Environmentally Benign Bio-Leaching Extraction of Rare Earth Elements from Non-Conventional Resources
Abstract
The bio-leaching approaches are environmentally benign and economically viable. We investigated the bacterial Acidothiobacillus ferrooxidans (Af) and the fungal Aspergillus niger (An) for bio-leaching of REEs from a phosphate ore, and examined the factors governing the leaching processes. Based on the shake flask leaching studies, an increase in surface area with the increase in pulp density level (1-15 wt.%) was found to lead to an increase in the amounts of REEs leached. In contrast, with an increase in the surface area by reducing the sizes of particles, from 75 µm to 32 µm, led to a decrease in the amounts of REEs leached. The maximum bioleached percentages of REEs from concentrate phosphate and tailings were (81% & 48%) by At. ferrooxidans and (65% & 38%) by A. niger, respectively using particle size of 75 µm and at a pulp density of 1 (g/50 mL).
Keywords
Florida phosphate tailing (FPT), Rare Earth Elements, Bio-leaching, Acidiothiobacillus ferrooxidans, Aspergillus niger
Introduction
Rare Earth Elements (REEs) are essential for many important applications, such as in electronics, energy generation and defense. The common resources for economic extraction of REEs are typically carbonates such as bastnaesite and phosphate as monazite, which are scarcely available on earth crust [1]. REEs are abundantly available in many clay and phosphate ores where REEs substitute Ca2+ or Si4+ of the mineral lattices. The conventional acid leaching of REEs from oxides are economically unfeasible for the clay and phosphate ores because of significantly low. REE contents in these ores [2-4]. In some cases, REEs could be extracted from these ores via chemical leaching routes as they constitute reasonable amounts of REEs (0.1-1% REEs). A major challenge faced in general in chemical leaching processes is the loss of REEs in the phosphate/gypsum precipitates/crystals, which are overcome in a step-by-step manner by sequentially removing impurities such as fluorine and calcium, including steps that carried out at elevated temperatures [5]. On the other hand, bio-leaching approaches are environmentally benign and economical alternatives. Acidophilic bacteria has been are used for bio-leaching of Au/Cu/Ni from low grade ores industrial scale. Studies on bioleaching of REEs from red mud (Al2O3) using Penicillium tricolor and Aspergillus niger (An), and, of bio-leaching (lab-scale) of phosphate rocks using Acidithiobacillus ferrooxidans (Af) have been reported [6,7]. We conducted bacterial (Af) bio-leaching of REEs from a phosphate ore in shake flasks and in columns, and we examined the key factors that govern leaching kinetics.
Methodology
Florida phosphate samples
FPT (Florida phosphate) samples including phosphate concentrate and tailings (with particle sizes about 197 µm and 147 µm), respectively were obtained from Florida University (USA). Phosphate concentrate (~80% fluorapatite) contained REEs about ~800 ppm and tailings (~80% clays) contained REEs about ~304 ppm, respectively. These samples were ground for different particle sizes by Fisher mortar grinder, model 155, U.S.A Table 1.
Microorganisms and growth conditions
At. ferrooxidans was supplied by the American Type Culture Collection (ATCC). At. ferrooxidans was grown in 9K medium [3.00 g/L (NH4)2SO4, 0.5 g/L MgSO4.7H2O, 0.5 g/L K2HPO4, 0.10 g/L KCL and 0.01 g/L Ca(NO3)2, 44.20 g/L FeSO4.7H2O] as previously by Silverman MP, Lundgren DG [8] at an initial pH of 2.5 obtained using 20% sulfuric acid. Also, A. niger was isolated from phosphate concentrate sample by the method of serial dilution using the Modified Czapek's- Dox agar (MCDA). MCDA medium comprised of 30 g/L sucrose, 10 g/L yeast extract, 3 g/L NaNO3, 1 g/L KH2PO4, 0.5 g/L MgSO4.7H2O, 0.5 g/L KCL, 0.01 g/l FeSO4.7H2O and 20 g/L agar and was autoclave sterilized at 121 ℃ for 15 min before use. The growth curve for bacterial and fungal culture was determined spectrophotometrically as optical density (OD) for bacterial cells at 600 nm while as a dry weight (mg/50 mL) for fungal cells.
1% FPT tailing samples were treated with At. ferrooxidans and A. niger in 9K and Modified Czapek's- Dox broth medium, respectively. All flasks were incubated at 30 ℃ using a Remi rotary shaker at 150 rpm/min for biomass measuring.
Volatile solids (VS) were determined as a measure of biomass production. VS was used rather than dry weight because monazite sand becomes entrapped in the biomass during bioleaching. By measuring VS, the organic portion of the total dry weight could be measured. Samples comprising the entire contents of a bioleaching flask were filtered on glass microfiber filters, dried overnight at 105 C, cooled to room temperature, and weighed. Samples were then ashed at 550 C for four hours, cooled to room temperature, and weighed again. The difference between the dried weight and the ashed weight was determined as VS.
Bioleaching experiments
The experiments were conducted with mesophilic acidophilic bacterial culture of At. ferrooxidans enriched in ferrous sulphate 9K medium. The culture was adapted to rare earth elements concentration of 1-10 ppm in 9K medium prior to experimentation. Bioleaching experiments were performed in a 500 mL Erlenmeyer flask with phosphate concentrate or tailings at 1% pulp density in 250 mL of 9K medium adjusted to pH 1.7 using 20% H2SO4 under aseptic conditions. Experiments were carried out for duration of (2-3) days using a Remi rotary shaker at 150 rpm/min and 30 ℃. In each flask 1 mL of start stationary phase bacterial culture (approximately 5 × 108 cells mL-1) was used as inoculum.
A. niger bioleaching experiments were carried out in 500 mL Erlenmeyer flasks with 250 mL where (3-5 days age) or one mL of fungal culture (approximately 1 × 107 spores mL-1) of Modified Czapek's- Dox liquid media and test samples (1%). Sterilization of the samples and media was done by autoclaving (121 ℃). Each flask was inoculated with 2 mL of spore suspension of the respective ore-adapted fungal strain. All flasks were incubated at 30 ℃ on a rotary shaker (150 rpm/min). Experiments were carried out for duration of 24 h. The resulting solutions were analyzed using a spectrophotometer to determine the percentage of REEs leached by the respective fungal strain.
Bioleaching of REEs by At. ferrooxidans and A. niger at different particle size and pulp density
To determine optimal particle size range where dissolution will be the highest, bioleaching tests were carried out with particles of 32, 53, 75 and 105 µm size for different pulp densities of 0.1, 0.5, 1, and 1.5 g/50 mL. The suspension was maintained at constant temperature of 30 ℃ and air sparged with simultaneous adjustment of solution pH with diluted sulphuric acid in the case of leaching with bacteria. Solution agitation was done at 150 rpm/min stirring speed. The inoculum volume fraction was 1% for bacteria and fungi.
Analytical method
For the determination of the REEs content in the bioleaching samples, the samples were filter through a Whatman No. 1 filter paper. Filtration of the samples allowed the separation of the mineral particulates with bacterial and fungal cells from the leaching media. The filtrate was further centrifuged at 7000 rpm for more than 10 minutes to remove micro-particulates. The filtrated was treated with 1-2 mL of ArsenazoI (obtained from Sigma Aldrich), where the addition of the amount of the chelating dye was standardized; 2-3 mL/L. The content of REEs was determined spectrophotometrically using the colorimetric technique (a Shimadzu model UV-260-UV visible spectrophotometer), where a characteristic peak at ~520 nm in the spectra allowed determination of REEs content in the liquor [9].
Results and Discussion
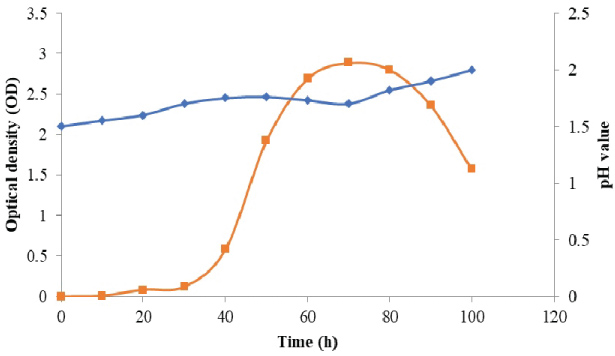
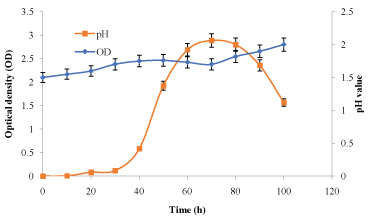
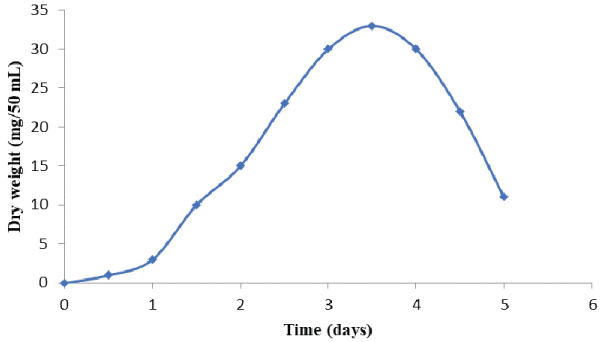
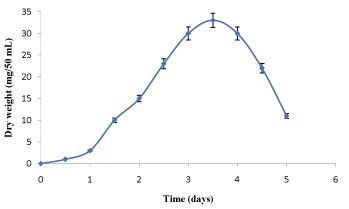
Growth behavior of At. ferrooxidans and A. niger with and without FPT tailing samples
The data in Figure 1 and Figure 2 show that the growth rate of At. ferrooxidans and A. niger. As the density of the culture increases, the rate of division decreases until the bacteria reaches a concentration at which they no longer divide but are viable. Estimation of optical density carried out using spectrophotometer allowed us to determine the bacterial and fungal growth. The exponential phase of bacterial growth started at 30 h after the lag phase and continued until 50 h when it reached the stationary phase. During the exponential phase, each microorganism is dividing at constant intervals. Thus, the population doubles as shown in Figure 1. Regarding the pH, initially increased due to the consumption of H+ by the bacteria and thereafter decreased due to the abiotic hydrolysis of ferric sulfate. The bacterial catalyzed oxidation of ferrous to ferric ion can be expressed as follows [6]:
The abiotic hydrolysis of ferric sulphate follows this reaction:
The following reaction shows the formation of jarosite:
The oxidation of ferrous ions by At. ferrooxidans is responsible for the large increase in the redox potential (Eh) of Fe3+/Fe2+.
The values of dry weight for At. ferrooxidans and A. niger in the presence of Florida phosphate tailing (FPT) samples, as the time of incubation increased. This result may be due to the attachment of bacterial/fungal cells to the surfaces of mineral samples. Also, it has to be noted that the toxicity of the leaching materials can affect the mechanism of bio-oxidation of sulphur and iron for At. ferrooxidans and thus reduce the metabolic products (citric acid, amino acids and polypeptides, etc.) for A. niger [7].
Shake flask bacterial leaching of REEs from Florida phosphate concentrate and tailing samples
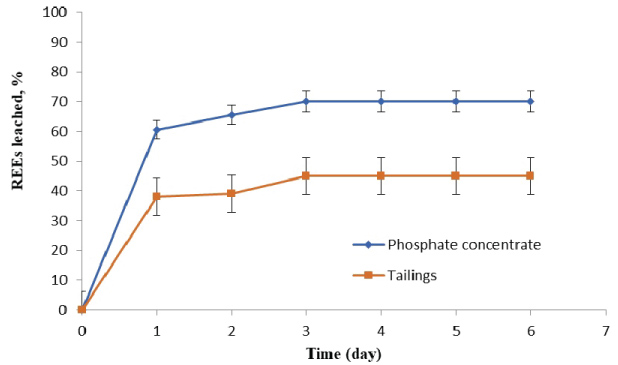
Based on bioleaching studies of REEs from FTP samples, by At. ferrooxidans and A. niger at different incubation periods, as shown in Figure 3 and Figure 4, it was found that the amount of REEs leached from phosphate concentrate and tailings by At. ferrooxidans increased with the time of incubation up to three days. The highest REEs leaching efficiencies were (70% & 45%) from phosphate concentrate and tailings, respectively at the mentioned conditions. After that, the amount of REEs leached was negligible. This decrease in leaching attributed to poor acid production capabilities of the bacteria after three days. The leaching reactions are considered to be via indirect action of At. ferrooxidans on bio-oxidation of ferrous sulphate (Fe2+) to form ferric sulphate (Fe3+) in leaching media. The indirect action of At. ferrooxidans suggests that H2SO4 and Fe2(SO4) were produced during of bio-oxidation reaction. The key reactions governing the leaching process are given below.
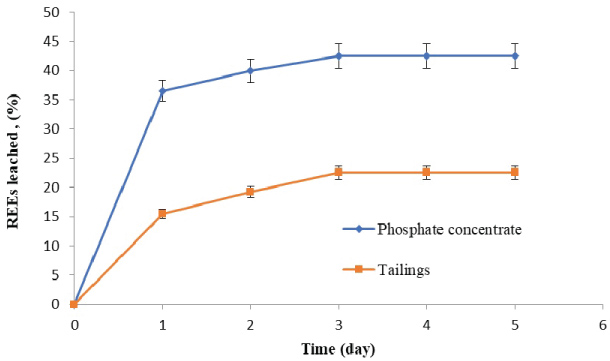
In addition to, the highest REEs leaching efficiencies by A. niger were (42.5% & 22.5%) from phosphate concentrate and tailings, respectively at the mentioned conditions. The leaching reactions are considered to be via direct action of A. niger on bio-dissolution of organic acids in leaching media. The leaching process with A. niger was attributed to a range of different type of reagents produced during its growth, which are low-molecular weight organic acids (LMWOAs), comprising aliphatic compounds with 1 - 3 carboxylic acid functional groups and methoxy, hydroxy, carboxylic acid substituted benzoic and cinnamic (aromatic) acids. Because they form stable complexes with metals, LMWOAs play a significant role in mineral transformation. Organic acids promote mineral dissolution by 1) Donating H+ to proton-promoted dissolution processes; 2) Forming inner-sphere surface complexes that dislodge structural metals from the mineral surface and 3) The formation of aqueous metal-ligand complexes that reduce the relative solution saturation with respect to minerals undergoing dissolution [4]. The key reactions governing the leaching process are given below.
Probable interaction pathways:
Citric acid is a tricarboxylic acid which contains three carboxylic moieties and one hydroxyle group (pka = 6.39) as the possible donor of a proton (H+) at 30 ℃.
(Citric acid) (Citrate)
Also, oxalic acid contains two carboxylic groups (pKa = 1.20 and pKa = 4.20) at 25 ℃. So, the possible complexes of rare earths reaction with oxalate anion are;
Bioleaching of REEs by At. ferrooxidans and A. niger with FPT particles of different size and pulp density
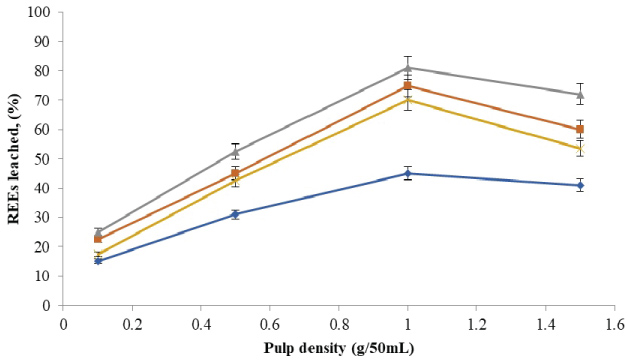
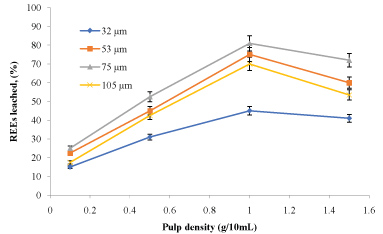
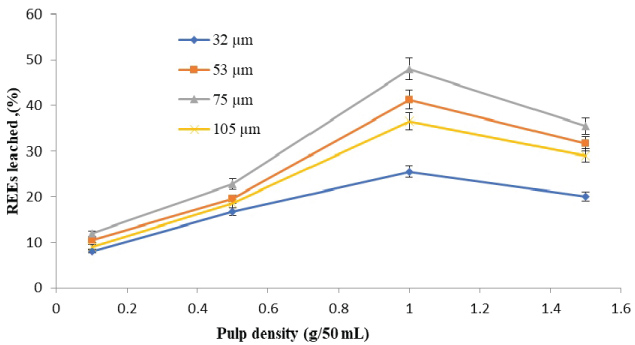
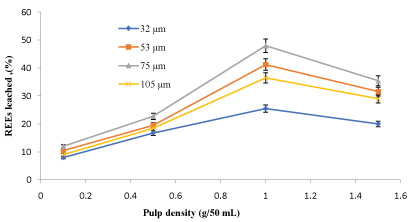
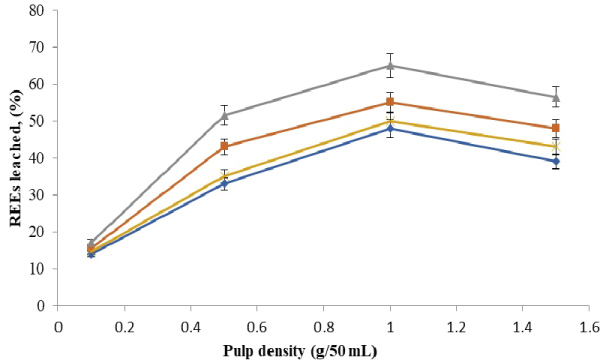
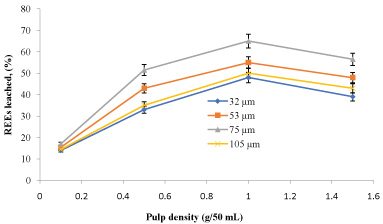
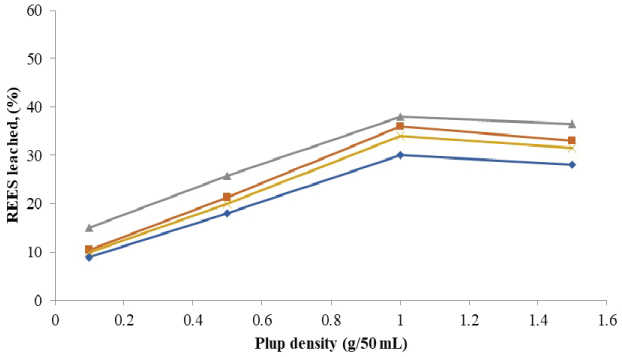
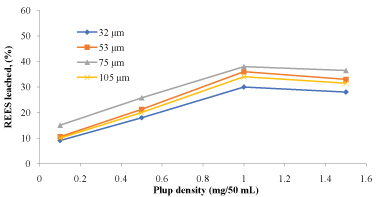
The amounts of rare earth elements bioleached at the varying size and pulp density fractions are shown in (Figure 5, Figure 6, Figure 7 and Figure 8). The maximum bio-dissolution of REEs from FPT were (81% & 48%) by At. ferrooxidans as previously appeared in Figure 5 and Figure 6 where (65% & 38%) by A. niger for concentrate phosphate and tailings as found in Figure 7 and Figure 8, respectively a particle size of 75 µm and at a pulp density of 1 (g/50 mL). The results reveal that dissolution generally increased with the decrease in particle size down to 75 µm but decreased as particle size decreased further to < 53 µm. Also, results showed that by increasing the pulp density from 0.1 to 1 (g/50 mL), the leaching was increased for three days of incubation. The bioleaching behavior of particles of different particle size can be explained by considering the surface area of the mineral that is available for the microbes.
The sizes of the particles determine the surface area, which in turn determined the amounts of REEs leached. Smaller surface area of the larger particle size fraction of 105 µm can be expected to result in a decrease in the number of active microbial attachment sites that could promote dissolution [10].
Lower recovery at finer particle size fractions might be attributed to possible cell damage and deactivation, which could be caused by finer particles arising from probable oxygen deficiency due to limiting air flow rate. Reduction of particle size below a critical level could increase the extent of the particle-particle collision and impose severe attrition which might disrupt the structure of the cells [11]. Optimal bio-dissolution at a particle size of 75 µm could be attributed to the combined effects of surface area and its overall suitability for microbial viability and activity. The results are consistent with finding of [12].
In a 2013 study, Qu and Lian examined bioleaching of REEs from red mud, a byproduct of bauxite ore processing for alumina production, by Penicillium tricolor RM-10 [7]. They reported total REE concentrations in the leachate of 20 to 60 mg/L, compared to 60 to 120 mg/L for monazite bioleaching by ML3-1 and WE3-F in this study. These concentrations corresponded to leaching efficiencies of 20% - 40%. Also, Brisson, et al. [13] mentioned that REE concentrations corresponded to leaching efficiencies of 3%-5% from monazite. Note that the red mud efficiency numbers are higher even though the overall concentrations are lower due to the lower starting concentrations of REEs in the red mud. Given the differences in the ores (red mud vs. monazite) and experimental time scales (50 days vs. 6 days), it is not surprising that leaching efficiencies differ.
Compared to (81% & 48%) by At. ferrooxidans and (65% & 38%) by A. niger, for concentrate phosphate and tailings in this study. This may be demonstrating that these organisms can phosphate as a source for growth.
Summary and Conclusions
From the work reported here the following interpretations are made:
• Bacteria/fungi grow well with minerals-where the generation time do not change.
• Particle size aspects smaller particle leaches better but due to larger amount of REEs leaching efficiency goes down. Larger particle sizes, less content of metals are exposed to the reacting solution, thereby reducing contact between the microbial cells and metal content of the ore that reducing the bioleaching rate.
• Bacterial/fungi grow and attach to surfaces of minerals from biofilm where attachment may occur via diffusion, convection and/or chemotaxis.
• Bacterial/fungi leaching shows promise in terms of the amount of REEs seen in the leached liquor.
• Bacterial/fungi appear to be ineffective at higher REE content. It is known that higher concentration of REEs in broth is likely to be toxic for bacterial survival and growth. These poor growth effects are seen to be prominent at lower particle size sample and high pulp density, where REEs were released in higher amounts. Regular removal of REEs from the bioreactor is critical for bacteria/fungi to be effective for bioleaching for a longer period of time.
Acknowledgment
The authors acknowledge the support from Florida Institute of Phosphate Research for their support in conducting this study.
References
- Kanazawa Y, M Kamitani (2006) Rare earth minerals and resources in the world. Journal of Alloys and Compounds 408-412: 1339-1343.
- Tian J, Chi R, Yin J (2010) Leaching process of rare earths from weathered crust elution-deposited rare earth ore. Transactions of Nonferrous Metals Society of China 20: 892-896.
- Moldoveanu GA, Papangelakis VG (2013) Recovery of rare earth elements adsorbed on clay minerals: II. Leaching with ammonium sulfate. Hydrometallurgy 131-132: 158-166.
- Goyne WK, LS Brantley, J Chorover (2010) Rare earth element release from phosphate minerals in the presence of organic acids. Chem Geol 278: 1-14.
- Chi R (1988) Extraction of rare earths from a low-grade, kaolinitic ore by percolation leaching. In: Bautista RG, Wong MM, Rare earths: Extraction, preparation and applications: The Minerals, metals and materials society. 227-234.
- Boon M, Heijnen JJ (1993) Mechanisms and rate limiting steps in bioleaching of sphalerite, chalcopyrite and pyrite with Thiobacillus ferrooxidans. In: Tormo AE, Lakshamanan VL, Biohydrometallurgical technologies. Miner. Met. Mat. Soc., Warrendale, Pennsylvania, 217-235.
- Qu Y, Lian B (2013) Bioleaching of rare erath and radioactive elements from red mud using Penicillium tricolor RM10. Bioresour technol 136: 16-23.
- Silverman MP, Lundgren DG (1959) Studies on the chemoautotrophic iron bacterium ferrobacillus-ferrooxidans .1. An improved medium and a harvesting procedure for securing high cell yields. J Bacteriol 77: 642-647.
- Marczenko Z (1976) Spectrophotometric Determination of Elements. John Wiley and Sons, New York.
- Hossain AM, Das M, Ananatharaman N, et al. (2004) Bioleaching of ZnS ore using Thiobacillus ferrooxidans. J Institution of Engineers (India) Chemical Division 85: 7-11.
- Deveci H (2002) Effect of solids on viability of acidophilic bacteria. Minerals Engineering 15: 1181-1189.
- Olubambi PA, Potgieter J, Ndlovu S, et al. (2009) Electrochemical studies on interplay of mineralogical variation and particle size on bioleaching low grade complex sulphide ores. Transactions of Nonferrous Metals Society of China 19: 1312-1325.
- Brisson VL, Zhuang WQ, Alvarez-Cohen L (2016) Bioleaching of rare earth elements from monazite sand. Biotechnol Bioeng 113: 339-348.
Corresponding Author
Shimaa Hussien, Department of Ore Processing, Nuclear Materials Authority, P.O. Box 530, El-Maadi, Cairo, Egypt.
Copyright
© 2018 Somasundaran P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.