Assessment of 'Bacterial (Acidic)-Leaching' of Rare Earth Elements from a Phosphate Ore
Abstract
Bioleaching methods are environmentally benign and economical options for extraction of rare earth elements (REEs) from low grade REE ores. We conducted shake-flask and column bio-leaching (Acidithiobacillus ferrooxidans) studies with a low grade phosphate ore constituting REEs, fluorapatite, and silicates. In shake-flask studies, the leaching rate relied mostly on the bacterial activity - acid producing ability of the bacteria; bacterial activity reduced according to F- ions content in the leached liquor. These ions leached rapidly from the corner/edge sites of the particles. This issue with F- ions was overcome in the column leaching studies, where the bacterial activity was improved by removing F- ions from the leached liquor at regular intervals. A key advantage was localization of the leached Ca2+ ions in the electrical double layers of the bacteria/mineral-water interfaces and, with differing culture constituents, gypsum/phosphate precipitation in liquor was prevented; a processing challenge in the chemical leaching methods.
Keywords
Bacterial growth, Leaching rate, Fluorine, Particle-size, Pulp-density
Introduction
Rare earth elements (REEs) are essential for many applications including electronics, energy generation and defense. The common resources of REEs are the oxide minerals such as bastnaesite and monazite. REEs are extracted from these ores using chemical leaching methods. However, these oxide minerals are scarcely available on earth crust. Therefore, much effort has gone into developing techniques to extract REEs from alternate resources such as low grade phosphate ore deposits, where REEs substitute Ca or Si of the lattices of the minerals [1,2]. Chemical leaching of these ores are economically unviable because they constitute trace amounts of REEs [3]. In cases, chemical leaching methods are adopted for ores constituting reasonable amounts of REEs. In general, a common challenge faced in chemical leaching processes is the formation of phosphate/gypsum precipitates in the leached liquor; these precipitates are removed from the leached liquor in a step-wise manner. These challenges however are significant in leaching of phosphate ores as they constitute large amounts of P and Ca [1,2]. On the other hand, bio-leaching approaches are economically viable alternatives, and are advantageous in eluding several challenges faced in the chemical leaching methods. The advantages of bioleaching approaches in have been reported. There are many application on the separation of REEs from black sands especially Monazite [4]. Rare earth elements also could be extracted from red mud (Al2O3) using Penicillium tricolor [5] and from phosphate rocks using Acidithiobacillus ferrooxidans (Af) [6].
We conducted a bio-leaching study using Af bacteria, and with a phosphate ore (fluorapatite/clays as main minerals). We studied the leaching kinetics, simplistically described as: Rt = [(k_1 A_p)/(k_2 C_t)], where, Rt is the leaching rate, Ap is the total particle surface area, and, Ct, the bacterial count at time t; k1 is the leaching rate constant for the surface area term (Ap), and k2 is the coefficient for the bacterial activity - H2SO4 producing ability of bacteria. We examined how the leaching rate differs with respect the bacterial growth behavior and bacterial activity [7,8]. Furthermore, we examined how microbe-mineral interactions, media/surface chemistry, and the electro-chemical aspects of the mineral-water interfacial regions influence leaching kinetics [8].
Materials and Methods
The froth flotation concentrate and tailing samples of a phosphate ore were obtained from Mosaic phosphate processing plant, Florida (Supplementary Material). The concentrate (~80% fluorapatite, < 200 µm) and tailing (clays, < 150 µm) samples contained REEs at ~826 ppm and ~304 ppm, respectively (Supplementary Table 1a and Supplementary Table 1b). The samples were ground to different sizes. The experimental details of the bioleaching studies and zeta-potential measurements are given in the supplementary section (Supplementary Figure 1, Supplementary Figure 2, Supplementary Figure 3, Supplementary Figure 4, Supplementary Figure 5, Supplementary Figure 6, Supplementary Figure 7 and Supplementary Figure 8).
Bacterial culture
At. ferrooxidans strain (AC9989) were obtained from the ATCC (American type culture collection), and were cultured in 9K medium [9] initial pH value of 1.7 was adjusted with 20% H2SO4. The bacterial culture was incubated in a Remi rotary shaker at 150 rpm/min, and at room temperature (25 ℃). The bacterial growth profile was monitored by measuring the cultures' optical density using a UV-visible spectrophotometer, from Perkin Elmer. The bacteria were also adapted to REEs at 100 ppm level, and growth was monitored for three days. Analytical grade of the chemicals (media constituents) were obtained from Sigma Aldrich, USA.
Bioleaching, chemical and column leaching studies
The Bioleaching experiments were conducted with A.f culture in 250 mL Erlenmeyer flasks. The flasks contained ore samples in desired amounts, to which 90 ml of 9K medium and 10 mL of A.f innocula was added; pH level adjusted to 1.7 with H2SO4 (25%). The pulp density (PD) levels were varied from 1-15 wt. %. The bio-leaching experiments were carried out for a period of 5 days, and in a rotary shaker (150-200 rpm/min). For each of the PD levels (1, 2, 5, 10 and 15 wt. %), the bioleaching tests were carried with differing Particle size distribution (PSD) levels (32, 53, 75 and 105 μm); PSD = 100% passing a particular size. The tests were carried out at room temperature (25 ℃).
Chemical leaching studies were carried out with the concentrate or tail samples in a 250 mL Erlenmeyer flask, where 100 ml of H2SO4 (25%) was used. The pulp density levels in these tests were 1-15 wt. %. The leaching studies were carried out in in a rotary shaker (150-200 rpm/min) and at room temperature (25 ℃), pH levels - below pH 2.
Column leaching studies were carried out in a glass column of 12 inch height and 2 inch inner diameter. The top and bottom end of this column was tapered. A column was packed to ¾ of its height with the concentrate samples of sizes 105 µm and, in another test, a mixture of 105 µm and 35 µm (1:1 wt. ratio). The bottom part of the column was plugged with glass wool. Fully grown bacterial culture was introduced from the top opening of the column, and onto the bed of the mineral sample. The culture percolated thorough the bed, where the leached liquor was collected at the bottom part of the column. The leaching process was continuous in terms of the amounts of the culture added and the leached liquor collected, maintaining a flow rate of 1 mL/min. The leached liquor was analyzed for REE content.
The leaching rate profile was first developed by plotting cumulative amounts of REEs leached as a function of time. A first derivative profile of this profile was used for extraction information on the leaching rates. The first derivative profile was developed using Origin Pro.8 software.
Determination of REE content in leached liquor
For the determination of the REE content in the bioleaching and chemical leaching samples, the samples were filter through a Whatman No. 1 filter paper. Filtration of the samples allowed the separation of the mineral particulates and bacteria from the leaching media. The filtrate was further centrifuged at 7000 rpm for more than 10 minutes to remove micro-particulates. The filtrated was treated with 1-2 mL of ArsenazoI (obtained from Sigma Aldrich), where the addition of the amount of the chelating dye was standardized; 2-3 mL/L. The REEs content in the filtrate was then determined using UV visible spectrometer, where a characteristic peak at ~520 nm in the spectra allowed determination of REE content in the liquor. A standard curve was developed using a range of solutions containing different REE levels, prepared with Standard solution obtained from Sigma Aldrich. 50 mg/L each: Sc, Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb and Lu in 2% nitric acid. The leached liquor samples obtained from the chemical leaching studies and column leaching studies were analyzed for REEs content in the manner described for the bio-leaching studies [10]. The fluorine content in the chemical leaching liquor was determined using a standard fluorine ions selective electrode.
Results and Discussion
Assessment of bio-leaching kinetics
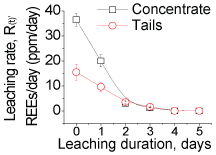
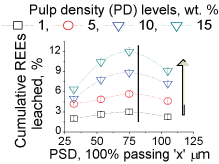
We determined the REEs leaching rates (Supplementary Figure 7) in our shake-flask bio-leaching studies, carried out with the froth flotation concentrates and tails of a phosphate ore (Supplementary Table 1a And Supplementary Table 1b). Irrespective of the PSD (35 - 100 µm) and PD (1-15 wt. %) levels in the leaching media, and other processing parameters such as bacterial count, shaking speed and aeration rate, the leaching rate at room temperature (25 ℃) declined (Supplementary Figure 1) progressively over the leaching period (5 days), and for both the concentrate and tail samples. The amounts of REEs leached were proportional to the PD levels (Supplementary Figure 2), confirming that the REEs leached in higher amounts as the total exposed surface area of the mineral particles were increased in leaching. This proportionality owes to lower bond-energies for association of the REEs at the surfaces of the minerals than in the bulk. Similarly, it was expected that the REEs would leach in higher amounts as the surface area increases with smaller particles in leaching. Though, reduction in particle sizes from 100 to 75 µm led to leaching of REEs in higher amounts, further reduction (< 75 µm) in sizes resulted a corresponding decrease in the REEs leached (Supplementary Figure 2); demonstrating an anomalistic trend of the leaching kinetics. This mean by increasing the surface area of the grinding ore sample abnormally, the bioleaching was deceased. Probably, due to increase the concentration of metals which decrease the bacterial activity.
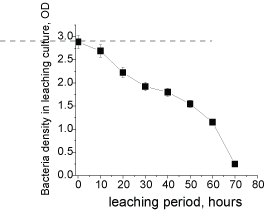
The factors responsible for the anomalistic trend were attributed to the bacterial growth behavior and the bacterial activity. Other factors such as bacterial attachment efficiencies onto the minerals (Supplementary Figure 7), pulp rheology, media/surface chemistry and the processing parameters were neglected, because nominal variations in the leaching rates were observed with respect to these factors. The bacterial growth behavior were also disregarded as a key factor as the growth curves were similar with respect to the differences in PSD and PD levels, and irrespective of the REE contents (Supplementary Figure 8) in the liquor. The toxicity attribute of REEs on growth behavior was also disregarded as a key factor as the bacterial growth behavior (the declining growth rate) was least affected (Supplementary Figure 8) with culture media (without minerals) constituted 100 ppm of REEs.
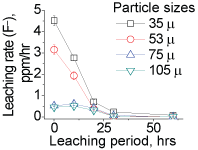
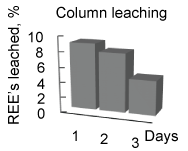
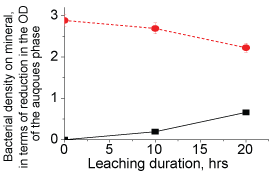
To this end, the factor governing the anomalistic trend was therefore ascribed to the bacterial activity. The decline in the leaching rates with smaller particles, demonstrating the anomalistic trend, indicated that the acid producing ability of bacteria differ with variations in the particle sizes. This decline in the bacterial activity was not due to the REEs content in the liquor as the bacteria were able produce acid in reasonable amounts to leach minerals even at high (> 100 ppm) REE content, and throughout the 5 days period, e.g. 15% solids (Supplementary Figure 2). Therefore, other ions such as Ca, Si, P and F (Supplementary Table 1a And Supplementary Table 1b), which constitute the concentrate sample, were considered to be responsible for the decline in bacterial activity. Of these ions, the F- ions strongly modulate bacterial activity, and even at lower concentrations (< 100 ppm), which is through deactivation a specific enzyme system responsible the acid production, oxygen deficiency, and modulation of metabolic pathways. These F- ions leach from the edge/corner sites at a faster rate than that from the planar sites [11]. The lattice energies of the F- ions at these sites are less in comparison to their strong binding energy (600 eV) at the planar/bulk sites. We examined F- ion's release rate by conducting chemical (H2SO4) leaching studies (Supplementary Figure 3), and with the concentrate samples [12,13]. These studies ascertained that the F- ions indeed leach at a faster rate from the edge/corner sites, 5-3 ppm/hr for particles < 75 µm and < 1 ppm/hr for particles > 75 µm. (Due to absorption of the F- ions in the cytosol of bacteria, F- ions content could not be determined reliably in bio-leaching studies). We then examined if removal of the F- ions from liquor would improve leaching rate and ascertain that the rate of F- ion leaching being responsible for the anomalistic trend. We conduced column leaching studies (Supplementary Figure 4), with smaller particles, where the leached liquor was removed and replaced with fully grown fresh culture at regular intervals (~4 mL/min), meaning that the F-ions were removed from the liquor at regular intervals. Resultantly, the REEs leaching rate (Supplementary Figure 2) was moderately affected, unlike that (Supplementary Figure 1) in the shake-flask studies. These results justified that the bacterial activity relied on the rate of F- ions release into the liquor. As the particle size becomes smaller, the F-ions content in the leached liquor become high enough at an early stage of leaching to reduce the bacterial activity, which in turn cause decline in the REEs leached.
Effects of bacterial attachment on to minerals and Media chemistry on leaching behavior
Bacterial attachment efficiencies onto the minerals typically govern the bio-leaching kinetics. Many studies relate the Langmuir isotherms demonstrating bacterial attachment onto minerals to bacteria's affinity towards a wide range of sulfide/silicate minerals [14]. Here, the negligible attachment efficiaes for bacteria (Supplementary Figure 8) indicated that the bacteria avail its energy from the media instead of that from the mineral surfaces via redox reactions. Therefore, the leaching of phosphate ore samples associated an indirect mechanism, where minerals were leached by the acid (H2SO4) produced by the bacteria; the leaching reactions are summarized as:
Concentrate: 10H2SO4 + Ca3(REE)5(PO4)6F2→3Ca2+ + 10SO42- + 5REE3+ +6H3PO4 + F2- + 4H2O
Tailing: 2H2SO4 + Ca(REE) Mg5Si8O22 (OH)2→Ca2+ + REE3+ + 2SO42- + Mg5Si8O22 + 2H2O
A major advantage of the bioleaching approaches over the chemical leaching methods, for phosphate ores, was realized to be related to the media chemistry. In chemical leaching processes, phosphate rocks are spontaneously digested in H2SO4, where the Ca2+ ions content in the leached liquor are significant within a shorter time period. Therefore, gypsum formation is more likely in these systems. On similar ground, in our chemical leaching studies, carried with 15 wt. % PD level, formation of gypsum crystal was evident (microscopy images of the samples) (Supplementary Figure 5). However, the bioleaching media contained Mg2+ (0.05 wt. %) and (NH4)2- (0.3 wt.%) ions. These ions suppress gypsum/phosphate precipitates/crystals formation in liquor though lowering of the solubility products. Substantiating this interpretation, gypsum/phosphate crystals (> 2 μm) were absent in the phase contrast microscopy images of the bio-leached liquor and, those taken on the particle surfaces [15]. Suppression of gypsum formation in the bioleaching studies therefore attributed to lowering of the solubility products of gypsum due to Mg2+ and (NH4)+ in the media.
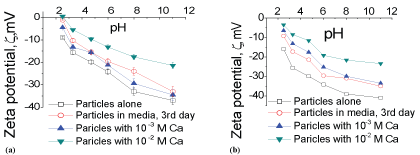
Unlike in chemical leaching, layer-by-layer dissolution of mineral lattices in bio-leaching occurs at slow rates, and due to that the clay (silicate) and phosphate minerals leach slowly across the pH range of 1.7-3. These slow leaching rates are advantageous because the Ca and P contents are low (in comparison to that in chemical leaching) in leached liquor to facilitate precipitate formation. Furthermore, due to slow leaching rates, selective removal of the leached ions from the media at regular intervals is option in bio-leaching, allowing optimization of the Ca2+ and P level in the liquor to circumvent gypsum/phosphate precipitation. Importantly, phosphate/gypsum crystallization could also be dealt with in bioleaching to a significant extent as the Ca2+/PO4 ions localize preferentially in the Electrical double layers (EDLs) of the mineral- and bacterial-water interfaces. We obtained evidence confirming localization of these ions in EDLs, where the zeta-potential () values were positive for concentrate/tailing samples and as function of leaching time (Supplementary Figure 6a, Supplementary Figure 6b). The value increases due to localization of Ca2+ ions in the Debye layer, which we verified by estimating higher values for concentrate and tailing samples that were suspended in water having a range of different levels of Ca2+ ions (regulated by Ca(NO3)2. A preferential distribution of the Ca2+ ions across the media volume also means that the solubility products of the Ca2+ and SO42- ions differ across the liquor phase. Thus, localization of leached ions in the EDLs are advantageous for the prevention of the precipitates formation in bio-leaching processes.
Conclusion
In bioleaching of phosphate ores, bacterial activity in presence of leached ions in the leached liquors a key factor. Generally, bioleaching increased as particles decreased down to 75 μm. The size of particles determines the surface area which also affects bioleaching. In particular, for ores constituting fluorine in their mineral lattices, the F-leaching rates largely contribute to bacterial activity and, therefore, the leaching kinetics. The effects of F- and REEs on bacterial activity could be markedly different, and it would be worth delineating their effects. A method to separate these leached ions from the liquor on a regular basis appears to a processing criterion. The bioleaching approaches also offer several advantages over chemical leaching methods, where the roles of media ions (Mg2+ and (NH4)+ions) and phenomena occurring at mineral- and bacteria- water interfaces can be appropriately considered.
These results indicate the challenges but also the promising aspects of bioleaching as alternative process for recovering REEs from phosphate ore.
Ackowledements
The authors acknowledge support of Florida Institute of phosphate research (FIPR) and National Science Foundation under the (Grant No. 0749481/1362060) for this research work.
References
- Jordens A, Cheng YP, Waters KE (2013) A review of the beneficiation of rare earth element bearing minerals. Miner Eng 41: 97-114.
- Kumari A, Panda R, Jha MK, et al. (2015) Process development to recover rare earth metals from monazite mineral: A review. Miner Eng 79: 102-115.
- Emsbo P, McLaughlin PI, Breit GN, et al. (2015) Rare earth elements in sedimentary phosphate deposits: Solution to the global REE crisis. Gondwana Res 27: 776-785.
- Hassanien WA, Desouky OA, Hussien SS (2014) Bioleaching of some Rare Earth Elements from Egyptian Monazite using Aspergillus ficuum and Pseudomonas aeruginosa. Walailak J Sci Tech 11: 809-823.
- Qu Y , Lian B (2013) Bioleaching of rare earth and radioactive elements from red mud using Penicillium tricolor RM-10. Bioresource Technol 136: 16-23.
- Chi R, Xiao C, Gao H (2006) Bioleaching of phosphorus from rock phosphate containing pyrites by Acidithiobacillus ferrooxidans. Miner Eng 19: 979-981.
- Haddadin J, Dagot C, Fick M (1995) Models of Bacterial Leaching. Enzyme Microb Tech 17: 290-305.
- Rohwerder T, Gehrke T, Kinzler K, et al. (2003) Bioleaching review part A. progress in bioleaching: fundamentals and mechanisms of bacterial metal sulfide oxidation. Appl Microbiol Biotechnol 63: 239-248.
- Silverman MP, Lundgren DG (1959) Studies on the Chemoautotrophic Iron Bacterium Ferrobacillus-Ferrooxidans .1. An Improved Medium and a Harvesting Procedure for Securing High Cell Yields. J Bacteriol 77: 642-647.
- Busev AI, Tiptsova VG, Ivanov VM (1981) Analytical Chemistry of Rare Elements. (1st edn), Mir Publishers, Moscow.
- Marquis RE, Clock SA, Mota-Meira M (2003) Fluoride and organic weak acids as modulators of microbial physiology. Fems Microbiol Rev 26: 493-510.
- Nathanael AJ, Mangalaraj D, Hong SI, et al. (2013) Influence of fluorine substitution on the morphology and structure of hydroxyapatite nanocrystals prepared by hydrothermal method. Mater Chem Phys 137: 967-976.
- Sevim F, Sarac H, Kocakerim MM, Yartasi A (2003) Dissolution kinetics of phosphate ore in H2SO4 solutions. Ind Eng Chem Res 42: 2052-2057.
- Devasia P, KA Natarajan (2010) Adhesion of Acidithiobacillus ferrooxidans to mineral surfaces. Inter J Miner Proc 94: 135-139.
- Guan BH, Yang LC, Wu ZB (2010) Effect of Mg2+ Ions on the Nucleation Kinetics of Calcium Sulfate in Concentrated Calcium Chloride Solutions. Ind Eng Chem Res 49: 5569-5574.
Corresponding Author
S Hussien, Ore Processing Department, Nuclear Materials Authority, P.O. Box 530 El-Maadi, Cairo, Egypt.
Copyright
© 2018 Hussien S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.