The Phytonutrients and Cytotoxicity Evaluation of Cinnamon Impressicostatum as Potential Antioxidant and Antibacterial in Food Supplement
Abstract
The fundamental quest for food safety and security fit for consumption cannot be underscore. Cinnamon has been known to possess wide variety of biological activities. We evaluated the phenolic and cytotoxity of C. impressicostatum as a potential additive to food to increase the shelf life and to prevent bacterial food infection. We used the Folin-Ciocailteu method for the total phenolic content quantification and MTT Assay using the Human Embryonic Kidney cells (HEK 298) for the cytotoxity evaluation of the crude extract of C. impressicostatum, ICP-MS was also used for the quantification of heavy metal ion in the crude extract. As shown in Table 1, the presence of essential metals in natural products is very important in its suitability as a food supplement and highlights the important role it plays in the normal functioning of the human body. Detection of toxic metals is pertinent as it may be a primary factor or accessory factor in influencing antibacterial activity against the target bacteria in this study. Toxic metals in Cinnamomum extracts may also potentially pose a risk to human health. This will eventually influence the ease of commercialisation of any extracts produced from cinnamon, in Table 2, the water extract of C. impressicostatum stem-bark showed a high level of phenolic content. The same extract also demonstrated highest antibacterial activity suggesting that it may be the phenolic compound(s) present in the extract responsible for bioactivity against MRSA and the other bacteria tested in this study.
Keywords
Phytonutrients, Cytotoxicity, Cinnamon, Antioxidant, Antibacterial, Food supplement
Introduction
It has been estimated that worldwide there are over 500,000 species of plants. Traditionally, the compounds produced by plants have been categorised as primary and secondary metabolites. Compounds contributing to fundamental metabolism are termed primary metabolites. In contrast, secondary metabolites are limited in their distribution, both throughout the plant and between different species [1]. Secondary metabolites were once thought to be waste compounds [1]. Our understanding of the important in-planta functions of many secondary metabolites is gradually expanding. It has been revealed that many secondary metabolites are potent repellents and even toxic agents to pests and herbivores and also possess antibacterial activity [2]. Secondary metabolites that are semiochemicals are relied on as a means of defense against pathogens and predators, as attractants to lure mobile creatures for fertilisation and dissemination and also for aerial allelopathy (interplant communication). On the other hand, secondary metabolites that are volatile organic compounds have been revealed to be attractive to insects and help with fertilisation whilst secondary metabolite pigments that give warning colouration defend against predators [2]. Other plant pigments provide protection against environmental damage such as free radicals and UV radiation [1]. Some secondary metabolites perform signalling functions as plant hormones and pheromones. Plants produce an incredible array of secondary metabolites and many of these have been developed into economically important products including oils, gums, resins, tannins, rubber, waxes, pigments, flavours, fragrances, surfactants, preservatives, pesticides and pharmaceuticals [1]. As such, plant secondary metabolites represent a tremendous resource for commerce, particularly if they or their derivatives are potentially bioactive against pathogenic bacteria and other infectious parasites.
Phytochemistry plays a fundamental role in the chemical investigation of plant metabolites, including secondary metabolites. Through phytochemical studies, directed characterisation of the chemical composition of complex secondary metabolite essential oils or plant extracts may be undertaken. Phytochemical screening can also assist in taxonomical classification whilst bioassay guided studies can target and identify biologically active compounds in complex plant extracts [3].
It is clearly documented in the literature that Cinnamomum species possesses potent antimicrobial activities against several microbes. Cinnamon occupied a pre-eminent position in the ancient world and was much sought after. In fact, it is often qualified as 'sensational cinnamon' and 'spice of life'. It contains important medicinal essential oils in its leaves, fruits, twigs, stems, inner and outer bark. It has enormous valuable pharmacological activities. Cinnamomum is a genus within the family Lauraceae. Many species within this genus are used as spices. Most of cinnamon biological activity exists in its essential oils, which are about 90% cinnamaldehyde [4]. Cinnamon as a plant possesses chemo-preventive, antibacterial, antifungal, antiviral, antispasmodic, antipyretic, anti-ulcer, choleretic, sedative, hypothermic, lipolytic, antiseptic, anesthetic, anodyne, cytotoxic, hypolipidemic, antiplatelet properties and also stimulates the immune system. It may be useful in reducing cardiovascular disease and the risk of cancer [5,6].
Materials and Methods
Determination of total phenolic content in the crude extracts
Growing evidence has shown in recent research into human health and nutrition that plant secondary metabolites play essential and critical roles in wellness [7-10]. Phenolic metabolites that are plant-based are quite interesting due to their potent antibacterial and antioxidative activity and have a host of pharmacological applications such as for anticancer therapy and platelet aggregation inhibition activity [11-15]. Phenolic metabolites are mostly components of vegetables and fruits and play a functional role in defense against insects and animal herbivores [16]. The synthesis or production of phenolic metabolites might be as a result of insect-induced stressors, UV light exposure and microorganism-induced stressors. This therefore suggests that phenolics have a protective role against insect predators, light-oxidative effects and microbial infection [1,17].
The total phenolics were quantified based on the method described by Singleton 1999 (20) using the Folin-Ciocailteu (FC) reagent. First, the calibration curve of aqueous gallic acid solutions of known concentrations was prepared.
Preparation of Gallic acid curve
25 mg of Gallic acid was dissolved in about 5 mL of 95% ethanol and made up to 50 mL in a volumetric flask with 95% ethanol. The solution was diluted with water to prepare 50, 100, 150, 200 and 250 μg/mL Gallic acid.
Construction of calibration curve
1.58 mL of distilled water was added to 20 μL of Gallic acid solution and blank solution (0.95% ethanol) in a cuvette. Then, 100 μL of FC reagent was added to each cuvette and mixed well. After 5 minutes, 300 μL of saturated sodium carbonate solution was added to each cuvette and mixed thoroughly followed by incubation in the dark at 40 °C for 30 minutes. The absorbance was measured at 760 nm. Each measurement was undertaken thrice and a calibration curve of absorbance versus concentration was generated.
Determination of the absorbance of extracts
1.85 ml of distilled water was added to 20 μL of extracts (250 μg/mL in ethanol) and blank solution (0.95% ethanol) in a cuvette. Then, 100 μL of FC reagent was added to each cuvette and mixed well. After 5 minutes, 300 μL of saturated sodium carbonate solution was added to each cuvette and mixed thoroughly followed by incubation in the dark at 40 °C for 30 minutes. The absorbance was determined at 760 nm. The total phenol in the extracts was calculated from the calibration curve and determined as percent phenolic content (g phenolics/g crude extract).
Determination of cytotoxic activity (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) (MTT) assay
The goal of this study was to determine whether the extracts from Cinnamomum species that showed antibacterial activity were cytotoxic. Quite interestingly, often the difference between a therapeutic and a toxic extract or compound is simply the dosage level [3].
In the measurement of cellular metabolic activity via the NAD (P)H-dependent cellular oxidoreductase enzyme, the MTT colorimetric assay is mostly used. The cellular enzyme reduces the MTT dye tetrazolium to an insoluble complex called formazan which is purple in colour. The dye tetrazolium can be used to assess cytotoxic and cytostatic activity of potential medicinal plants and other toxic materials. The MTT reagent is light sensitive, thus the processes of the MTT assay are performed in the dark [4]. 99 ul of cell suspensions of Human Embryonic Kidney cells (HEK 298) containing 1 × 106 cells were seeded into each well of a sterile 96 well plate and then incubated for 24 h at 37 °C in a humidified incubator. Crude extracts of C.impressicostatum were added to concentrations ranging from 50 to 250 μg mL-1. The final volume in each well was 100 μl and the final concentration of DMSO in each well was 0.5%. The plates were further incubated for 48 h. 5 ml of MTT reagent was added to each well and incubated for 4 h. The plates were centrifuged at 1000 rpm for 5 min at 4 °C. The MTT solution and the medium were aspirated from the wells without disturbing the formazan crystals at the bottom of the wells. Buffered DMSO (200 μL) was added into each well to dissolve the formazan crystals. The plates were shaken for 5 min and the absorbance was recorded on a microtitre plate reader at the wavelength of 570 nm and a reference wavelength of 630 nm. The percentage of viable cells in the treatments was calculated using the following formula:
5-fluorouracil was used as a positive control while 0.5% DMSO was used as the negative control (DMSO did not affect cell proliferation). Cell proliferation at each concentration of each extract was tested in triplicate in each respective 96 microwell plate and the whole experiment was repeated three times.
Inductively coupled plasma - mass spectrometer (ICP-MS)
Inductively coupled plasma - mass spectrometry (ICP-MS) systems are currently being applied in different areas of research studies, which include environmental, clinical, metallurgical, geochemical, chemical etc. The advances made by this technique are due to its rapid multi-element detection limit even at an ultra-trace level, isotopic capacity and the pace of analysis.
Sample preparation
2g of C. impressicostatum leaves, stem-bark and stem-wood were transferred into Teflon digestion vessels using a spatula. All digestion vessels were pre-cleaned by soaking in 1% nitric acid for over 12 h, followed by rinsing with ultrapure water.
Microwave digestion
The sample was digested using the microwave digestion system START D (Milestone Inc., USA) equipped with 10 Teflon vessels. Two grams of each sample were mixed with 7 ml of 65% nitric acid (Merk, Darmstadt, Germany) and 1 ml of 30% hydrogen peroxide (Merk, Darmstadt, Germany) in a separate digestion vessel. The digestion vessels were assembled and placed into a microwave digester. All samples and blanks were subjected to two steps of 15-minute digestion at 200 °C at a power of 1200 W. When the digestion process had completed, the vessels were removed from the microwave digester and cooled to room temperature. All digestates were transferred into separate glass bottles.
Preparation of working solution and determination of metal content
Five ml of each digestate was diluted with ultrapure water to a final volume of 50 ml after being mixed well; 13 ml of the final sample solution was transferred into separate auto sampler tubes for subsequent ICP-MS analysis. Sixteen (16) metals of interest were included in this study i.e. Cu, Zn, Mn, Ni, Fe, Co, Cr, Pb, Cd, As, Se, Al, Na, Mg, Ca, and Hg. Multi-element standard solutions (PerkinElmer Pure Atomic Spectroscopy Standard) were used for external calibration. Metal analysis was performed using the PerkinElmer ELAN® ICP-MS system equipped with an ASX-520 Auto-sampler (CETAC Technologies, Omaha, USA). The whole system was flushed with a solution of highly pure 1% HNO3 (PerkinElmer Pure Atomic Spectroscopy Standard) prior to analysis of standards, blanks and samples. Then, metal analysis was performed for all standards by ICP-MS. A five-point calibration curve was automatically generated for each metal of interest by computer software. After that, all thirty samples were subjected to metal analysis using ICP-MS (Table 3).
External calibration
Each metal of interest was calibrated using five working standards at concentrations of 5, 10, 50, 100 and 200 ppb. The correlation coefficient (r2) values for all calibration curves ranged from 0.9926 to 1.0000. 14 out of 16 calibration curves showed satisfactory linearity (r2 > 0.995 with reference to at least four points), hence the method used in this study is linear within the range of 5 to 200 ppb.
Statistical analysis
Microsoft excel 2010 was employed to derive graphs and summary statistics such as standard deviation values. Statistical Package for Social Sciences (SPSS) software version 19 was used to analyse the data. Results obtained from this study were analysed using one-way analysis of variance (ANOVA) and Post hoc least significant Difference (LSD). The level of significance (p-value) was set at 0.05.
Results
Cytotoxicity assay
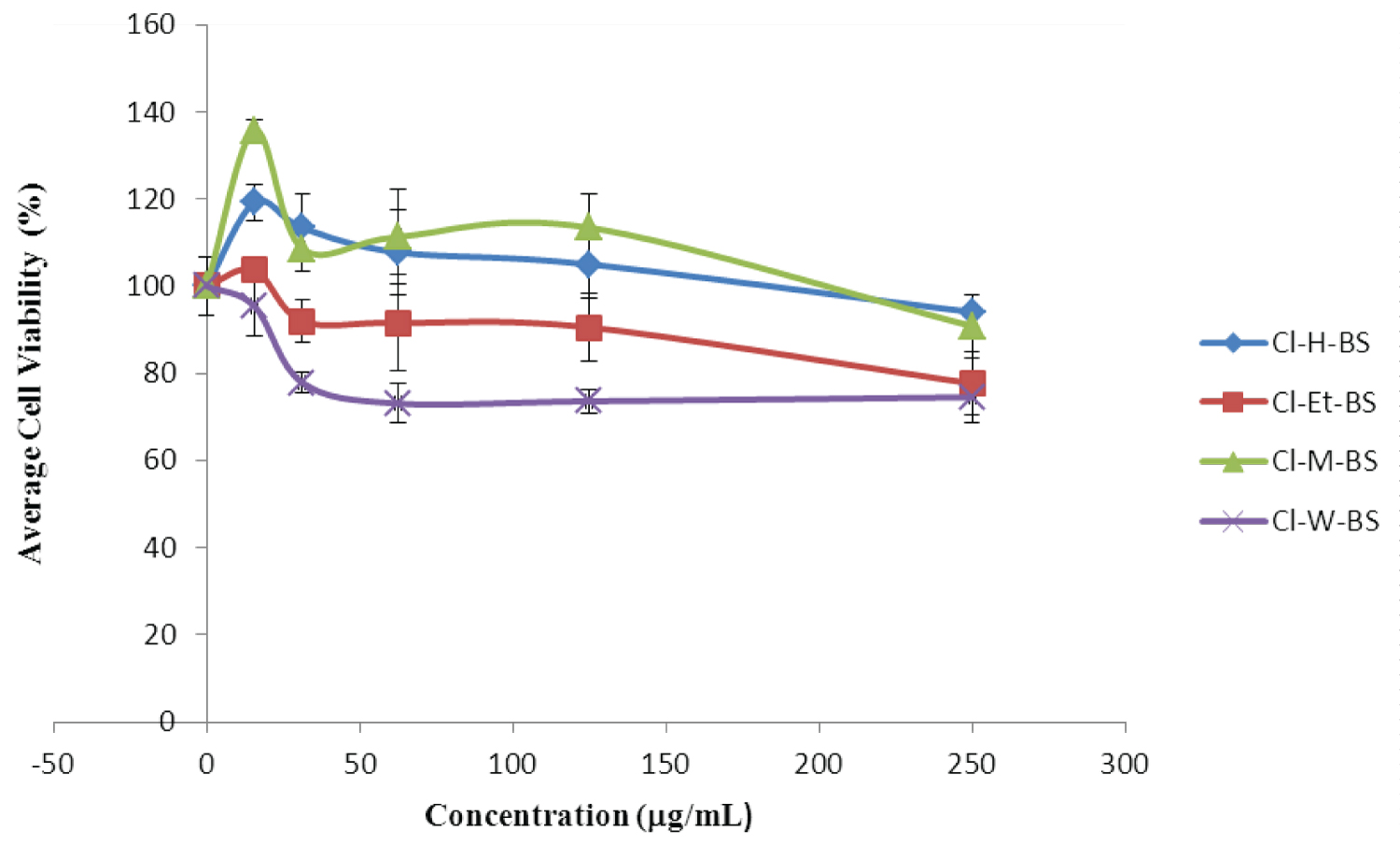
The cytotoxic effect of C. impressicostatum extracts was evaluated using Human Embryonic Kidney Cells (HEK298). Cell viability was slightly but significantly reduced at different concentrations of the extracts tested, in a dose dependent manner, which indicated that the crude extracts of C. impressicostatum hexane bark stem (CI-H-BS), C. impressicostatum ethyl acetate bark stem (CI-Et-BS), C. impressicostatum methanol bark stem (CI-M-BS) and C. impressicostatum water bark stem (CI-W-BS) were slightly but significantly cytotoxic to normal human embryonic kidney cells (HEK 298), at the minimal inhibitory concentration of our extract (Figure 1).
Inductively-Coupled Plasma Mass Spectrometry (ICPMS)
The concentrations of 16 metals (Cu, Zn, Mn, Ni, Fe, Co, Cr, Pb, Cd, As, Se, Al, Na, Mg, Ca, Hg) in the digested C. impressicostatum bark stem sample were analysed by IC-PMS as shown in Table 4. The analysed metals were grouped into three categories: macro-elements, trace metals and toxic metals.
Total phenolic content (TPC)
The total phenolic content (TPC) evaluations of C. impressicostatum stem-bark hexane, ethyl acetate, methanolic and water extracts as well as volatile oils are shown in Table 2. The water and methanolic extracts were found to have the highest TPC values.
Discussion
There is the belief that natural medicines are much safer than synthetic drugs. This has caused exceptional growth and interest in the natural product industry, comprising phytotherapeutic agents and phytopharmaceutical products. This fact has led to a resurgence of scientific interest in their biological effects. There is no universal regulatory system insuring the safety and activity of natural products and in most countries they are not sufficiently investigated analytically or toxicologically [1]. Contrary to popular belief, herbal medicines can be potentially toxic to human health. In fact, scientific research has shown that many plants used in traditional and folk medicine are potentially toxic, mutagenic and carcinogenic [2,3].
Part of the current challenges being faced in ethno-therapeutic research is the toxicity of some constituent of medicinal plants and plant-related products. The potential cytotoxicity of Cinnamomum species was evaluated in this study using Human Embryonic Kidney cells (HEK 298) Extracts from C. impressicostatum were shown not to be cytotoxic to the HEK cells and this confirms the reason why, over the years, it has been used as a spice in global cuisine. This also clearly indicates that C. impressicostatum stem bark extract can be safely used for human therapy.
Metal ions are known to be essential and function in part in over one third of all proteins and also as an abundant cofactor in fundamental life processes, it include biological processes such as: respiration, photosynthesis, carbon, hydrogen, nitrogen and sulfur metabolism, biosynthesis of antibiotics, replication and repair of DNA, antioxidant defense, gene regulation, and neurotransmission. The understanding of the multi-functional role of metals and their presence in natural product is of utmost importance. Though, toxic metals such as Hg, Cd, and Pb at elevated concentrations can be toxic to the cell the roles of metal in biological processes and in human health have greatly accelerated in past three decade. The quantification of metal ions in plants offer enormous potential for understanding life processes and human diseases at both the cellular and molecular levels.
In this study we evaluated the presence of essential, non-essential and toxic metals in C. impressicostatum. As shown in Table 1, the presence of essential metals in natural products is very important in its suitability as a food supplement and highlights the important role it plays in the normal functioning of the human body. Detection of toxic metals is pertinent as it may be a primary factor or accessory factor in influencing antibacterial activity against the target bacteria in this study. Toxic metals in Cinnamomum extracts may also potentially pose a risk to human health. This will eventually influence the ease of commercialisation of any extracts produced from cinnamon.
Currently, evidence has shown that secondary plant metabolites play critical roles in human health and may be nutritionally important [21-24]. Of special interest are plant-based phenolic metabolites due to their potent antibacterial and antioxidant activity and wide range of pharmacological properties including anticancer and platelet aggregation inhibition activity [25-29]. Phenolic metabolites are common constituents of fruits and vegetables that function in the defense against insects and animal herbivores [30]. The synthesis of phenolics in plants correlates to insect-induced stressors, UV light exposure and microorganism-induced stressors, which suggests they have a protective role in preventing insect predation, photo-oxidation and bacterial and fungal infection [31,32]. In this study, as shown in Table 2, the water extract of C. impressicostatum stem-bark showed a high level of phenolic content. The same extract also demonstrated highest antibacterial activity suggesting that it may be the phenolic compound(s) present in the extract responsible for bioactivity against MRSA and the other bacteria tested in this study.
Competing Interests
The author(s) declare that they have no financial and/or non-financial competing interests.
Acknowledgements
This study was funded by the International Medical University, Malaysia, under Research Grant 2010/222. The reference strain used for this study, Methicillin Resistant Staphylococcus aureus, ATCC 700698, was obtained from the Faculty of Medicine and Health Sciences, Department of Medical Microbiology and Parasitology, Universiti Putra, Selangor, Malaysia.
References
- Iwu MW, Duncan AR, Okunji CO (1999) New antimicrobials of plant origin. In: J. Janick, Perspectives on new crops and new uses. ASHS Press, Alexandria, VA, 457-462.
- Raven PH, Evert RF, Eichhorn SE (2005) Biology of Plants. (7th edn), WH Freeman and Company, New York.
- Dewick PM (1992) Medicinal natural products: A biosynthetic approach. (2nd edn), John Wiley & Sons Ltd, West Sussex.
- Harborne JB (1998) Phytochemical methods: A guide to modern techniques of plant analysis. (3rd edn), Chapman and Hall, London.
- Bown D (1995) The royal horticultural society encyclopedia of herbs and their uses. (1st edn), Dorling Kindersley Publishers Ltd, London.
- Jayaprakasha GK, Rao LJ, Sakariah KK (2002) Chemical composition of volatile oil from Cinnamomum zeylanicum Z Naturforsch C J Biosci 57: 990-953.
- Hiramatsu K, Asada K, Suzuki E, et al. (1992) Molecular cloning and Nucleotide sequence determination of the regulator region of mecA gene in methicillin-resistant Staphylococcus aureus (MRSA). FEBS let 298: 133-136.
- Berger-Bachi B, Barberis-Mai L, Strassle A, et al. (1989) FemA, a host-mediated factor essential for methicillin resistant in Staphylococcus aureus: Molecular cloning and characterization. Mol Gen Genet 219: 263-269.
- Iinuma M, Tsuchiya H, Sato M, et al. (1994) Flavonones with potent activity against methicillin-resistant Staphylococcus aureus. J Pharm Pharmacol 46: 892-895.
- Sato M, Tsuchiya H, Takase I, et al. (1995) Antibacterial activity of flavonone isolated from sophora exigua against methicillin-resistant Staphylococcus aureus and its combination with antibiotics. Phytotherapy Research 9: 509-512.
- Tanaka H, Sato M, Fujiwara S, et al. (2002) Antibacterial activity of isoflavonoids isolated from Erythrina variegate against methicillin-resistant Staphylococcus aureus. Lett Appl Microbiol 35: 494-498.
- Llarrull LI, Testero SA, Fisher JF, et al. (2010) The future of β-lactams. Curr Opin Microbiol 13: 551-557.
- Draw SM, Bonomo RA (2010) Three decades of beta-lactamase inhibitors. Clin Microbiol Rev 23: 160-201.
- Wilke MS, Lovering AL, Strynadka NC (2005) Beta-lactam antibiotic resistance: A current structural perspective. Curr Opin Microbiol 8: 525-533.
- Bebrone C (2007) Metallo-β-lactamases (classification, activity, genetic organization, structure, zinc coordination) and their superfamily. Biochem Pharmacol 74: 1686-1701.
- Avneet Saini, Rohit Bansal (2012) Insights on the structural characteristics of NDM-1: The journey so far. Advances in Biological Chemistry 2: 323-334.
- http://theconversation.com/new-antibiotics-whats-in-the-pipeline-10724
- http://www.sci-news.com/medicine/science-antibiotics-methicillin-resistant-staphylococcus-aureus-01548.html
- Ogston A (1984) Classics in Infectious Diseases. "On Abscesses". Rev Infect Dis 6: 122-128.
- Rosenbach A (1884) Mikro-Qrganismen bei den Wund-Infections-Krankheiten des Menschen. Wiesbaden JF Bergmann.
- Lindsay J (2008) Staphylococcus - molecular genetics. Caister Academic Press.
- http://www.textbookofbscteriology.com
- http://www.bacterio.cict.fr/allnamessz.html
- Lowy FD (1998) Staphylococcus aureus New England Journal of Medicine 339: 520-532.
- Trülzsch K, Grabein B, Schumann P, et al. (2007) Staphylococcus pettenkoferi sp nov., a novel coagulase-negative staphylococcal species isolated from human clinical specimens. Int J Syst Evol Microbiol 57: 1543-1548.
- Murray PR, Rosenthal KS, Pfalter MA (2005) Staphylococcus and related organisms. In: Mandell GI, Bennett JE, Polin R, Medical Microbiolog. (5th edn), Elservier Mosby, Edinburgh, United Kingdom, 221-236.
- Deresinski S (2005) Review of MRSA. Clinical Infectious Diseases 40: 562-573.
- Wilkinson BJ (1997) Biology. In: KB Crossley, Archer GL, The Staphylococci in human disease. Churchill Livingstone, New York.
- Kloos WE, Schleifer KH (1986) Genus 4. Staphylococcus Rosenbach 1984. In: J.G. Holt, P. Sneath HA, Mair NS, Sharpe MS, Bergey’s Manual of Systematic Bacteriology. (2nd edn), MD. Williams and Wilkins, Baltimore, 1013-1035.
- Waldvogel F (2000) Staphylococcus aureus (including toxic shock syndrome). In: Mandell, Douglass, Bennet’s Principles and Practice of Infectious Diseases. (5th edn), Churchhill living stone, New York, USA, 2069-2092.
- http://www.life.umd.edu/CBMG/faculty/asmith/Staphylococcus.jpg
- Kloos WE, Lambe DW (1991) Staphylococcus. (5th edn), American Society for Microbiology, Washington DC.
Corresponding Author
Ayuba Sunday Buru, Department of Medical Laboratory Science, College of Medicine and Health Sciences, Afe Babalola University, Ado-Ekiti, Ekiti State, Nigeria
Copyright
© 2022 Buru AS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.