Four Weeks of Omega-3 Supplementation does not Improve Cycling Time Trial Performance in Trained Cyclists
Abstract
Objectives
This study examined whether omega-3 polyunsaturated fatty acid (n-3 PUFA) supplementation lowered the heart rate (HR), rating of perceived exertion (RPE) and oxygen uptake (V̇O2) and accordingly improved cycling performance in a time trial.
Design
In a randomised, crossover, double-blind study, trained male cyclists (n = 10) were supplemented for 4 weeks with n-3 PUFA (5.7 g/day of eicosapentaenoic acid (EPA) and docosahexaenoic (DHA)) and 4 weeks with placebo (6g olive oil), with a 4-week washout period.
Methods
Cycling performance trials (45 min preload at 70% maximal work rate (Wmax) followed by 15 min time trial) were carried out prior to and following both supplementation periods. Fatty acid composition of blood total lipids was analysed prior to and in response to supplementation.
Results
Whole blood n-3 PUFA (% total fatty acids) increased from 1.67% (SD = 0.99%) to 3.72% (SD = 1.22%) (p < 0.05) following 4 weeks n-3 PUFA supplementation. Submaximal measures of V̇O2, HR, respiratory exchange ratio (RER) and RPE were unaffected by supplementation. Time trial performance (mean power W) was unchanged by n-3 PUFA (pre 239 W, SD = 34 W vs post 243 W, SD = 33 W), as were measures of V̇O2, HR, RER and RPE during the time trial.
Conclusions
High dose n-3 PUFA supplementation for 4 weeks did not improve cycling performance or attenuate the physiological variables usually associated with improved cycling performance, i.e. V̇O2 and HR, in a repeated-measures, placebo-controlled, crossover design study. It is possible that the exercise protocol used in the study was of insufficient intensity for the n-3 PUFA to show beneficial affects due to the highly trained nature of the cyclists.
Keywords
Fish oil; n-3 PUFA, Exercise, Oxygen uptake, Heart rate, Time trial
Abbreviations
DHA: Docosahexaenoic Acid; EPA: Eicosapentaenoic Acid; FAME: Fatty Acid Methyl Esters; HR: Heart Rate, n-3 PUFA: Omega-3 Polyunsaturated Fatty Acid; PT: Performance Test; RER: Respiratory Exchange Ratio; RPE: Rating of Perceived Exertion; V̇CO2: Carbon dioxide Production; VESTPD: Minute Ventilation; V̇O2: Oxygen Uptake; Wmax: Maximal Work Rate
Introduction
It is well documented that omega-3 long chain polyunsaturated fatty acids (n-3 PUFAs) have beneficial effects on human health; supplementing with n-3 PUFA has demonstrated positive effects on atherosclerosis [1] cardiovascular disease [2], rheumatoid arthritis [3], asthma [4], brain function [5], and the prevention of acute and chronic inflammation [6]. These affects are due to the anti-inflammatory, antithrombotic, antiarrhythmic, hypolipidemic and antiproliferative properties of n-3 PUFA [7] and it is for this reason that n-3 PUFA is one of the most popular dietary supplements used by elite athletes [8].
Nonetheless, evidence that n-3 PUFA supplementation can be advantageous for athletes is equivocal. Maximum oxygen uptake (V̇O2 max) appears to be unaffected by n-3 PUFA supplementation [9-12]. Conversely, some studies have reported a trend for increased time to fatigue [13] and a reduction in submaximal V̇O2 following periods of n-3 PUFA supplementation [11,14]. The effects of n-3 PUFAs on heart rate (HR) has also produced equivocal results, with some studies showing a reduction in submaximal HR [10,14,15] and others reporting no effect on submaximal [9,11,13] or maximal HR [15-17]. The incongruities in the results could simply be due to small sample sizes and lack of power in the findings of Huffman, et al. [13] as only 10 participants were investigated with only 5 taking n-3 PUFA supplements, compared with 9 and 10 in the studies by Peoples et al [14] and Kawabata, et al. [11]. With regards to exercise performance, studies that have used cycling time trials as an indicator of cycling performance, have failed to find an effect of regular n-3 PUFA supplementation [18-20]. However, Hingley, et al. [21] found a decrease in the mean oxygen consumption expressed relative to workload over a 5-minute time trial following 8 weeks of docosahexaenoic acid (DHA) rich tuna oil suggesting an improved cycling economy during a physiologically demanding time trial. An important factor to note is that except for the study by Bloomer, et al. [9], all of these previous studies have been parallel groups design, not repeated measures.
Previous studies conducted on the effects of n-3 PUFA supplementation on cycling performance have used relatively low dosages (≤ 3g/day) that may not have sufficiently increased circulating eicosapentaenoic acid (EPA) and DHA content to observe an impact on performance [18,20], or they have neglected to measure incorporation of n-3 PUFA [13,19]. Moreover, evidence has highlighted a large degree of inter-individual variation in the capacity to metabolise n-3 PUFAs [22]. Burke, et al. [23] suggests conducting repeated trials in the same individual to confirm the robustness of a measured response to a supplement is important. Therefore, a crossover design would allow individual responses to be substantiated against incorporation rates. Accordingly, the aim of the present randomised, double-blind, placebo-controlled, crossover design study was to determine whether 4 weeks of n-3 PUFA supplementation could improve cycling performance. We hypothesised that n-3 PUFA supplementation would lower the oxygen cost of exercise, reduce heart rate and Ratings of Perceived Exertion (RPE) and accordingly improve cycling performance in a time trial scenario.
Methods
Participants
The experimental protocol followed the Declaration of Helsinki principles and was approved by Loughborough University Ethics Human Participants sub-committee (Study ID: R14-P72) and registered as a clinical trial on www.clinicaltrial.gov (Study ID: NCT03205241). An a priori power calculation was performed using G*Power version 3.1.9.2 [24] based on data from a published study by Buckley, et al. [15] (N = 25) investigating the effect of n-3 PUFA supplementation on submaximal heart rate (time trial performance was not used due to previous findings being negative). The effect size was considered to be extremely large using Cohen's (1998) criteria. With an alpha = 0.05 and power = 0.80 the projected sample size required with this effect size is N = 10 (5 per group). Thus, the proposed sample size of N = 10, with all participants completing both n-3 PUFA and placebo conditions in a repeated measures design, is adequate for this secondary outcome of the study. Ten trained (V̇O2 max = 54 ± 5 ml. kg∙ min-1, training age 8 years) male cyclists, aged 38 ± 7 years participated in the study.
All participants were healthy, non-smokers with no history of metabolic, cardiovascular or neurological disease. Participants reported that they had not taken fish oil, antioxidant or anti-inflammatory supplements in the six months prior to commencing the study and agreed to not consume them throughout the duration of the study. Prior to participation, informed written consent was provided by the participants and a health screen questionnaire was used to determine the suitability to participate in the study. All testing took place during the competition phase of the cycling season and all participants were regularly competing in category 3/4 road races.
Protocol
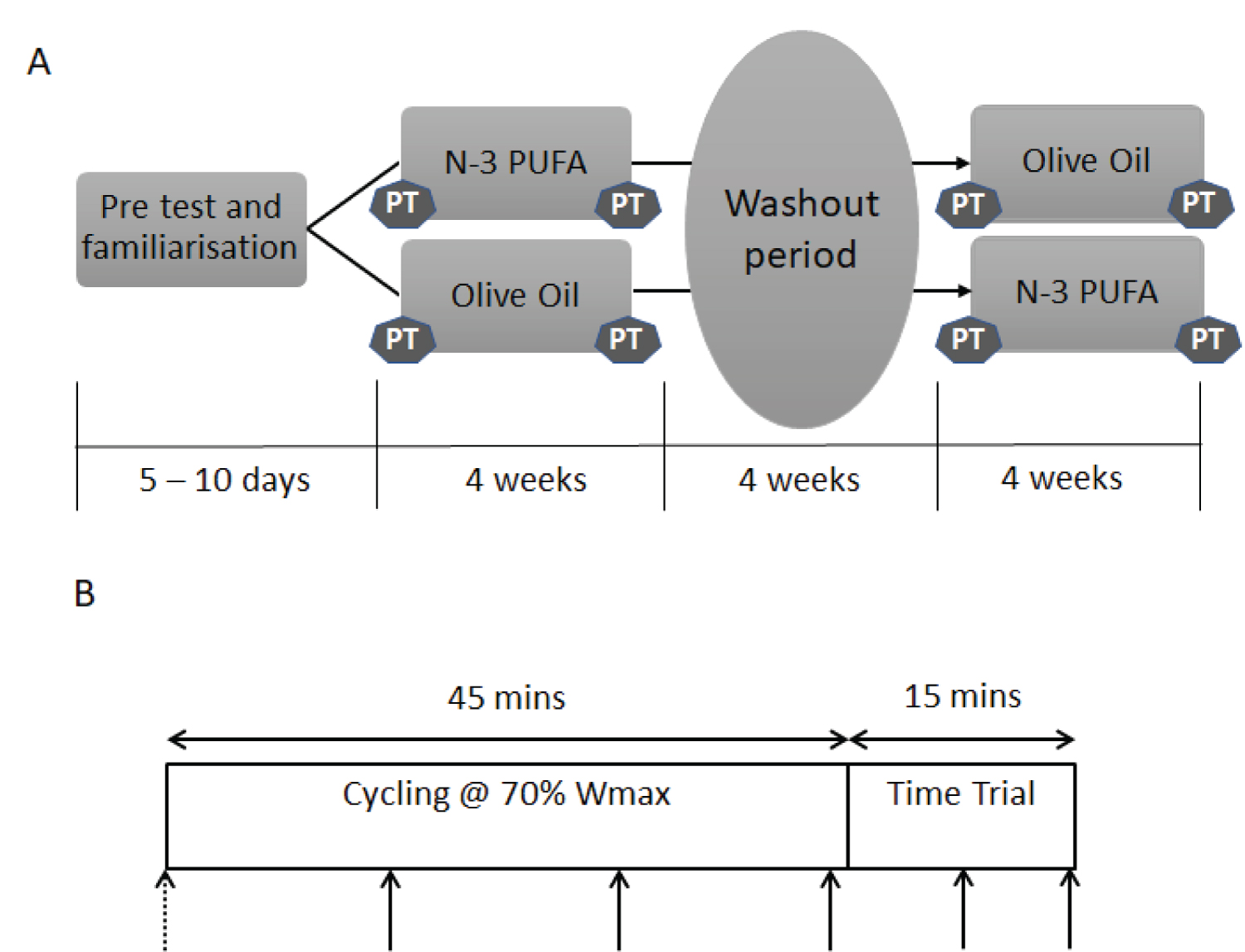
The duration of the study was 14 weeks, including a pre-test and familiarisation 5-10 days before the first performance trial. Four cycling performance trials were performed: pre and post four weeks n-3 PUFA supplementation and pre and post four weeks olive oil supplementation, in a double blind, randomised crossover design (Figure 1A). The supplementation periods were separated by a four-week washout period. Prior to performance trials, participants were required to keep a three-day food diary and replicate the day prior to each performance test to ensure energy intake did not influence performance or substrate oxidation. During the testing period, the cyclists were asked to maintain a constant training load, so that training status was consistent throughout the study. A training diary was used to log type of activity, duration, distance and session Rating of Perceived Exertion (sRPE).
Both placebo (Olive Oil, Puritan's Pride, New York, USA) and n-3 PUFA (Holland and Barrett, Warwickshire, UK) were provided in capsule form. Participants were instructed to take six capsules per day (two with breakfast, lunch and dinner) providing 5.7 g of n-3 PUFA and 0.01 g per day of α-Tocopherol or 6 g per day of olive oil. The composition of the capsules was determined using Gas Chromatography Mass Spectrometry (GC - MS) with n-3 PUFA supplements containing 601 mg of EPA and 253 mg of DHA and olive oil capsules containing 1000 mg of oleic acid per capsule. The n-3 PUFA dose was chosen based on previous findings [25] showing a similar dose in 10 males can induce significant changes over 4 weeks in the lipid profile of human blood. The dose of α-Tocopherol used to stabilise the PUFAs within the supplement is substantially lower than in studies demonstrating a potent antioxidant effect in the context of blunting training adaptations [26]. Many previous studies investigating the effects of n-3 PUFAs on sports performance have typically used olive oil as a placebo [20,27,28] as olive oil does not appear to have an effect on performance and the associated physiological variables [14,20,29]. Compliance for taking the capsules was monitored by capsule counting upon return of supplement pots by participants and verified with whole blood fatty acid incorporation.
Maximal work rate and maximal oxygen uptake (V̇O2 max) were determined. Following a warm-up period of 5 min at 100 W, workload was increased by 50 W every 3 min until volitional exhaustion. Participants pedalled at a self-selected pedal cadence between 80 and 120 rpm and were given verbal encouragement throughout to maintain their preferred pedal cadence. The maximal work rate (Wmax) was determined using the formula:
Wmax = Wout ÷ [(t/180) × 50].
Wout is the workload of the last completed stage and t is the time in seconds in the final stage. The Lode Excalibur Sport electromagnetically braked ergometer (Lode B.V, Groningen, Netherlands) was used in hyperbolic mode for the Wmax test. Hyperbolic mode allows a constant pre-set work rate to be imposed on the participant, independent of the cadence. Volitional exhaustion was deemed to have occurred when there was a precipitous decline of 20 rev•min-1 below their self-selected pedal cadence, at which point they were instructed to stop pedalling. On completion of the graded exercise test, participants were given a 10min rest period before performing a 15-min familiarisation session. This was conducted to improve the reliability of the Performance Tests, and involved the participant cycling at their predicted workload (70% Wmax) for the future laboratory visits (see below). All performance tests were conducted in the morning (7-9 am) following a 10 hour overnight fast. Participants' subsequent tests were performed at the same time of day to minimise diurnal variation, with stable climatic conditions (20-22 ℃ and humidity between 45-55%). Participants were asked to refrain from strenuous exercise and the consumption of alcohol and caffeine 24 h prior to testing and to arrive at each trial in a fully rested and hydrated state. Participants were advised to stay hydrated by drinking water ad-libitum throughout the tests. The performance trial consisted of 45 min submaximal cycling at 70% Wmax, followed by 15 min time trial (see Figure 1B). During the first 45 min the electromagnetically braked ergometer was in the hyperbolic mode, so that work rate (70% Wmax) was independent of pedalling rate. The ergometer was changed to linear mode for the 15-min time trial so that with increasing pedalling rate the work rate increased, allowing the participant to use their speed to effectively increase the gear/power output on the cycle ergometer. The linear factor setting was chosen to allow a pedal rate identical to the individuals mean pedalling rate from the V̇O2 max test at 70% Wmax using the following formula:
W = [L × (RPM)2]
where the RPM is the mean RPM calculated from the V̇O2 max test. The participants were given verbal encouragement to perform as much work as possible in the time trial. The cycle ergometer was connected to a computer that measured work rate every second and the mean power performed over the 15-min time trial was calculated as a measure of performance. The participants received no feedback on the power output, heart rate or cadence, and were only provided feedback through elapsed time, to avoid test retest influence. The same investigator provided encouragement to the participant across all tests. HR, RPE and 1-minute expired air samples were collected according to solid arrows denoted on Figure 1B at 14, 29 and 44 minutes in the submaximal cycling and at 7 and 14 minutes in the time trial. Minute ventilation (VESTPD), V̇O2 and Carbon dioxide production (V̇CO2) and Respiratory Exchange Ratio (RER) were determined using a Servomex 1500 (Servomex, UK) and Harvard Dry Gas Meter (Harvard Apparatus, Massachusetts, USA).
During all performance trials, an intravenous cannula was inserted into an antecubital vein of the non-dominant arm for the collection of blood samples. Whole blood (10ml) was collected prior to commencing each performance test (dashed arrow, Figure 1B) for the determination of fatty acid composition of blood total lipids. Fatty acids were identified with GC-MS using an adapted method of Gravina, et al. [30]. Briefly, fatty acid methyl esters (FAME) were prepared by incubating whole blood with 3.4 ml BHT-methanol standard and 200 μl of acetyl chloride at 70 ℃ for 60 minutes. The reaction was cooled and 5 ml of 6% (K2CO3) was added to stop and neutralise the reaction. n-hexane (1.5 ml) was then added and centrifuged. The washing step was repeated, and the supernatant evaporated until dry using nitrogen gas and reconstituted in 100 µl of hexane with 20 µg/ml of internal standard. The derivatisation released fatty acids from both membranes and phospholipids. A 1 µl sample was injected on the GC-MS. The individual FAMEs were identified by comparing to the retention times of a Supelco 37 Mix FAME standard (Sigma Aldrich) complemented with the MS NIST library. Fatty acids were grouped by type (saturated, monounsaturated and polyunsaturated split into n-3 and n-6) and expressed as whole blood % total fatty acids.
Statistical analysis
Results are expressed throughout as means (x) ± standard deviation (SD). All data were tested for normality of distribution using the Sharipo-Wilk test and data was all normally distributed. For the main analysis, all physiological dependent variables (HR, RPE, V̇O2, RER) were analysed using a 2 (group) × 2 (trial) × 5 (time) repeated measures analysis of variance (ANOVA). Whole blood total fatty acids and time trial mean power were analysed using a 2 (group) × 2 (trial) ANOVA. P ≤ 0.05 was used to establish statistical significance. When Mauchly's assumption of sphericity was violated, degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity. When significant changes were identified over time, Bonferroni's post hoc correction was used to determine which time points were different. Dietary and training data were analysed using a paired samples t-test. IBM SPSS Statistics version 22 (IBM Corporation, New York, USA) was used for all statistical analysis.
Results
No differences were found in participants' dietary intake for total kilocalories, total grams of protein, carbohydrate and fat between all phases of the study in the 3-day food diaries completed prior to each trial. No differences were found in mean training duration (1303 ± 899 vs. 1398 ± 617 mins), training distance covered (369 ± 270 vs. 467 ± 285 km) or sRPE (14 ± 1 vs. 13 ± 2) for n-3 PUFA trial vs olive oil trial, respectively.
Compliance as estimated from mean return capsule count was 89% for both n-3 PUFA olive oil, with no difference noted (p = 0.955). There were no differences in saturated, monounsaturated, n-6 or n-3 PUFAs expressed as % total fatty acids in whole blood, pre- n-3 PUFA or olive oil supplementation. Following 4 weeks of n-3 PUFA supplementation there was an increase in % n-3 PUFA fatty acids in the whole blood (p = 0.043, Table 1). Three participants experienced the adverse effect of fishy burps during the n-3 PUFA supplementation phase.
Participants were able to maintain a mean power output of 70% Wmax throughout all trials, this equated to an oxygen consumption of 74 ± 8% V̇O2 max. There was no difference (p = 0.07) in the mean power after taking n-3 PUFA supplementation (pre 239 ± 34 W vs. post 243 ± 33 W) compared with olive oil supplementation (pre 246 ± 38 W vs. post 235 ± 36 W) in the time trial. No difference was determined for V̇O2, HR, RER or RPE during the 45-min steady state cycling or during the 15 min time trial prior to either n-3 PUFA or olive oil supplementation. Following supplementation there remained no difference between supplements (Table 2).
Discussion
The current study found that n-3 PUFA supplementation did not affect HR, RER, RPE or V̇O2 during both submaximal cycling and maximal cycling during the time trial or time trial performance, despite a significant increase in the % n-3 PUFA incorporated into the blood in trained cyclists. Despite the potential benefits associated with n-3 supplementation for endurance athletes, the trained cyclists in the present study experienced no beneficial effects compared with olive oil. These findings are in accordance with Nieman, et al. [31] and Toft, et al. [32], who both demonstrated incorporation of n-3 PUFAs into plasma and peripheral blood mononuclear cells, respectively, but did not find an improvement in performance in trained individuals. Our results extend and confirm the conclusions of Nieman, et al. [31] and Toft, et al. [32] using a stronger research design with a placebo control, employing a double-blind crossover method and using an exercise intensity more likely to be employed in the training programmes of trained cyclists. Reductions in submaximal HR [14,15,17] and V̇O2 [11,14] found in previous n-3 PUFA supplementation studies have been shown, which would suggest an effect on performance, although this was not evidenced in the present study. A potential rationale for the lack of effect on the physiological parameters measured during submaximal exercise is that the exercise intensity employed in previous studies has generally been lower (i.e. ~ 55% peak workload) than the 70% peak workload used in the present study. Despite finding reductions in submaximal heart rate following n-3 PUFA supplementation, Macartney, et al. [17] found no differences in heart rate between conditions during repeated sprints.
Together with findings from the present study, this data suggests that n-3 PUFA supplementation is only effective at decreasing the heart rate response during submaximal exercise, not at higher exercise intensities such as the present study. It is possible that n-3 PUFA supplementation increases fat oxidation during lower intensity exercise, reducing carbohydrate oxidation [33], therefore allowing individuals to use predominantly fats for energy at a higher absolute work rate, meaning they could essentially go for longer due to the greater fat stores than carbohydrate [34] and consequently heart rate is lowered along with perceived effort. However only increases in basal fat oxidation, not fat oxidation during exercise, have been previously reported following 3 weeks of n-3 PUFA supplementation [35].
Moreover, there was no difference in the RER in the present study, suggesting fat and carbohydrate oxidation were unaffected by supplementation. The participants in the present study were trained cyclists (VO2 max = 54 ± 5 ml∙ kg∙ min-1, training age = 8 years). Therefore, it is likely that they are accustomed to the exercise intensity employed in this study. Their bodies have adapted to overcome the exercise-induced inflammation through years of training, and it is likely that a small modification, such as 4 weeks n-3 PUFA supplementation, is unable to further overcome the inflammation. In the current study, cyclists were rested (to ensure standardisation) prior to commencing the performance tests, therefore likely to have lower levels of circulating markers of oxidative stress and inflammation.
Trained cyclists may rarely be in this situation and therefore the potential benefit of n-3 PUFA on performance could be context dependent. Supplementing with n-3 PUFA is more likely to exert anti-inflammatory and immunomodulatory effects in patient populations [4]. It could therefore be argued that n-3 PUFA supplementation may have a similar effect on an untrained population as they have not been through adaptation to training. Previous studies that have investigated the use of n-3 PUFAs on trained cyclists have found a similar lack of effect on performance [7,18], suggesting n-3 PUFA supplementation should not be recommended to trained cyclists as a means of improving performance, although other potential health benefits should not be disregarded. Classic crossover design studies with extended supplementation washout periods are prohibitive, particularly in exercise studies where training over a lengthy washout period could result in improved performance, confounding the effect of the supplementation. In the present study a 4 week washout period was chosen in order to allow time for n-3 PUFA to return to baseline levels [25], whilst minimizing the impact of training effects due to study duration. Analysis of a potential order effect was also conducted, and no differences were found between the randomised conditions. Whilst the study was double-blinded, three participants did experience fishy burps which may have inadvertently indicated that they were consuming fish oil potentially unblinding them from the study.
Whilst we acknowledge a sample size of 10 is a limitation of this study, we feel this is mitigated by the randomised crossover design employed and the fact that a performance test was completed prior to any supplementation period, giving a true baseline to compare with the post-supplementation data. Previous repeated measures investigations into n-3 PUFA supplementation and cycling performance have generally given participants either n-3 PUFA or placebo supplement for a time period and then assessed the difference between groups rather than comparing to a performance test conducted prior to any supplementation [36], which provides little indication of whether the supplementation had an effect on individual performance. Alternatively, they have used an independents groups design [11,17,31,37], which does not account for individual differences in incorporation of n-3 PUFA. This is the first study to investigate n-3 PUFA supplementation and cycling performance in a repeated-measures, placebo-controlled, cross over design, and has demonstrated that 4 weeks of n-3 PUFA supplementation does not improve cycling performance or attenuate the physiological variables associated with improved cycling performance, i.e. oxygen uptake RPE and heart rate, despite a significant increase in the % n-3 PUFA incorporated into the blood.
Acknowledgments
The authors would like to thank Glenda Anderson and Fiona Simm from Loughborough University for their assistance in data collection for the study. The authors would also like to thank the cyclists who volunteered to participate in the study.
Funding Sources
The research was supported by the National Institute for Health Research (NIHR) Diet, Lifestyle & Physical Activity Biomedical Research Unit based at University Hospitals of Leicester and Loughborough University. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
References
- PC Calder (2012) The role of marine omega-3 (n-3) fatty acids in inflammatory processes, atherosclerosis and plaque stability. Mol Nutr Food Res 56: 1073-1080.
- RN Lemaitre, IB King, D Mozaffarian, et al. (2003) n-3 polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: The Cardiovascular Health Study. Am. J Clin Nutr 77: 319-325.
- RJ Goldberg, J Katz (2003) A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain 129: 210-223.
- TD Mickleborough, R.L Murray, AA Ionescu, et al. (2003) Fish oil supplementation reduces severity of exercise-induced bronchoconstriction in elite athletes. Am. J Respir Crit Care Med 168: 1181-1189.
- JM Bourre (2004) Roles of unsaturated fatty acids (especially omega-3 fatty acids) in the brain at various ages and during ageing. J Nutr Health Aging 8: 163-174.
- PC Calder (2015) Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta 1: 469-484.
- M Da Boit, I Mastalurova, G Brazaite, et al. (2015) The effect of krill oil supplementation on exercise performance and markers of immune function. PLoS One 10: e0139174.
- G Shaw, G Slater, L Burke (2016) Changes in the Supplementation Practices of Elite Australian Swimmers Over 11 Years. Int J Sport Nutr Exerc Metab 26: 565-571.
- RJ Bloomer, DE Larson, KH Fisher-Wellman, et al. (2009) Effect of eicosapentaenoic and docosahexaenoic acid on resting and exercise-induced inflammatory and oxidative stress biomarkers: A randomized, placebo controlled, cross-over study. Lipids Health Dis 8: 36.
- A Boss, V Lecoultre, C Ruffieux, et al. (2010) Combined effects of endurance training and dietary unsaturated fatty acids on physical performance, fat oxidation and insulin sensitivity. Br J Nutr 103: 1151-1159.
- F Kawabata, M Neya, K Hamazaki, et al. (2014) Supplementation with eicosapentaenoic acid-rich fish oil improves exercise economy and reduces perceived exertion during sub maximal steady state exercise in normal healthy untrained men. Biosci Biotechnol Biochem 78: 2081-2088.
- F Delodder, L Tappy, L Liaudet, et al. (2015) Incorporation and washout of n-3 PUFA after high dose intravenous and oral supplementation in healthy volunteers. Clin. Nutr 34: 400-408.
- D M Huffman, T S Altena, T P Mawhinney, et al. (2004) Effect of n-3 fatty acids on free tryptophan and exercise fatigue. Eur J Appl Physiol 92: 584-591.
- GE Peoples, PL McLennan, PRC Howe, et al. (2008) Fish oil reduces heart rate and oxygen consumption during exercise. J Cardiovasc Pharmacol 52: 540-547.
- JD Buckley, S Burgess, KJ Murphy, et al. (2009) DHA-rich fish oil lowers heart rate during sub maximal exercise in elite Australian Rules footballers. J Sci Med Sport 12: 503-507.
- T Raastad, A Hostmark, S Stromme (1997) Omega-3 fatty acid supplementation does not improve maximal aerobic power, anaerobic threshold and running performance in well-trained soccer players. Scand J Med Sci Sport 7: 25-31.
- MJ Macartney, L Hingley, MA Brown, et al. (2014) Intrinsic heart rate recovery after dynamic exercise is improved with an increased omega-3 index in healthy males. Br J Nutr 112: 1984-1992.
- GS Oostenbrug, RP Mensink, MR. Hardeman, et al. (1997) Exercise performance, red blood cell deformability, and lipid per oxidation: effects of fish oil and vitamin E. J Appl Physiol 83: 746-752.
- S Poprzecki, A Zajac, M Chalimoniuk, et al. (2009) Modification of blood antioxidant status and lipid profile in response to high-intensity endurance exercise after low doses of omega-3 polyunsaturated fatty acids supplementation in healthy volunteers. Int J Food Sci Nutr 2: 67-79.
- E Lewis, P Radonic, T Wolever, et al. (2015) 21 days of mammalian omega-3 fatty acid supplementation improves aspects of neuromuscular function and performance in male athletes compared to olive oil placebo. J Int Soc Sports Nutr 12: 28.
- L Hingley, MJ Macartney, MA Brown, et al. (2017) DHA-rich fish oil increases the omega-3 index and lowers the oxygen cost of physiologically stressful cycling in trained individuals. Int J Spor Nutr Exerc Metab 27: 335-343.
- ML Nording, J Yang, K Georgi, et al. (2013) Individual variation in lipidomic profiles of healthy subjects in response to omega-3 fatty acids. PLoS One 8: e76575.
- LM Burke, P Peeling (2018) Methodologies for investigating performance changes with supplement use. Int J Sport Nutr Exerc Metab 28: 159-169.
- F Faul, E Erdfelder, AG Lang, et al. (2007) G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39: 175-191.
- AH Metherel, JM Armstrong, AC Patterson, et al. (2009) Assessment of blood measures of n-3 polyunsaturated fatty acids with acute fish oil supplementation and washout in men and women. Prostaglandins Leukot Essent Fat Acids 81: 23-29.
- M Ristow, K Zarse, A Oberbach, et al. (2009) Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A 106: 8665-8670.
- P Gray, A Chappell, AME Jenkinson, et al. (2014) Fish oil supplementation reduces markers of oxidative stress but not muscle soreness after eccentric exercise. Int J Sport Nutr Exerc Metab 24: 206-214.
- TD Mickleborough, JA Sinex, D Platt, et al. (2015) The effects PCSO-524®, a patented marine oil lipid and omega-3 PUFA blend derived from the New Zealand green lipped mussel (Perna canaliculus), on indirect markers of muscle damage and inflammation after muscle damaging exercise in untrained men: A randomized, placebo controlled trial. J Int Soc Sports Nutr 12: 10.
- S Jannas-Vela, K Roke, S Boville, et al. (2017) Lack of effects of fish oil supplementation for 12 weeks on resting metabolic rate and substrate oxidation in healthy young men: A randomized controlled trial. PLoS One 12: 1-14.
- L Gravina, FF Brown, L Alexander, et al. (2017) n-3 fatty acid supplementation during 4 weeks of training leads to improved anaerobic endurance capacity, but not maximal strength, speed, or power in soccer players. Int J Sport Nutr Exerc Metab 27: 305-313.
- DC Nieman, DA Henson, SR McAnulty, et al. (2009) n-3 polyunsaturated fatty acids do not alter immune and inflammation measures in endurance athletes. Int J Sport Nutr Exerc Metab 19: 536-546.
- AD Toft, M Thorn, K Ostrowski, et al. (2000) N-3 polyunsaturated fatty acids do not affect cytokine response to strenuous exercise. J Appl Physiol 89: 2401-2406.
- J Delarue, C Couet, R Cohen, et al. (1996) Effects of fish oil on metabolic responses to oral fructose and glucose loads in healthy humans. Am J Physiol 270: E353-E362.
- J Achten, AE Jeukendrup (2004) Optimizing fat oxidation through exercise and diet. Nutrition 20: 716-727.
- C Couet, J Delarue, P Ritz, et al. (1997) Effect of dietary fish oil on body fat mass and basal fat oxidation in healthy adults. Int J Obes Relat Metab Disord 21: 637-643.
- J Delarue, F Labarthe, R Cohen (2003) Fish-oil supplementation reduces stimulation of plasma glucose fluxes during exercise in untrained males. Br J Nutr 90: 777-786.
- EJH Lewis, F Stucky, PW Radonic, et al. (2017) Neuromuscular adaptations to sprint interval training and the effect of mammalian omega-3 fatty acid supplementation. Eur J Appl Physiol 117: 469-482.
Corresponding Author
Lynsey S James, School of Sport, Health and Exercise Science, Loughborough University, Epinal Way, Loughborough, LE11 3TU, England.
Copyright
© 2020 James LS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.