Coniferous Tree Species Effects on Soil Chemical Characteristics in Goro-Gutu District, Eastern Ethiopia
Abstract
The fast-growing natures of conifer tree species and favorable economic returns from these trees have encouraged the conversion of natural grasslands in open forests into tree plantations in Ethiopia. The dominant tree species used for this purpose in open forest areas of Eastern Oromia were Podocarpus falcatus, Juniperus procera and Cupressus lusitanica. These tree species plantations are spreading rapidly in mountain areas, specially, in protected open forest areas. However, how different tree species affect soil chemical nutrients of the open areas after plantation is remain largely unknown. Thus, the study was conducted at Keramile open forest of Goro-gutu district, Eastern Ethiopia, to evaluate the effect of coniferous tree species on grassland or open areas found in the open forest. The coniferous tree species used for our study were Podocarpus falcatus, Juniperus procera and Cupressus lusitanica. The current study investigated the soil chemical contents under the three tree species canopies, outside canopies as well as the interactions between tree species and canopy covers. Our results showed that the evaluated coniferous tree species had significant effect on soil pH, cation exchange capacity (CEC), organic matter (OM), total nitrogen (TN), organic carbon (OC), available phosphorous (av. P) and sodium (Na);in which soil pH, TN,OM and OC contents were significantly higher under P. falcatus than J. procera and C. lusitanica tree species. But no significant difference was detected in soil pH, TN, OM and OC contents between J. procera and C. lusitanica tree species. The present results also revealed that the canopy cover and their interaction had significant effect on soil OM, pH, OC, av. K, TN, EC, Na and CEC; were significantly higher OM, pH, OC, av. K, TN and CEC were obtained in the outside canopy than under the tree canopy cover, whereas soil EC and Na contents were higher under canopy than outside canopy cover. However, no significant difference was observed between under canopy and the outside canopy in soilav. P, Ca and Mg contents. Generally, our study showed that the coniferous tree species evaluated had negative impact on soil chemical properties (OM, pH, OC, av. K, TN and CEC) of the open or grassland of the study area. Therefore, management and monitoring of soil chemical characteristics of grassland found in open forest is crucial in Keramile open forest, Goro-gutu district, Eastern Ethiopia and other open woodlands receiving similar practice.
Keywords
Tree Species; Canopy; Grassland; Inside Canopy; Outside Canopy; Open Forest
Introduction
Land use change considerably influences on soil quality. Vegetation affects the amount and type of organic matter added to a soil [1,2] and this tends to significantly affect soil structure, color, pH, cation exchange capacity (CEC), infiltration and water holding capacity of soils [3]. In fact, the different land cover such as afforestation with native and non-native species can potentially cause changes in the evolution of ecosystems, including changes in soil carbon storage, reduced soil nutrients, increased biomass; soil acidification and return the nutrients to trees [4]. Different tree species, through their different properties in terms of produced litter, released nutrient and chemical composition of litter, play a substantial role in nutrient cycling [5].
Tree canopies alter the abiotic environment for understory vegetation directly by affecting light availability, temperature, humidity, etc. and indirectly by influencing soil processes [6]. The interception of solar radiation is a major factor affecting the understory. Canopy is the key regulator of solar radiation absorption and can prevent over 95% of light radiation from reaching the Earth's surface. The negative effects of tree canopy include reduced light availability for photosynthesis, tree root competition for water and soil nutrients, allopathic effects of trees, increased phytophagous fungi and pests and rainfall interception [6]. Further, low soil temperatures beneath a shade-tolerant canopy can also reduce decay rates, leading to accumulation of organic matter and further decreases in decomposition rates [7].
In evaluating the effects of afforestation with coniferous species in the temperate zones it was observed that afforestation caused changes in some soil chemical properties such as reduced pH and increased nutrient uptake [8]. Conifer litter, with higher amounts of secondary compounds such as lignin and polyphenols, decays more slowly than that of grass, which typically has lower phenolic and lignin concentrations and may promote acidification and a decline in soil fertility [9]. The effect of afforestation on grassland soil properties showed that exchangeable cations (Ca, Mg, K) and N were lower in plantations than in adjacent native vegetation [10]. The results of research on soil acidification and organic carbon (C) in these plantations have shown that afforestation in tropical regions leads to deep acidic litter layer and higher cation concentrations [11]. Additionally, Farley et al. [12] reported that soils have frequently been shown to be more saline and acidic with afforestation. Despite the Forestry Commission's aim to substantially increase timber production and sustain yields for the future, coniferous afforestation has consequently led to significant changes in soil chemistry across an ecosystem level [13]. There had been a dramatic increase in coniferous forest throughout the world [14].
Due to their important in ecosystem services [15]; grasslands are particularly valuable habitats that are in the focus of nature conservation and ecosystem restoration [16]. Grasslands face immense pressure from human-induced environmental change but are widely perceived to be of low conservation priority relative to forests [17,18]. The undervaluation of grassland ecosystem is reflected in national and international [19] environmental policies that inadvertently exacerbate conversion for agriculture, degradation caused by inappropriate management and increasingly, tree planting [20]. Thus, the future threats to grasslands appear high, given a need to feed a rapidly growing human population. Therefore, the grassland biome, specifically, is critically endangered [21] and it is the biome most in need of conservation. However, due to the resulting widespread clearance of forest, the newly established plan of the country, rapidly acquired and managed previous grassland sites across East Hararghe zone as coniferous plantation forests. This rapid increase in coniferous afforestation was dominated by the plantation of the faster growing, high yielding, exotic and indigenous coniferous tree species such as Cupressus lusitanica, Juniperus procera and Podocarpus falcatus.
The fast growing natures of those trees and favorable economic returns from tree plantations have encouraged the conversion of natural grasslands into tree plantations. Despite the increasing number of studies looking at the stability of tree plantations [22,23], there have been few efforts comparing the forests established and grasslands [24]. Furthermore, a lack of scientific management guidelines, soil degradation, decline in biological diversity, and low resilience of the ecosystem are common problems in afforested areas [25]. The effects of tree species on the associated understory herbaceous productivity vary with the environment or the climatic conditions [26]. Additionally, factors that are critical for vegetation diversity are not consistent across regions or locals [27]. Thus, understanding how they affect the soil chemical properties is critical to the understanding of ecological functions of plantations and to the improvement in their management [28].
For maintaining sustainability, soil nutrient should not be significantly decreased, and no significant negative changes in soil properties should happened [29]. Therefore, considering the different effect of each species on soil properties, understanding the soil characteristics is a principle of suitable management [30] because for creating a sustainable maintaining of soil nutrients has great importance. Many studies have been done about the effect of tree species on soil chemical and physical properties [31].
The Keramile open forest vegetation comprises of conifer trees having scattered distribution interspersed with open patches. A number of studies on herbaceous communities have been carried out in semi-arid areas of the zone; most of them have focused on floristic diversity, understory biomass yield and soil chemical properties [2, 32]. However, no information is available on the influence of conifer tree species on soil chemical properties in Keramile open forest vegetation of Goro-gutu district, East Ethiopia; where levels of protection for grasslands at high altitude are so low and failed to inspire governments to protect them. Therefore, the current study was carried out to investigate the effect of Cupressus lusitanica, Juniperus proceraand Podocarpus falcatus trees species on grassland soil chemical properties in the open forest ecosystem of Keramile, Goro-gutu district, Ethiopia.
Material and Methods
Description of the study area
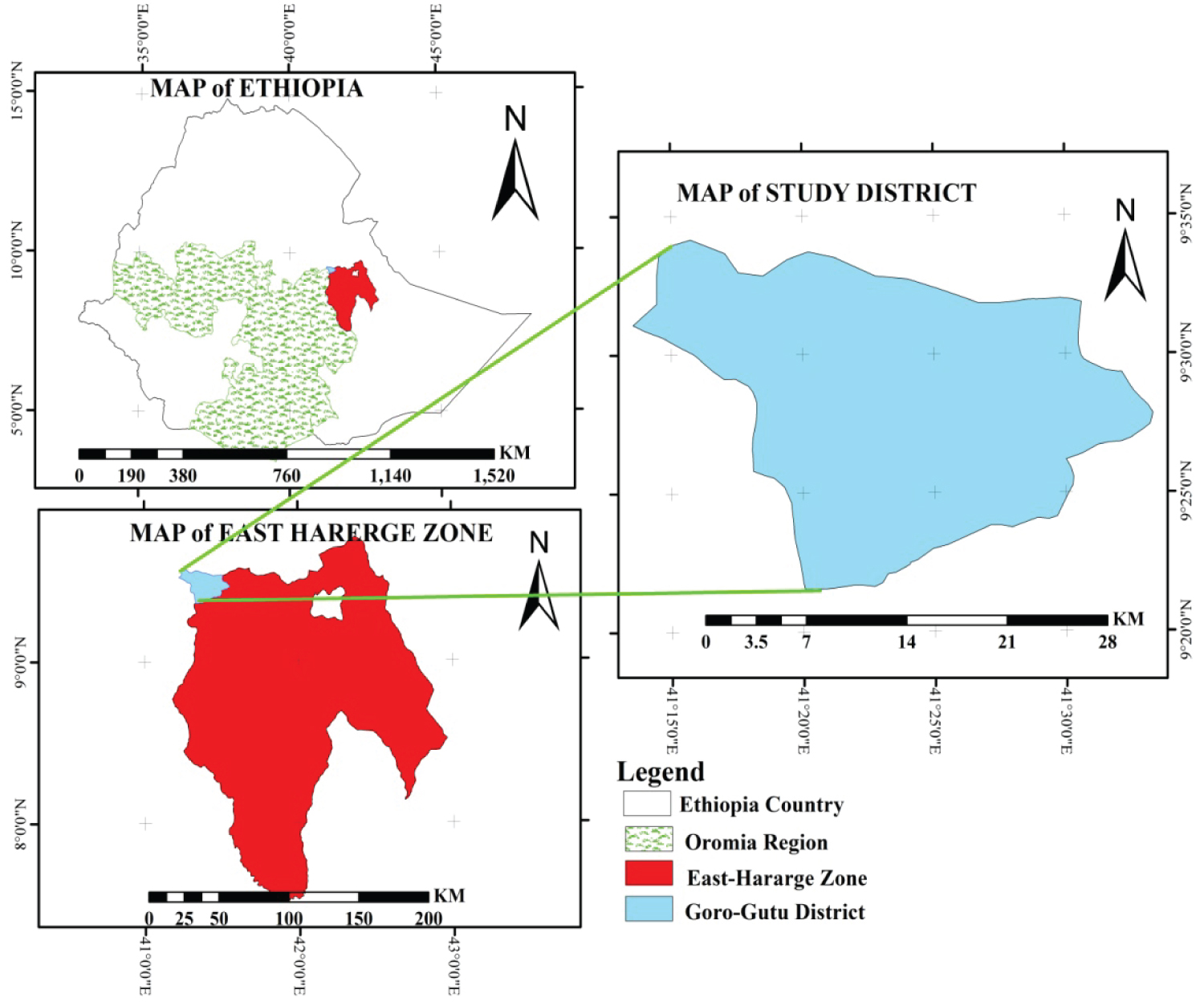
Location: The study was conducted at Keramile exclosure in Goro-gutu district. Goro-gutu district is found in eastern highlands of Ethiopia, Eastern Hararghe Zone of the Oromia National Regional State. It is 408 km from Addis Ababa at 9o35'N, 38o18'E; on the main road to Harar and it is located 107 km from the Zonal capital Harar. The district shares long boundary with Western Hararghe Zone of Oromia and Shinile Zone of the Somali National Regional State (Figure 1).
Climate: According to the district's bureau of agriculture report, the agro-ecological classification of the Goro-gutu district indicated that 28% of the total area is classified as mid-land "woina-dega ", 49% as lowland "kola" and 23% as highland "dega". The area is characterized by mountain, plateau, dissected gullies and degraded hills. It has bimodal rainfall patterns. The shortest season is from mid-February to April and the longest season is from June to October having annual average rain fall of 900mm with the corresponding temperature range of 16-20oc and 20-240c during the coldest and warmest months respectively.
Flora: The study area is characterized by open forest which is made up of trees or shrubs and dominated with grass species. The vegetation description presented by east harangue planning and economic development (2001) shows that the study area is characterized by Dry Evergreen Montane Forest and Grassland Complex on the basis that the vegetation type occurring in an altitudinal range of 2000 - 2300 m, with average annual temperature and rainfall of 16-24° C and 800-1200 mm, respectively.
The dominant tree species of the study area Juniperus procera, Cupressus lusitanica, Podocarpus falcatus, Croton macrostachyus, Cordia africana, Ficussycomorus, Hageniaabyssinica, Oleaeuropaea, Acacia abyssinica, Acacia decurrens, Acacia saligna, Eulcalyptus globules, Psidiumguajava, Schinusmolle, Gravillea robusta and Casuarinas cunninghamiana. The whole area is dominated by Juniperus procera, Cupressus lusitanica and Podocarpus falcatus. The area is well known by its natural vegetation and plantations of exotic tree species.
Selection of sampling sites
Based on visual field observation three dominant coniferous tree species, representing one exotic (Cupressus lusitanica) and two indigenous (Podocarpus falcatus and Juniperus procera), found in isolation, were selected for this study. The tree species used in this study are representative of the dominant trees in the study area. Based on their abundance, canopy sizes and tree heights, compared to other woody species, they represent suitable tree species for a purposive study of the effects of tree species on herbaceous plants. Accordingly, 20 matured trees from each species were purposively selected based on their similar canopy size and tree height. No shrubs or termite mounds found under or close to the selected trees. In total, 60 trees (3 tree species x 20 trees for each species) were selected for the study.
Tree height was measured using clinometers. The canopy cover of the trees was measured by using the measuring tape on ground level through the canopy length and then canopy area was calculated by using perpendicular diameters in two dimensions at right angle according to Savadogo and Elfving [33].
Where: -
CA= Canopy/crown area
CD1 and CD2 = Canopy diameters in two dimensions at right angle
Soil sampling and analysis
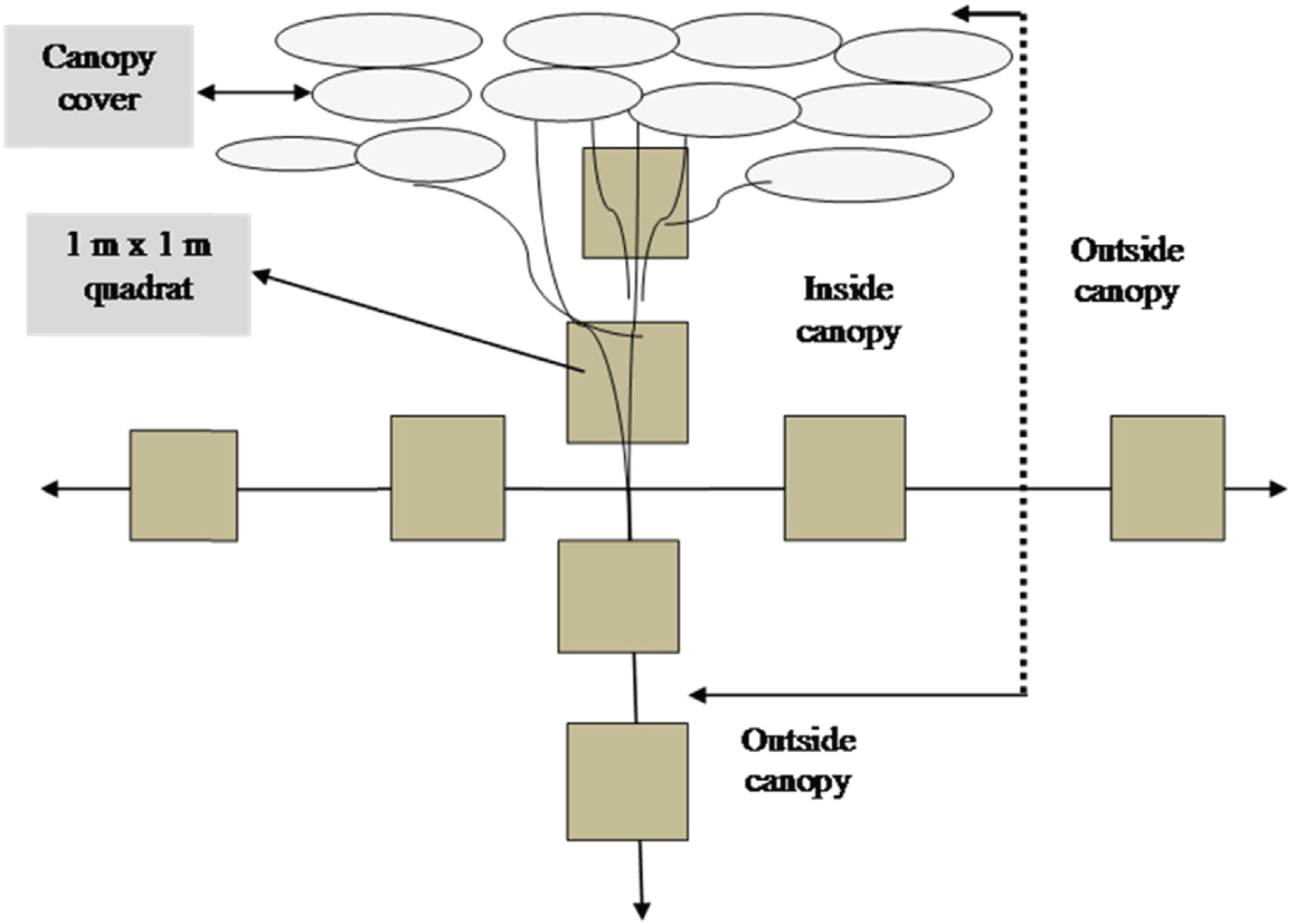
Soil sample data collected using a soil auger at depth of 0-20 cm in four directions (north, south, east and west) under and outside canopies of each tree. An area of 1 m2 used for each quadrat (Figure 2). Soil samples collected from eight quadrats (four quadrants for each canopy type) for a single tree (Figure 3). Totally, 480 samples (3 tree species x 20 trees/species x 2 canopy cover x 4 directions as sample quadrats) of soil were selected. The soil samples from the same tree species under and outside canopy cover were pooled and mixed together separately to form composite sample. Finally, 24 composite soil samples (3 tree species x 2 canopy covers x 4 directions as quadrat samples) were stored in plastic bags, labeled, sealed and transported to the soil laboratory of Bedele Soil laboratory for chemical analyses.
The soil samples were analyzed using different techniques. Soil pH and electrical conductivity (EC) was analyzed using the procedures of Peech [34], while his percentage organic carbon (OC) was determined according to the Walkley and Black [35] method. Organic matter (OM) was estimated as OC % *1.724 and total nitrogen was analyzed using the Houba et al, [36] procedure. Available phosphorus (P) was determined using Olsen et al, (1954) method. Exchangeable potassium (K) by flame photometer method as described by Rowell (1994) and Calcium (Ca) and magnesium (Mg) were analyzed by atomic absorption spectrophotometer method [37]. Sodium (Na) was analyzed according to flame photometer as described by Rowell (1994). Cation exchangeable capacity (CEC) was analyzed using the method of Chapman (1965).
Data analysis
The soil samples data from all quadrats were combined tree species separately to it's under canopy and outside canopy. The data obtained were subjected to two ways ANOVA (Analysis of Variance) in the factorial experiment, with tree species as one factor and canopy type as the other factor. All statistical analyses were performed using SAS software (SAS, 2009, version 9.1.3) by the General Linear Models (GLM) procedure. The model included the effects of tree species, canopy cover and their interaction as independent factor. Mean separations were tested using the least significance difference (LSD) and significant levels considered at P< 0.05.The statistical model used for this study was:
Where: - = over all observation
= over all mean
= tree species effect
= canopy effect
= interaction effect
= error effect
Results and Discussions
Heights and crown diameters of the three coniferous tree species
The mean heights and crown diameters of the three dominant tree species, Cupressus lusitanica, Podocarpus falcatus and Juniperus procera selected for this study is presented in Table 1.
Tree species, canopy cover and their interaction influence on the soil chemical properties
Soil chemical analysis carried out during the present study revealed particular significant in soil chemical attributes (Table 2).
Soil pH: The pH of soil in the study area was significantly affected (P< 0.01) by tree species (Table 2). Soil pH was neutral under P.falcatus, while it was slightly acidic under J. proceraandC. lusitanica. This result might be due to the low understory vegetation of J. proceraandC. lusitanica. Herb layer species richness and cover increased was positive effect on pH [38]. Similarly, Ahmed [3] reported that vegetation tends to significantly affect pH of soils. Additionally, the difference in soil Ph might be associated with the difference in litter quality and quality of the tree species. Similar to the current finding, Macdonald and Fenniak [39] reported the change in soil physicochemical properties depends on the litter quality and quantity and the canopy architecture, which in turn depends on the tree species. The significant difference in soil pH under the three tree species examined may also indicate that the decomposition rate of their litter differs, as the addition of organic matter is known to reduce soil pH [40, 41]. The result obtained may suggest that the organic matter from P.falcatus is probably more easily decomposed than that from J. proceraand C. lusitanica tree species. The lower pH values recorded in the soils collected from under. Procera and C. lusitanica than under the other tree species suggests that the presence of these two trees may have had acidifying effect on soils under the trees, which probably may have been attributable to the formation of organic acid and releases of carbon dioxide due to litter decomposition. This result suggests that the mechanism of acidification under tree canopies and the actual impact of the change in pH depend on the tree species.
Comparing soils under the tree canopies with the outside canopies, soils under J. procera and C. lusitanica tree species tend to be acidic than outside canopy. The pH of soil was also significantly influenced by crown cover of trees species showing lower pH value under canopy cover than the open area. Consistent with the findings of Zemmrich et al. [42], canopies are the main factors affecting soil pH. The difference in soil pH might be due to their litter quality. Conifer litter decays more slowly than that of grass, which typically may promote acidification and a decline in soil fertility [9].The widespread input of highly acidic pine needles onto the tree floor also creates an extremely acidic litter, aiding soil acidification. Berthrong et al. [4] also reported the presence of a coniferous tree canopy has lead to considerable acidification and long term changes in soil chemistry. Similarly, Berthrong.et al. [4] reported that afforestation with native and non-native tree species can potentially cause soil acidification. However, this result was inconsistent to findings of Kahi et al. [26] and Tessema and Belay, [32] who obtained higher pH of soil under canopy than outside canopies in rainfall scarcity areas, in a semi-arid savanna of Ethiopia. However, Conifers in general are known for their ability to chemically alter the environment, specifically by lowering the pH (Bol and Vroomen2008). Soil pH was also significantly affected by the interaction of tree species and canopy cover.
Soil electrical conductivity: Electrical conductivity which is important to measure in soil in order to determine the relative amount of salt in soil which may have adverse effect on plant growth at a maximum level was no significant difference (P > 0.05) among the tree species, as well as the interaction of tree species and canopy cover (Table 2). However, the electrical conductivity of soil was significantly influenced by canopy cover of tree species showing higher EC value under canopy cover than the open grassland. The result is in agreement with Farley et al. [12], who reported that soils have frequently been shown to be more saline with afforestation. Similarly, soil salinization has been reported worldwide after afforestation was reported by Berthrong et al. [10].
Soil cation exchange capacity: The cation exchange capacity (CEC) of soil in the study area was significantly difference (P < 0.04) among the tree species (table 2). The soil CEC content under P. falcatus species was higher than under J. procera and C. lusitanica tree species. The soil CEC content was also significantly influenced by canopy cover of tree species. Relatively higher CEC content was recorded outside canopy than under the tree species canopies. An important factor contributing to the improvement of cation in outside canopy or grassland might be the higher organic matter contents compared to under tree canopy. Gao and Chang [43] showed that CEC is highly correlated with organic matter content of the soil, which is in turn, is affected by different soil management practices such as changes in land use. Similarly, Teshome et al. [44] reported the soil CEC values in agricultural land uses decreased mainly due to the reduction in organic matter content. Soil cation exchange capacity was also significantly affected by the interaction of tree species and canopy cover.
Soil exchangeable Ca, Mg, K and Na contents of the tree species did not differ significantly ((P > 0.05)). Soil Ca, Mg, K and Na contents were also not significantly affected by the interaction of tree species and canopy cover. However, there was a significant difference in the soil P content among the tree species evaluated, where higher value was recorded from P. falcatus than J. proceraand C. lusitanica trees species. This difference may be explained by the observed difference in soil pH (Table 2) between the tree species, since pH is known to influence the status and availability of soil phosphorus [45,46]. No significant differences between canopies types were detected in soil Ca, P and Mg contents. But there was a significant difference in available K and Na between canopies where higher available K content in the outside canopies than under canopy areas of the tree species. This result agrees with the conclusions of Farley et al. [12] and Berthrong et al. [10] who found that exchangeable cations (K) were lower in plantations than on grassland soil.
Soil organic matter, organic carbon and total nitrogen: Soil OM, OC, TN contents were significantly affected by tree species (Table 2). These soil properties showed higher concentrations under P.falcatus species than J. procera and C. lusitanica. This might be due to the higher herbaceous species richness and ground cover under P. falcatus canopies [46]. Increased herbaceous vegetation ground cover under P. falcatus may reduce the impact of soil erosion, decreases run-off and reduced top soil loss which leads to elevated levels of soil fertility. High biomass yield under P. falcatus tree increased the residues added to soil surface. Because these vegetation contributes to the stocking of soil organic carbon, which is essential for good soil structure and nutrient availability that supports aboveground productivity. The herbaceous layer plays a key role in improving soil fertility, stimulating soil nutrient cycles [48]. Additionally, it might be also due to different in their litter quality. Different tree species, through their different properties in terms of produced litter, released nutrient and chemical composition of litter, play a substantial role in nutrient cycling [5].
Soil OM, OC and TN contents were significantly affected by canopy covers (Table 2). The higher percent of the three soil nutrients were obtained in the open grassland/outside than under canopy cover. The difference might be related to the amount and quality of litter between the under the trees canopies and the open grassland. Similar to the current study, De Schrijver et al. [9], reported conifer litter decays more slowly than that of grass and may promote acidification and a decline in soil fertility. In addition, the difference might be related to the higher herbaceous vegetation richness and ground cover which accumulates higher litter biomass and increases the contents of soil organic matter and protects the soil from erosion. Consistent with the findings of Tessema et al. [2], the higher herbaceous cover adds organic matter and protects the soil from erosion. Also similar to the present study, evaluated that soils in afforested areas with Cupressus spp had lower TN and OM compared to grasslands soils. The present finding was also in agreement with that of Franzluebbers [49] who reported that soil organic carbon under grasslands is usually greater than under other land uses. This is why Pontes [50] point out that prevention of soil loss due to water and air erosion, maintenance of soil fertility is a very im¬portant non-market value of grasslands. The low light and moisture availability which slows down the decomposition rate of litter might be also the factors that brought lower soil OC, OM and TN contents under the tree canopies. The amount of OM in soil is the product of diverse factors over a period of time on the relative rates of return of organic residues to the soil and their subsequent breakdown in the soil [51]. The higher growth rates of these conifer tree species may lead to more drastic changes in soils than the other a native tree species. The fast growth rates can lead to higher demand for soil nutrients [52].
Summery and Conclusion
In recent times, the realization that coniferous tree species plantations leads to deterioration in soil quality has been widely recognized. The negative influence of all tree species on the soil chemical properties led to decrease in soil fertility. The present steady revealed that tree species showed significant effect on the soil chemical properties. However, the soil chemical nutrients under the canopy of P. falcatustree had significantly higher than under J. procera and C. lusitanica tree species, but little differences observed between J. procera and C. lusitanica tree species. These results indicate the potential of P. falcatus for maintaining soil chemical fertility while J. procera and C. lusitanicatree species affect negatively soil chemical nutrients of the study area. On the other hand, significantly higher concentrations of soil chemical nutrients were found outside canopy than under canopy. This showed that canopy cover of the tree species was also found to be negatively affected soil chemical nutrients of the study area. Therefore, proper management of grassland soil nutrients in open woodland is very crucial under the rapidly growing human population, changing climate and global warming. Our finding showed that there is a need for further understanding of the coniferous tree species and soil interactions in high altitude open forest ecosystems of Eastern Oromia, which serve as a home to grassland herbaceous vegetation. Furthermore, efforts to conserve and manage forests and grassland should be integrated. Also a further study of changes in soil chemical properties in the long term of different coniferous tree species in grassland is needed to understand ecological consequences of tree plantation and to promote sustainable management.
Acknowledgements
The authors would like to acknowledge the Oromia Agricultural Research Institute (OARI) for the financial support to this research and Fed is Agricultural Research Center (FARC) for providing logistical support during the sampling trips. The author is very grateful of Goro-gotu district administration for the security support during the field work. Also the author is grateful to Professor Tessema Zewdu for his assistance during the field data collection of which the research was a success and critical reading of the manuscript, valuable suggestions, and consistent forbearance during the research period. Lastly, a very deep admiration and special thanks goes to my friends especially my lovely friend, Mr. Shifera Gelana for his unforgettable kindness, generosity, encouragement, and valuable support during my study.
References
- Parker R (2000) Introduction to plant science. Delmar, Thomson Learning, USA, 704.
- Tessema ZK, de Boer WF, Baars RMT, et al. (2011) Change in soil nutrients, vegetation cover, structure and herbaceous biomass in response to grazing in semiarid, savanna of Ethiopia. Journal of Arid Environments 75: 662-670.
- Ahmed Hussein (2002) Assessment of spatial variability of some physicochemical properties of soils under different elevations and land use systems in the western slopes of Mount Chilalo, Arsi, Ethiopia.MSc Thesis, Haramaya University, Haramaya, Ethiopia.
- Berthrong ST, Schadt CW, Piñeiro G, et al. (2009) Afforestation alters the composition of functional genes in soil and biogeochemical processes in South American grasslands. Appl Environ Microbiol 75: 6240-6248.
- Kooch Y, Hosseini SM, Mohammadi J, et al. (2012) Effects of uprooting tree on herbaceous species diversity, woody species regeneration status and soil physical characteristics in a temperate mixed forest of Iran. Journal of Forestry Research 23: 81-86.
- Valladares Fernando, Lauri Laanisto, Ülo Niinemets, et al. (2016) Shedding light on shade: ecological perspectives of understorey plant life. Plant Ecology & Diversity 9: 237-251.
- Crawford RMM, Jeffree CE, Rees WG (2003) Paludification and forest retreat in northern oceanic environments. Ann Bot 91: 213-226.
- Farley KA, Kelly EF (2004) Effects of afforestation of a páramo grassland on soil nutrient status. Journal of Forest Ecology and Management 195: 281-290.
- De Schrijver A, De Frenne P, Staelens J, et al. (2012) Tree species traits cause divergence in soil acidification during four decades of post agricultural forest development. Global Change Biology 18: 1127-1140.
- Berthrong ST, EG Jobbagy, RB Jackson (2009) A global meta-analysis of soil exchangeable cations, ph, carbon, and nitrogen with afforestation. Ecological Applications 19: 2228-2241.
- Lima AM, Silva IR, Neves JC, et al. (2006) Soil organic carbon dynamics following afforestation of degraded pastures with eucalyptus in southeastern Brazil. Journal of Forest Ecology and Management 235: 219-231.
- Farley KA, G Pineiro, SM Palmer, et al. (2008) Stream acidification and base cation losses with grassland afforestation. Water Resources Research 44: 03.
- Convery I, T Dutson (2008) Rural Communities and Landscape Change: A Case Study of Wild Ennerdale. Journal of Rural and Community Development 3: 104-118.
- Macdonald CA, N Thomas, L Robinson, et al. (2009) Physiological, biochemical and molecular responses of the soil microbial community after afforestation of pastures with Pinusradiata. Soil Biology and Biochemistry 41: 1642-1651.
- Allan E, Manning P, Prati D (2015) Land use intensification alters ecosystem multifunctionality via loss of biodiversity and changes to functional composition. Ecol Lett 18: 834-843.
- Veen P, Jefferson R, De Smidt J, et al. (2009) Grasslands in Europe of high nature value, KNNV Publishing, Zeist: 320.
- Parr CL, LehmannCER, BondWJ, et al. (2014) Tropical grassy biomes: Misunderstood, neglected, and under threat. Trends Ecol Evol 29: 205-213.
- Veldman JW, OverbeckGE, NegreirosD, et al. (2015) Toward an old-growth concept for grasslands, savannas, and woodlands. Frontiers in Ecology and the Environment 13: 154-162.
- Putz FE, Redford KH (2010) The importance of defining “forest”: Tropical forest degradation, deforestation, long-term phase shifts, and further transitions. Biotropica 42: 10-20.
- Veldman JW, Overbeck GE, Negreiros D, et al. (2015) Tyranny of trees in grassy biomes. Science 347: 484-485.
- B Reyers, DHK Fairbanks, AS Van Jaarsveld, et al. (2001) Priority areas for the conservation of South African vegetation: a coarse-filter approach. Diversity and Distributions 7: 79-95.
- Urrego DH, Niccum BA, La Drew CF, et al. (2011) Fire and drought as drivers of early Holocene tree line changes in the Peruvian Andes. Journal of Quaternary Science 26: 28-36.
- Lutz M, Wolf S, Eugster W, et al. (2013) Contrasting response of grassland versus forest carbon and water fluxes to spring drought in Switzerland. Environmental Research Letters 8: 035007.
- Zimmermann NE, Edwards TC, Graham CH, et al. (2010) New trends in species distribution modelling. Ecography 33: 985-989.
- Wingfield MJ, J Roux, B Slippers, et al. (2013) Established and new technologies reduce increasing pest and pathogen threats to Eucalypt plantations. Journal of Forest Ecological and Manage 301: 35-42.
- Kahi CH, Ngugi RK, Mureithi SM, et al. (2009) The canopy effects of Prosopis juliflora (DC) and Acacia tortilis (HAYNE) on herbaceous plant species and soil phsico-chemical properties in Njempts, Kenya. Tropical and Subtropical Agro ecosystems 10: 441-449.
- Duan WJ, H Ren, SL Fu, et al. (2010) Community comparison and determinant analysis of understory vegetation in six plantations in South China. Restor Ecol 18: 206-214.
- Berthrong ST, G Pineiro, EG Jobb´Agy, et al. (2012) Soil C and N changes with afforestation of grasslands across gradients of precipitation and plantation age. Ecological Application 22: 76-86.
- Rouhi-Moghadam E, et al. (2011) Investigation of some soil properties in pure and mixed plantations of oak (Quercuscastanifolia). Iranian J Soil Res 1: 39-48.
- Chen YM, Y Cao (2014) Response of tree regeneration and understory plant species diversity to stand density in mature Pinus tabulaeformis plantations in the hilly area of the Loess Plateau, China Ecol Eng 73: 238-245.
- Luan J, Liu S, Zhu X, et al. (2012) Roles of biotic and abiotic variables in determining spatial variation of soil respiration in secondary oak and planted pine forests. Soil Biology and Biochemistry 44: 143-150.
- Tessama Zewdu, Belay Ejigu (2017) Effect of tree species on understory vegetation, herbaceous biomass and soil nutrients in a semi-arid savanna of Ethiopia. Journal of Arid Environments 139: 76-84.
- Savadogo P, Elfving B (2007) Prediction models for estimating available fodder of two savanna tree species (Acacia dudgeon and Balanites aegyptiaca) based on field and image analysis measures. African Journal of Range Forage Science 24: 63-71.
- Peech, Michael (1965) Hydrogen-ion activity. Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties 9: 914-926.
- Walkley A, Black C A (1934) An examination of different methods for determining soil organic matter and the proposed modifications by the chromic acid titration method. Journal of Soil Science 37: 29-38.
- Houba V J G, Lexmond T M, Novozamsky I, et al. (1996) State of the art and future developments in soil analysis for bioavailability assessment. Science of the Total Environment 178: 21-28.
- Hesse PR, P Hesse (1971) A textbook of soil chemical analysis. Chemical Publishing Company, London.
- Kooijman A M (2010) Litter quality effects of beech and hornbeam on undergrowth species diversity in Luxembourg forests on limestone and decalcified marl. Journal of Vegetation Science 21: 248-261.
- Macdonald, T E Fenniak (2007) Understory plant communities of boreal mixed wood forests in western Canada: natural patterns and response to variable-retention harvesting. Journal of Forest Ecology and Management 242: 34-48.
- Kumar G, Hegde KR, Luckins CB (2001) Decomposition and Nutrient Release Pattern of Leaf Litters of Mangium (Acacia Mangium Wild). Indian Journal of Agroforestry 3: 11-22.
- Landon J R (2014) Booker Tropical Soil Manual: A Handbook for Soil Survey and Agricultural Land Evaluation in the Tropics and Subtropics. Routledge.
- Zemmrich, M Manthey, S Zerbe, et al. (2010) Driving environmental factors and the role of grazing in grassland communities: a comparative study along an altitudinal gradient in Western Mongolia. Journal of Arid Environments 74: 1271-1280.
- Gao G, Chang C (1996) Changes in cation exchange capacity and particle size distribution of soils associated with long-term annual applications of cattle feed lot manure. Soil Science 161: 115-120.
- Teshome Yitbarek, Heluf Gebrekidan, Kibebew Kibret, et al. (2013) Impacts of land use on selected physicochemical properties of soils of Abobo area, western Agriculture, Forestry and Fisheries 2: 177-183.
- Haggar JP, Warren GP, Beer JW, et al. (1991) Phosphorus Availability under Alley Cropping and Mulched and Un mulched Sole Cropping Systems in Costa Rica. Plant and Soil 137: 275-283.
- Hands MR, Harrison AF, Bayliss-Smith T (1995) 10 Phosphorus Dynamics in Slash-and-Burn and Alley Cropping Systems of the Humid Tropics. John Wiley: 155-170.
- Tolera F, Tessema Z (2021) Influences of tree species and canopy cover on aboveground biomass an ground cover of herbaceous plants in eastern Oromia, Ethiopia. American Journal of agriculture and forestry 9: 233-240.
- Wang HF, M Lencinas, C Ross Friedman, et al. (2011) Understory plant diversity assessment of Eucalyptus plantations over three vegetation types in Yunnan, China. New Forests 42: 101-116.
- Franzluebbers AJ (2009) Soil organic carbon in managed pastures of the southeastern United States of America.In: Grassland carbon sequestration: management, policy and economics. Food and Agricultural Organization, Roma 11: 163-175.
- Pontes L da S (2011) Plant functional traits and nutrient gradients on grassland. Grassland Science in Europe 16: 470-483.
- Tilahun Chibsa, Asefa Ta'a (2009) Assessment of Soil Organic Matter under Four Land Use Systems in the Major Soils of Bale Highlands, South East Ethiopia b. Factors Affecting Soil Organic Matter Distribution. World Applied Sciences Journal 6: 1506-1512.
- Zhang X Q, M U F Kirschbaum, Z H Hou, et al. (2004) Carbon stock changes in successive rotations of Chinese fir (Cunninghamia lanceolata (Lamb) Hook) plantations. Journal of Forest Ecology and Management 202: 131-147.
Corresponding Author
Tolera Fikadu, Range Ecology and Biodiversity, Fedis Agricultural Research Center, Oromia Agricultural Research Institute, P.O. box 904, Harar, Ethiopia
Copyright
© 2022 Fikadu T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.