gadd GM (2003) Fungal involvement in bioweathering and biotransformation of rocks and minerals. Mineral Mag 67: 1127-1155.
- Rosling A, Lindahl BD, Finlay RD (2004) Carbon allocation to ectomycorrhizal roots and mycelium colonising different mineral substrates. New Phytol 162: 795-802.
- Banfield JF, Barker WW, Welch SA, et al. (1999) Biological impact on mineral dissolution: Application of the lichen model to understanding mineral weathering in the rhizosphere. Proc Natl Acad Sci USA 96: 3404-3411.
- Mark MS, Anke MH, Michael D, et al. (2009) The fungal-mineral interface: Challenges and considerations of micro-analytical developments. Fungal Biology Reviews 23: 122-131.
- Smits MM (2006) Mineral tunnelling by fungi. In: Gadd GM, Fungi in Biogeochemical Cycles. Cambridge University Press, Cambridge, 311-327.
- Hoffland E, Kuyper TW, Wallander H, et al. (2004) The role of fungi in weathering. Front Ecol Environ 2: 258-264.
- Rawlings (2002) Heavy metal mining using microbes. Annu Rev Microbiol 56: 65-91.
- Rohwerder T, Gehrke T, Kinzler K, et al. (2003) Bioleaching review part A: progress in bioleaching: fundamentals and mechanisms of bacterial metal sulfideoxidation. Appl Microbiol Biotechnol 63: 239-248.
- Edwards KJ, Bond PL, Gihring TM, et al. (2000) An archaeal iron-oxidizing extreme acidophile important in acid mine drainage. Science 279: 1796-1799.
- Edwards KJ, Hu B, Hamers RJ, et al. (2001) A new look at microbial leaching patterns on sulfide minerals. FEMS Microbiol Ecol 34: 197-206.
- Gehrke T, Telegdi T, Thierry T, et al. (1998) Importance of extracellular polymeric substances from thiobacillus ferrooxidans for bioleaching. Appl Environ Microbiol 64: 2743-2747.
- Barreto M, Jedlicki E, Holmes DS (2005) Identification of a gene cluster for the formation of extracellular polysaccharide precursors in the chemolithoautotroph acidithiobacillus ferrooxidans. Appl Environ Microbiol 71: 2902-2909.
- Stanley NR, Lazazzera BA (2004) Environmental signals and regulatory pathways that influence biofilm formation. Mol Microbiol 52: 917-924.
- McCready RG, Gould WD (1990) Microbial Mineral Recovery. McGraw Hill, New York.
- Mishra D, Kim D, Ahn JG, et al. (2005) Bioleaching: A microbial process of metal recovery; A review. Metal Miner Inter 11: 249-256.
Corresponding Author
Shimaa S Hussien, Professor of Biotechnology, Ores Processing Department, Nuclear Materials Authority, P.O. Box 530, El-maadi, Cairo, Egypt.
Copyright
© 2024 Hussien SS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.