Physiological Adaptations of Apnea-Conditioned Athletes and their Implications for Synchronized Swimmers' Performance
Abstract
Introduction
Apneas form an essential tool for training in aquatics including swimming, fin swimming and synchronized swimming (SS). We examine the direct responses and the long-term physiological adaptations to acute apnea and apnea training respectively in order to determine their potential impact on SS athletes' performance.
Evidence acquisition
A literature review was conducted using as keywords apnea, hypoxia, diving response and synchronized swimming.
Evidence synthesis
Apnea-trained individuals demonstrate more effective responses to acute hypoxia compared to controls including stronger diving response and other hematological variables. The latter involves an elevated hematocrit and hemoglobin and reduced blood acidosis. Long-term physiological adaptations to apnea training largely in divers include bigger lungs, stronger respiratory muscles and increased blood circulation in the brain possibly accountable for their superior reaction to hypoxic conditions.
Conclusions
Physiological responses to acute apnea and apnea training may favourably influence SS athletes' performance. Apnea adaptations are manifested not only in improved tolerance to hypoxia and oxygen conservation but also, in delayed blood acidosis. Attempts to illuminate apnea-conditioned athletes' physiological adaptations to hypoxia may refer to the trigeminovagal reflexes e.g., the diving response and to the cold shock response. Arguably, these interrelated yet not elucidated reflexes are activated with apnea and cold water immersion. As apnea-training adaptations resemble altitude acclimatisation, future research may compare the impact of the exposure between these two conditions on athletes' performance. Finally, attention should be drawn to the reduced chemo-sensitivity to hypoxia found in apnea-conditioned individuals as it might be life threatening.
Keywords
Apnea, Physiological adaptations, Synchronized swimming, Diving response
Introduction
Synchronized swimming (SS) is an Olympic sport that encompasses swimming, dancing and gymnastics combined with repeated water submersions. SS programs are performed in solos, duets, trios, combos or in teams of men and women athletes. SS demands excellent swimming and sport-specific skills e.g. sculling, aerobic endurance and anaerobic power and strength, extraordinary flexibility and agility [1-3]. Performance is scored equally for technical and artistic skills by a panel of judges [4].
SS athletes' ability to control their breath while in motion to perform routines and figures is vital for achieving high scores. Figures are specific lifts, throw and move while routines are divided into (a) technical and (b) free. Technical routines are compulsory rudiments executed in a specific order though; "free routines" are open to athletes' creativity. Routine duration increases with the number of competitors but it is also determined by their age and skill level. It ranges from 2.5 min to 5 min i.e., minimum duration for solos and maximum for teams, combos etc. (International Olympic Committee). Routine structure varies in terms of its duration, apnea episodes and the degree of immersion using different body positions. The inverted vertical position accounts for approximately 43% of total routine time whereas, apnea sessions last on average 21 s [5].
Undoubtedly SS athletes' progress is heavily dependent on their water skills coupled with apnea training. Therefore, both apneas and swimming drills form the backbone of their daily training programs.
We analyze the impact of acute apneas and apnea training via the examination of individuals' short-term responses and long-term physiological adaptations to conditions of hypoxia (Figure 1).
As both apnea and hypoxia might lead to loss of consciousness [6], we hypothesize that adjustments to hypoxic conditions might involve risks. Understanding the mechanisms concerning apnea adaptations should ensure safe training [7,8]. The overarching aim of this review is to establish how apnea and hypoxia training can safely improve SS athletes' performance.
Evidence Acquisition
This review is based on scientific articles in Sport Discus, Google Scholar and PubMed electronic search engines using as keywords apnea, hypoxia, diving response and synchronized swimming.
Evidence Synthesis
Apnea and hypoxia
Apnea relates to breath control causing retention of carbon dioxide (CO2) within the body and an increase of its partial pressure (PCO2) in the alveoli faster than in the venous blood [9]. Peripheral chemo-receptors in carotid and aortic bodies subsequently release afferent messages to the brainstem respiratory centre to restore oxygen (O2) homeostasis [10]. Triggers would be (a) the fall in the oxygen's partial pressure (PO2) and/or (b) an increase in hydrogen ions (H+) caused by the increased CO2 in the blood [11]. Apnea terminates in the "struggle phase" where respiration restarts involuntarily after respiratory muscles' contraction [12-14]. Interestingly, psychological factors may also determine individual's tolerance to an increased breathing drive [14]. Increased apnea duration can be achieved by either delaying physiological conditions leading to the breakpoint and/or increasing individuals' tolerance to such conditions [15].
Apnea characteristics resemble conditions of hypoxia experienced in high altitudes. At sea level, barometric pressure is 760 mmHg. As oxygen constitutes 20.93% of the atmospheric air, its partial pressure (PO2) is ~ 159 mmHg. Although the association between partial pressures of air gases remains stable, at high altitudes, their absolute values are reduced. Boyle's Law states: "in stable temperature, gas volumes are inversely related to their pressure". Therefore, at high altitude, gas volumes increase due to the fall in the barometric pressure, e.g., at mountain Everest-9000 m it is ~ 250 mmHg and accordingly, PO2 equals to one third of its value at sea level [16]. The reduced barometric pressure is translated for human respiration as a diminished PO2 in the alveoli and reduced oxygen saturation in the arterial blood [17]. Semenza [18], proposes that apnea relates to oxygen deficiency in the blood and tissues irrespective of changes in the barometric pressure as opposed to Millet, et al. [19]. We adopt Semenza's terminology of apnea as more generic.
Trained divers demonstrated increased tolerance to lower PO2 and a corresponding higher PCO2 in their arterial blood as opposed to non-divers i.e., 35 mmHg-50 mmHg and 60 mmHg-45 mmHg respectively [20]. These results along with additional longer-term adaptations to apnea are systematically discussed in the sections that follow. Furthermore, conditions of apnea-hypoxia cause a rise in PCO2 and a corresponding fall in pH, i.e., characteristics of the diving reflex or diving response-a more generic term including several reflexes as discussed below.
Apnea and the diving response
The Diving Response (DR) is intrinsically linked with apnea, particularly when accompanied with face immersion in cold water [21]. It is characterized by bradycardia and increased peripheral vasoconstriction. It is found in all mammals but also in birds and constitutes a survival mechanism against hypoxia [22]. Factors such as face immersion or otherwise [23], depth of water immersion and body position [24], water and environmental temperatures and fitness level [9] might be influencing DR's intensity. Individual's age may have a negative impact on the intensity of bradycardia possibly due to the reduced contraction ability of blood vessels [9]. Although, older individuals demonstrated remarkable free diving results, these might be attributed to their technical skills and experience [8]. Lung volumes and consequently breath-holding capacity may also be relevant in this context [24]. Empirical evidence confirms the predictive ability of "hypoxic ventilatory response" to the maximal breath-hold [25].
DR is the result of a complex neural network integration of respiratory and cardiovascular system controls [21]. It is arguably linked to the trigeminocardiac reflex characterised as peripheral or a sub-form of the latter [26]. Like DR, this also survival mechanism against hypoxia characterised by increases in the cerebral blood flow providing brain with oxygen in an efficient and effective way. Arguably, the triggering of the DR may cause a fatal biphasic drop in the heart rate [27]. At the moment, it appears that central stimulation of trigeminocardiac reflex is accompanied with hypotension whereas a peripheral stimulation as with DR evidences a gradual increase of mean arterial blood pressure. Elucidating on both trigeminocardiac reflex and DR might be important considering the strong response that elite breath-hold divers develop on the latter [26].
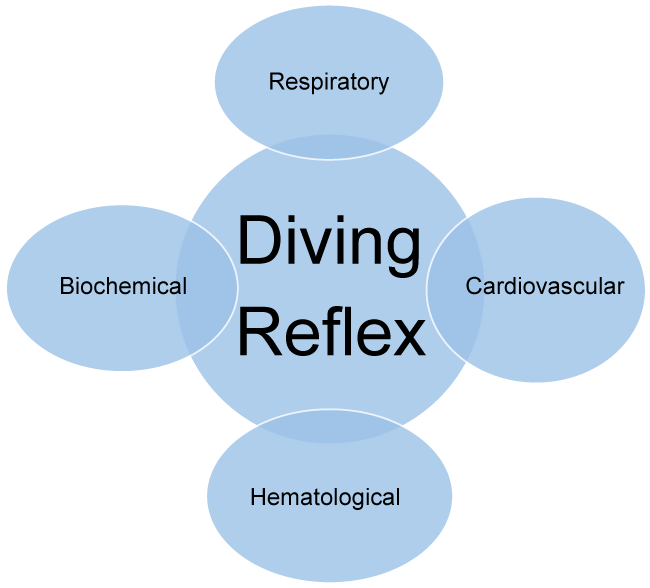
Despite that the physiological responses of the DR are strongly interrelated, to facilitate discussion, we categorize those into the following distinct sections (Figure 2).
The diving response and respiration
DR is presumed an oxygen conservation mechanism protecting humans from conditions of hypoxia [28]. The redirection of blood flow from periphery to our vital organs is an indisputable proof of this allegation [29]. The rise of the central blood flow might be attributed to the rise in PETCO2 and a corresponding fall in the PETO2; but also to the simultaneous increase in arterial blood pressure [30]. Bradycardia may be the result of a rise in the total peripheral resistance followed by a blood accumulation in the aorta and the arteries which in turn might be activating baroreceptors leading to a reduced venous return [31].
Researchers initially noticed a direct relationship between DR and a lower rate of fall in the arterial blood oxygen saturation [28,32,33] but, adjusted experimental designs revealed a causal relationship between these two parameters [34,35]. Apnea with face immersion leads to greater oxygen conservation via a more intense DR and a higher oxygen saturation [23,36]. Under conditions of dynamic apnea-cycling on an ergometer-an increased anaerobiosis and a greater fall of venous oxygen led to an oxygen conservation mechanism [33]. This mechanism delays the fall of the PO2 both in the alveoli and in the arterial blood and it consequently delays hypoxia to vital organs. The percentage of oxygen saturation confirmed the activation of the DR in these experiments.
Although divers and underwater hockey players exhibited reduced chemo-sensitivity under conditions of hypoxia, results are inconclusive concerning hypoxic adaptations to their respiratory drive [37,38].
The diving response and cardiovascular variables
Bradycardia and an elevated arterial blood pressure form DR's primary characteristics [23,39-42]. Heart rate changes due to the DR appear far more intense compared to those in the arterial blood pressure [37,43] although contradictory evidence exists [44]. Increases in the arterial blood pressure are manifested only in humans and not in aquatic mammals [21]. DR causes arterial blood pressure increases as high as 280 mmHg and 200 mmHg for systolic and diastolic respectively [41]. However, contradictory evidence exists concerning arterial blood pressure during water submersion partly due to the water temperature per se [9] and partly possibly due to methodological differences [45]. For example, Ferrigno, et al. [41] measured changes in arterial blood pressure using invasive methods during simulated dives of 10 m in hyperbaric chamber whereas Sieber, et al. [46] used non-invasive blood pressure measurement methods during actual dives of ≥ 10 m. It should be noted also that "pulse pressure" i.e., the difference between systolic and diastolic pressure depends on stroke volume and its ejection speed, but also on arterial compliance [10]. Although the first two factors may be influenced by DR, arterial compliance is a characteristic of each individual and particularly it relates to his/her state of health e.g., atherosclerosis.
Breath-hold divers exhibited a drop in their heart rate of 20-30 beats·min-1 [41] and a reduction in the limb blood flow due to peripheral vasoconstriction [44,47].
Low water temperature is inversely related to the intensity of the DR particularly for fit individuals [48,49]. However, the "Cold Shock like Response" might overshadow the DR when the water is below 10 °C [48,50]. The "Cold Shock like Response" and/or the "cold shock response" cause an increase in both heart rate and blood arterial pressure via the arousal of the sympathetic nervous system [51]. Arguably, the simultaneous activation of both "Cold Shock like Response" and DR might lead to an "autonomic conflict" [52,53]. Others suggest a synergistic effect on the parallel activation of these two systems leading to an "oxygen conservation breaking point" [54].
Dry apnea i.e., apnea in the air as opposed to wet apnea-apnea with face immersion-causes only minor [55] or no bradycardia [44]. On the contrary, wet apnea leads to maximum bradycardia particularly when the water is cold [56]. Environmental temperature is positively related to the intensity of the DR particularly when accompanied with face immersion in cold water [9,48]. Lung volumes and intrapleural pressure might also be influencing the degree of bradycardia [57].
Dynamic exercise causes more intense DR as opposed to isometric exercise or rest [40,58,59] although there also exist inconclusive results [60,61]. Jay, et al. [50], attributed DR's cardiovascular shifts to the cold shock response, by demonstrating that the lower the water temperature-for face immersion-the higher the heart rate response and the lower the apneic time. Short intensive exercise followed by face immersion in cold water (10 ℃) led to a reduction of apneic time by 75% compared to apnea at rest [62]. This might be due to competing needs for an increased blood flow between muscles and vital organs resulting in: a) an abrupt cessation of apnea, and b) an exaggerated rise of systolic blood pressure.
The diving response and biochemical variables
The DR is directly related to the rise of anaerobiosis and the consequent increase of lactic acid and a fall in pH [23,33,34]. Individuals' familiarity with conditions of apnea such as divers is confirmed through an augmented DR [59,63]. Elite divers demonstrate higher anaerobic metabolism during DR activation as opposed to controls [20]. However, trained divers also exhibit lower oxidative stress after dynamic apnea as opposed to controls [58]. Divers' and endurance athletes' physiological adjustments to apnea appear to lead to lower acidosis i.e., plasma lactate analogous to altitude acclimatization [58,59].
The diving response and hematological variables
The activation of the DR leads to a temporary rise in erythropoietin resulting in an elevated hematocrit and hemoglobin [23,64-69]. These in turn cause an improved apnea duration through the increased number of red cells circulating in the blood [65,70]. Hematological changes are caused by spleen contraction as a response to apnea and the consequent rise of erythropoietin. Spleen contraction is confirmed via ultrasonic measurements of spleen volume [39] or radionuclide measurements [65]. It should be noted that splenectomized individuals did not demonstrate similar results [64,70]. Further, plasma protein concentration did not change suggesting that the elevation of hematocrit and hemoglobin are not artificial [70]. De Bruijn, et al. [64] demonstrated a 24% (p < 0.01) rise in erythropoietin in their apnea experiments [66,68]. Arguably, spleen contraction can be trained as in repeated apneas it becomes faster whereas recovery slows down [39,69]. Finally, the hematological responses as described above may be linked to apnea per se and not necessarily to the diving response [23].
Table 1 presents an indicative list of studies on the physiological responses to "dry apnea" and "wet apnea" during exercise and/or at rest for various groups of individuals:
Apnea-trained individuals demonstrate stronger tolerance to conditions of hypoxia via a more intense DR [71-73]. Table 2 summarizes physiological responses to apneas for individuals not accustomed to hypoxic conditions after following apnea training programs:
Below we examine studies on SS athletes and the physiological changes brought in by apnea training.
Synchronized swimming athletes' adaptations to apnea
SS athletes exhibited physiological adaptations during apnea sessions similar to those of professional divers such as longer apnea durations, more intense bradycardia and greater oxygen conservation compared to controls [4,74]. DR was more intense in SS athletes during static and dynamic apneas vs. controls [75]. There were no differences between the two groups on respiratory variables such as oxygen consumption, oxygen saturation etc. at the end of apneas [74]. During a static apnea of 50 seconds, in a reverse body position, SS athletes exhibited a remarkable fall in heart rate from 98 ± 14 bpm to 70 ± 7 bpm [76]. It should be noted that, SS athletes exhibited an anticipatory tachycardia before competition followed by a bradycardic response both of which are evidenced on the variability of their performance [77-79].
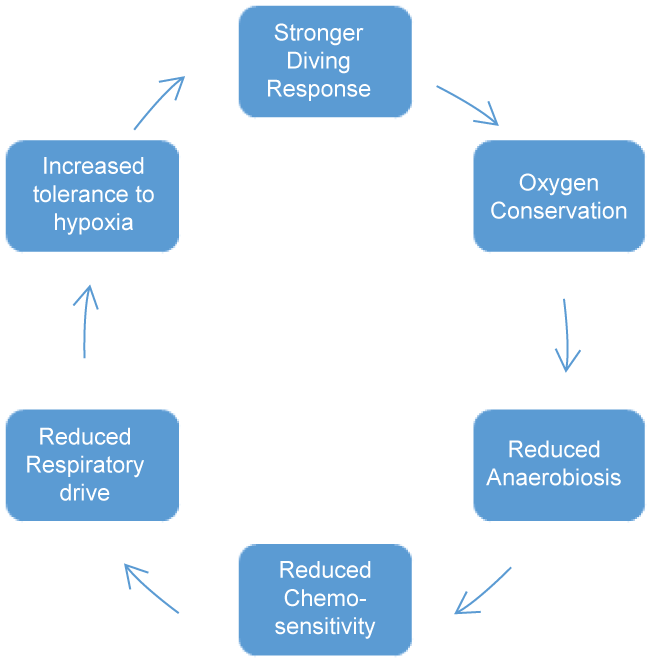
Researchers advocate that, SS athletes had augmented lung volumes and vital capacity compared to controls. Further, their respiratory response to hypoxia was lower [4]. However, hypercapnic response i.e., ventilation in liters per minute to alveolar PCO2 was similar for both groups. Naranjo, et al. [80], stress the importance of functional respiratory adaptations induced by apnea for boosting SS athletes' performance. Consequently in the long-term apnea training may favourably alter the DR as follows: (Figure 3).
Discussion
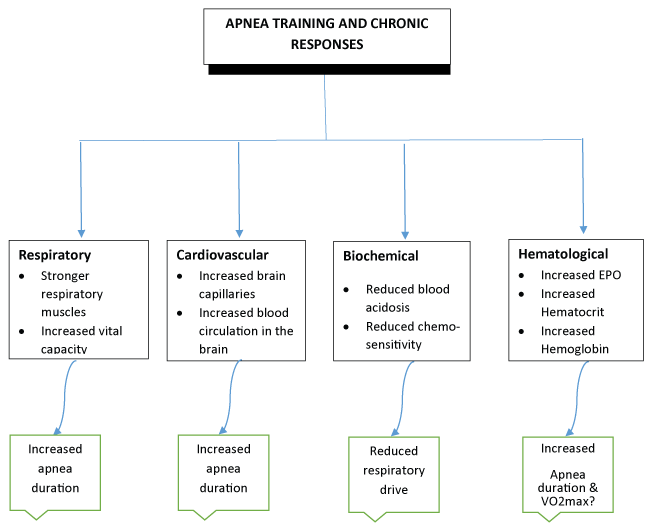
Trained divers have bigger lungs and stronger respiratory muscles compared to controls. We may hypothesize that apnea training delays respiratory muscles' fatigue during prolonged and peak exercise [81]. Similar respiratory adaptations were confirmed for swimmers including improved lung elasticity leading to an improved forced vital capacity [82]. Elite divers develop protective mechanisms against hypoxia in conjunction with mechanisms that reduce total oxygen consumption [73]. Prolonged exposure to hypobaric hypoxia is found to cause an increase in brain capillaries improving tolerance to hypoxia [83]. Divers exhibit more intense DR and a higher blood circulation in their brain during apneas [71]. Individuals involved with breath holding activities demonstrate a reduced chemo-sensitivity to hypoxia and hypercapnia leading to a reduced respiratory drive and corresponding increases in cardiovascular responses. These lead to longer breath holding times [21]. Undoubtedly, the aforementioned functional adaptations characterizing divers would have a positive impact on SS athletes' performance during competitive events.
In brief, empirical evidence suggests that apnea training causes increases in lung volumes and vital capacity and improves the performance of respiratory muscles by delaying fatigue during prolonged intensive exercise. It also delays blood acidosis during static and/or dynamic apneas but it also increases tolerance to hypoxia. Individuals' trained in apneas exhibit longer apnea durations, elevated DR responses and arguably a rise in their maximum oxygen consumption (VO2max) i.e., their aerobic capacity. However, hypoxic compared to normoxic training in swimmers did not enhance their aerobic or anaerobic ability [84].
An oxygen conservation effect during apneas is evident through a reduced arterial oxygen desaturation coupled with a reduced rate of oxygen store depletion in the lungs [34,35,85]. Further, an elevated haematocrit, haemoglobin and erythropoietin have been confirmed in apnea trained individuals [23,64,86]. These characteristics found in trained divers also appear in SS athletes. The physiological adaptations to apnea are extremely valuable for SS athletes performing repeated and occasionally prolonged dynamic apneas during competitive events. Arguably, half of the time during a competitive event is underwater [87]. Therefore, long apnea duration, tolerance to hypoxia, delayed blood acidosis coupled with increased lung volumes and vital capacity are essential for their successful performance. Synchronized swimming requires aerobic and anaerobic power [88] in conjunction with extraordinary breath control [89]. Therefore, an exaggerated DR combined with structural lung adaptations are essential for succeeding in competitions.
Rodriguez-Zamora, et al. [78], highlight the impact of psychological factors in SS athletes' performance and particularly the anticipatory tachycardia due to their perceived intensity of exercise demands. They suggest an interrelationship exists between psychological factors and the physiological stress that SS athletes undergo during competitive events. An anticipatory tachycardia was evidenced as a response to specific routines to be performed during training or competition according to their perceived difficulty (RPE) [79]. Therefore arguably, this stress-related anticipatory tachycardia may blunt athletes' habituation to hypoxia on their performance in SS competitions.
In brief, apnea-conditioned individuals, exhibit the following characteristics that undoubtedly account for their increased tolerance to hypoxia and possibly their enhanced aerobic and anaerobic power. (Figure 4).
Concerning the possible dangers involved in apnea training, one relates to extreme breath-hold divers' reduced ventilatory response to breathing CO2 due to their frequent exposure to high PCO2 [90]. However, is the alleged reduced chemo-sensitivity to hypoxia and hypercapnia that characterizes divers and other apnea-trained athletes unsafe [15,21,38]? According to Grassi, et al. [91], divers exhibit a diminished ventilatory response only to hypercapnia but not to hypoxia. Further, as the former might not represent an adaptive mechanism but an inherited characteristic [92], thus, it should not raise any issues of concern. On the contrary, what could be life threatening is the commonly used hyperventilation prior to apnea and it should be applied with caution [6,15].
Conclusions
Apnea induces spleen contraction and a rise in hematocrit and hemoglobin lasting upto ten minutes [65,68,69], sufficient for SS athletes to successfully perform their competition program. In essence, repeated apnea sessions before competition would be a legitimate and a safe way to boost performance [93]. In the long-term, apnea training not only increases its duration but it also boosts tolerance to hypoxia [71]. Apnea training reduces blood acidosis and oxidative stress, factors that may clearly influence SS athletes' performance [58,59]. Allegedly, apnea training may also increase VO2max, i.e., individuals' aerobic capacity [73].
In conclusion, apnea training causes physiological adaptations allowing individuals not only to exert an improved breath control but also, to cope with conditions of hypoxia in an effective and efficient manner. These adaptations may be described as aquadicity [94]. They are manifested in an elevated DR coupled with other structural and functional changes such as increased lung volumes and vital capacity. SS athletes' ability to control their breath definitely affects both their functional performance and their artistry. Caution should be taken as apnea training arguably reduces the chemically initiated drive to breathe [95]. Further research should meticulously examine the implications of this suspected respiratory adaptation.
Postscript
This review highlighted the differences in apnea responses between non-apnea trained individuals and athletes familiar with hypoxic conditions. It also identified the similarities among apnea-trained athletes. The underlying mechanisms of these differences/similarities are yet not fully understood although researchers attribute those to the diving response and hypoxia conditioning. Others claim that there is no discernible interrelationship between respiration and hemodynamic variables under various conditions of breathing-apnea [96]. Future research should clarify those physiological mechanisms that lead to altered responses to hypoxia between apnea-trained and non-apnea trained individuals. The purpose of such research should be not only with a view of boosting athletes' performance but foremost to ensure their safety. A robust theoretical framework is required to guide research but also, to render empirical evidence comparable and most importantly, to facilitate its interpretation. So far, three related yet not fully understood reflexes have been identified in this context namely, the diving response, the cold shock response and the trigeminocardiac reflex. Research may attempt to clarify these reflexes, their interrelationships including their similarities and differences. Finally, as apnea-training adaptations resemble altitude acclimatisation future research may compare the impact of the exposure to these two conditions on athletes' performance.
References
- Bante S, Bogdanis GC, Chairopoulou C, et al. (2007) Cardiorespiratory and metabolic responses to a simulated synchronized swimming routine in senior (>18 years) and comen (13-15 years) national level athletes. J Sports Med Phys Fitness 47: 291-299.
- Labudová J (2014) Motor factors of sport performance in synchronized swimming of younger competitors. Comenius University in Bratislava.
- Mountjoy M (2009) Injuries and medical issues in synchronized Olympic sports. Curr Sports Med Rep 8: 255-261.
- Bjurstrom RL, Schoene RB (1987) Control of ventilation in elite synchronized swimmers. J Appl Physiol (1985) 63: 1019-1024.
- Iglesias X, Rodríguez-Zamora L, Clapés P, et al. (2014) Multidimensional analysis of the structure of competitive routines in synchronized swimming. Revista DE Psicologia Del Deporte 23: 173-180.
- Davies BN, Donaldson GC, Joels N (1995) Do the competition rules of synchronized swimming encourage undesirable levels of hypoxia? Br J Sports Med 29: 16-19.
- Greco M, Quaranta B (1996) Heart rate variations in response to apnea during synchronized swimming. Part I. Med Sport 49: 261-269.
- Ostrowsksi A, Strzala M, Stanula A, et al. (2012) The role of training in the development of adaptive mechanisms in freedivers. J Hum Kinet 32: 197-210.
- Geladas Ν (2008) Free diving: the physiology of apnea. Medical Publishers P.X. Paschalides, Athens.
- Vander A, Sherman J, Luciano D (2001) Human physiology: The mechanisms of body function. (8th edn), Medical Publishers P.X. Paschalides, Athens.
- Prabhakar NR, Peng Y (2004) Peripheral chemoreceptors in health and disease. J Appl Physiol 96: 359-366.
- Agostoni E (1963) Diaphragm activity during breath holding: factors related to its onset. J Appl Physiol 18: 30-36.
- Courteix D, Bedu M, Fellmann N, et al. (1993) Chemical and nonchemical stimuli during breath holding in divers are not independent. J Appl Physiol (1985) 75: 2022-2027.
- Lin YC, Lally DA, Moore TO, et al. (1974) Physiological and conventional breath-hold breaking points. J Appl Physiol 37: 291-296.
- Pollock NW (2008) Breath-hold diving: performance and safety. Diving Hypob Med 38: 79-86.
- Kleisouras V (2007) Exercise Physiology (Ι). P.X. Paschalides Publishing, Athens, 382-383.
- West JB (1984) Human physiology at extreme altitudes on Mount Everest. Science 223: 784-788.
- Semenza GL (2009) Regulation of oxygen homeostasis by hypoxia-inducible factor. Physiology (Bethesda) 24: 97-106.
- Millet GP, Faiss R, Pialoux V (2012) Point: Hypobaric hypoxia induces different responses than normobaric hypoxia. J Appl Physiol (1985) 112: 1783-1784.
- Ferretti G (2001) Extreme human breath-hold diving. Eur J Appl Physiol 84: 254-271.
- Foster GE, Sheel AW (2005) The human diving response, its function, and its control. Scand J Med Sci Sports 15: 3-12.
- Alboni P, Alboni M, Gianfranchi L (2011) Diving bradycardia: a mechanism of defence against hypoxic damage. J Cardiovasc Med 12: 422-427.
- Schagatay E, Andersson JP, Nielsen B (2007) Haematological response and diving response during apnea and apnea with face immersion. Eur J Appl Physiol 101: 125-132.
- Manley L (1990) Apnoeic heart rate responses in humans. Sports Med 9: 286-310.
- Feiner JR, Bickler PE, Severinghaus JW (1995) Hypoxic ventilatory response predicts the extent of maximal breath-holds in man. Respir Physiol 100: 213-222.
- Lemaitre F, Chowdhury T, Schaller B (2015) The trigeminocardiac reflex-a comparison with the diving reflex in humans. Arch Med Sci 2: 419-426.
- Wolf S (1965) The Bradycardia of the Dive Reflex-A Possible Mechanism of Sudden Death. Trans Am Clin Climatol Assoc 76: 192-200.
- Lindholm P, Sundbland P, Linnarsson D (1999) Oxygen-conserving effects of apnea in exercising men. J Appl Physiol 87: 2122-2127.
- Brown CM, Sanya EO, Hilz MJ (2003) Effect of cold stimulation on cerebral blood flow in humans. Brain Res Bull 61: 81-86.
- Jiang ZL, Yamaguchi H, Tanaka H, et al. (1994) Blood flow velocity in common carotid artery in humans during breath-holding and face immersion. Aviat Space Environ Med 65: 936-943.
- Pan AW, He J, Kinouchi Y, et al. (1997) Blood flow in the carotid artery during breath-holding in relation to diving bradycardia. Eur J Appl Physiol Occup Physiol 73: 388-395.
- Andersson J, Schagatay E (1998) Arterial oxygen desaturation during apnea in human. Undersea Hyperb Med 25: 21-25.
- Andersson JP, Liner MH, Runow E, et al. (2002) Diving response and arterial oxygen saturation during apnea and exercise in breath-hold divers. J Appl Physiol (1985) 93: 882-886.
- Andersson JP, Liner MH, Fredsted A, et al. (2004) Cardiovascular and respiratory responses to apnea with and without face immersion in exercising humans. J Appl Physiol (1985) 96: 1005-1010.
- Lindholm P, Nordh J, Linnarsson D (2002) Role of hypoxemia for cardiovascular responses to apnea during exercise. Am J Physiol Regul Integr Comp Physiol 283: 1227-1235.
- Andersson JP, Evaggelidis L (2009) Arterial oxygen saturation and diving response during dynamic apneas in breath-hold divers. Scand J Med Sci Sports 19: 87-91.
- Breskovic T, Valic Z, Lipp A, et al. (2010) Peripheral chemoreflex regulation of sympathetic vasomotor tone in apnea divers. Clin Auton Res 20: 57-63.
- Dujic Z, Breskovic T, Bakovic D (2013) Breath-hold diving as a brain survival response. Transl Neurosci 4: 302-313.
- Bakovic D, Valic Z, Eterovic D, et al. (2003) Spleen volume and blood flow response to repeated breath-hold apneas. J Appl Physiol (1985) 95: 1460-1466.
- Bergman SA Jr, Campbell JK, Wildenthal K (1972) "Diving reflex" in man: Its relation to isometric & dynamic exercise. J Appl Physiol 33: 27-31.
- Ferrigno M, Ferretti G, Ellis A, et al. (1997) Cardiovascular changes during deep breath-hold dives in a pressure chamber. J Appl Physiol (1985) 83: 1282-1290.
- Tocco F, Crisafulli A, Melis F, et al. (2012) Cardiovascular adjustments in breath-hold diving : comparison between divers and non-divers in simulated dynamic apnoea. Eur J Appl Physiol 112: 543-554.
- Jaja SI, Etemire E (2006) Blood pressure and electrocardiographic changes during face immersion in water in young men on regular exercise. NQJHM 16: 1-5.
- Heusser K, Dzamonja G, Tank J, et al. (2009) Cardiovascular regulation during apnea in elite divers. Hypertension 53: 719-724.
- Breskovic T, Uglesic L, Zubin P, et al. (2011) Cardiovascular changes during underwater static and dynamic breath-hold dives in trained divers. J Appl Physiol (1985) 111: 673-678.
- Sieber A, L'Abbate A, Passera M, et al. (2009) Underwater study of arterial blood pressure in breath-hold divers. J Appl Physiol (1985) 107: 1526-1531.
- Gooden BA (1994) Mechanism of the human diving response. Integr Physiol Behav Sci 29: 6-16.
- Schagatay Ε, Holm Β (1996) Effects of water and ambient air temperatures on human diving bradycardia. Eur J Appl Physiol Occup Physiol 73: 1-6.
- Speck DF, Bruce DS (1978) Effects of varying thermal and apnoeic conditions on the human diving reflex. Undersea Biomed Res 5: 9-14.
- Jay O, Christensen JP, White MD (2007) Human face-only immersion in cold water reduces maximal apnoeic times and stimulates ventilation. Exp Physiol 92: 197-206.
- Tipton MJ (1989) The initial responses to cold-water immersion in man. Clin Sci (Lond) 77: 581-588.
- Alboni P, Alboni M, Gianfranchi L (2011) Simultaneous occurrence of two independent vagal reflexes: a possible cause of vagal sudden death. Heart 97: 623-625.
- Shattock MJ, Tipton MJ (2012) Autonomic conflict: a different way to die during cold water immersion? J Physiol 590: 3219-3230.
- Costalat G, Pichon A, Joulin F, et al. (2015) Modeling the diving bradycardia: toward an "oxygen-conserving breaking point"? Eur J Appl Physiol 115: 1475-1484.
- Lundgren CEG, Ferrigno M (1985) Physiology of breath-hold diving. 31st Undersea and Hyperbaric Medical Society Workshop. Undersea and Hyperbaric Medical Society.
- Costalat G, Coquart J, Castres I, et al. (2013) Hemodynamic adjustments during breath-holding in trained divers. Eur J Appl Physiol 113: 2523-2529.
- Song SH, Lee YK, Chung YA, et al. (1969) Mechanism of apneic bradycardia in man. J Appl Physiol 27: 323-327.
- Joulia F, Steinberg JG, Wolff F, et al. (2002) Reduced oxidative stress and blood lactic acidosis in trained breath-hold human divers. Respir Physiol Neurobiol 133: 121-130.
- Joulia F, Steinberg JG, Faucher M, et al. (2003) Breath-hold training of humans reduces oxidative stress and blood acidosis after static and dynamic apnea. Respir Physiol Neurobiol 137: 19-27.
- Kiviniemi AM, Breskovic T, Uglesic L, et al. (2012) Heart rate variability during static and dynamic breath-hold dives in elite divers. Auton Neurosci 169: 95-101.
- Tocco F, Marongiu E, Pinna M, et al. (2013) Assessment of circulatory adjustments during underwater apnoea in elite divers by means of a portable device. Acta Physiol (Oxf) 207: 290-298.
- Konstantinidou S, Soultanakis E (2016) Cardiorespiratory responses and reduced apneic time to cold water face immersion after high intensity exercise. Respir Physiol Neurobiol 220: 33-39.
- Schagatay Ε, van Kampen Μ, Emanuelsson S, et al. (2000) Effects of physical and apnea training on apneic time and the diving response in humans. Eur J Appl Physiol 82: 161-169.
- de Bruijn R, Richardson M, Schagatay E (2008) Increased erythropoietin concentration after repeated apnoeas in humans. Eur J Appl Physiol 102: 609-613.
- Espersen K, Frandsen H, Lorentzen T, et al. (2002) The human spleen as an erythrocyte reservoir in diving-related interventions. J Appl Physiol (1985) 92: 2071-2079.
- Hurford WE, Hong SK, Park YS, et al. (1990) Splenic contraction during breath-hold diving in the Korean ama. J Appl Physiol (1985) 69: 932-936.
- Palada Ι, Eterovic Ι, Obad Α, et al. (2007) Spleen and cardiovascular function during short apneas in divers. J Appl Physiol 103: 1958-1963.
- Richardson M, de Bruijn R, Holmberg HC, et al. (2005) Increase of hemoglobin concentration after maximal apneas in divers, skiers, and untrained humans. Can J Appl Physiol 30: 276-281.
- Schagatay E, Haughey H, Reimers J (2005) Speed of spleen volume changes evoked by serial apneas. Eur J Appl Physiol 93: 447-452.
- Schagatay EK, Andersson JP, Halle M, et al. (2001) Physiological and genomic consequences of intermittent hypoxia-selected contribution: Role of spleen emptying in prolonging apnoeas in humans. J Appl Physiol 90: 1623-1629.
- Joulia F, Lemaitre F, Fontanari P, et al. (2009) Circulatory effects of apnoea in elite breath-hold divers. Acta Physiol (Oxf) 197: 75-82.
- Lemaitre F, Polin D, Joulia F, et al. (2007) Physiological responses to repeated apneas in underwater hockey players and controls. Undersea Hyperb Med 34: 407-414.
- Lemaitre F, Seifert L, Polin D, et al. (2009) Apnea training effects on swimming coordination. J Strength Cond Res 23: 1909-1914.
- Alentejano TC, Marshall DA, Bell GJ (2010) Breath Holding with Water Immersion in Synchronized Swimmers and Untrained Women. Res Sports Med 18: 97-114.
- Alentejano TC, Bell GJ, Marshall DA (2012) Comparison of the Physiological Responses to Underwater Arm Cranking and Breath Holding Between Synchronized Swimmers and Breath Holding Untrained Women. J Hum Kinet 32: 147-156.
- Figura F, Cama G, Guidetti L (1993) Heart rate, alveolar gases and blood lactate during synchronized swimming. J Sports Sci 11: 103-107.
- Rodríguez-Zamora L, Iglesias X, Barrero A, et al. (2012) Physiological responses in relation to performance during competition in elite synchronized swimmers. PLoS One 7: e49098.
- Rodríguez-Zamora L, Iglesias X, Barrero A, et al. (2014) Perceived Exertion, Time of Immersion and Physiological Correlates in Synchronized Swimming. Int J Sports Med 35: 403-411.
- Rodríguez-Zamora L, Iglesias X, Barrero A, et al. (2014) Monitoring internal load parameters during competitive synchronized swimming duet routines in elite athletes. J Strength Cond Res 28: 742-751.
- Naranjo J, Centeno RA, Carranza MD, et al. (2006) A test for evaluation of exercise with apneic episodes in synchronized swimming. Int J Sports Med 27: 1000-1004.
- Nygren-Bonnier M, Gullstrand L, Klefbeck B, et al. (2007) Effects of glossopharyngeal pistoning for lung insufflation in elite swimmers. Med Sci Sports Exerc 39: 836-841.
- Basavaraj R, Satish M, Noor Jehan Begaum, et al. (2014) A Study on Pulmonary Functions among Swimmers-A Descriptive Study. J Evolut Med Dental Sci 3: 2680-2686.
- Chavez JC, Agani F, Pichiule P, et al. (2000) Expression of hypoxia-inducible factor-1alpha in the brain of rats during chronic hypoxia. J Appl Physiol (1985) 89: 1937-1942.
- Truijens MJ, Toussaint HM, Dow J, et al. (2003) Effect of high-intensity hypoxic training on sea-level swimming performances. J Appl Physiol (1985) 94: 733-743.
- Schagatay E, Anderson J (1998) Diving Response and apneic time in humans. Diving Hyperb Med 25: 13-19.
- Richardson MX, Lodin A, Reimers J, et al. (2008) Short-term effects of normobaric hypoxia on the human spleen. Eur J Appl Physiol 104: 395-399.
- Homma M (1994) The Components and the Time of 'Face In' of the Routines ins synchronized Swimming. Med Sci Aquatic Sports 39: 149-154.
- Yamamura C, Zust S, Takata K, et al. (1999) Physiological Characteristics of Well-Trained Synchronized Swimmers in Relation to Performance Scores. Int J Sports Med 20: 246-251.
- Jamnik V, Gledhill N, Hunter I, et al. (1987) Physiological assessment of syncrhonized swimming and elite synchronized swimmers. Med Sci Sports Exerc 19: S65.
- Ferretti G, Costa M, Ferrigno M, et al. (1991) Alveolar gas composition and exchange during deep breath-hold diving and dry breath holds in elite divers. J Appl Physiol (1985) 70: 794-802.
- Grassi B, Ferretti G, Costa M, et al. (1994) Ventilatory responses to hypercapnia and hypoxia in elite breath-hold divers. Respir Physiol 97: 323-332.
- Weil JV (2003) Variation in human ventilatory control-genetic influence on the hypoxic ventilatory response. Respir Physiol Neurobiol 135: 239-246.
- Lemaitre F, Julia F, Chollet D (2010) Apnea: A new training method in sport? Med Hypotheses 74: 413-415.
- Varveri D, Karatzaferi C, Pollatou E, et al. (2015) Aquadicity: a discussion of the term and of how it applies to humans. J Bodyw Mov Ther 20: 219-223.
- Ferretti G, Costa M (2003) Diversity in and adaptation to breath-hold diving in humans. Comp Biochem Physiol A Mol Integr Physiol 136: 205-213.
- Badra LJ, Cooke WH, Hoag JB, et al. (2001) Respiratory modulation of human autonomic rhythms. Am J Physiol Heart Circ Physiol 280: 2674-2688.
Corresponding Author
Henry N Williford, Ed.D, FACSM, ACSM EP-C, Department of Kinesiology, Auburn University Montgomery, PO Box 244023, Montgomery, AL 36124, USA, Tel: +334-244-3548, Fax: +334-244-3198.
Copyright
© 2017 Williford H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.