Sensitivity towards Nitrate: Comparison of Groundwater versus Surface Water Crustaceans
Abstract
Groundwater pollution with Nitrate represents a rising environmental problem, which might even be intensified by climate change. In this study, the acute (24 h) and chronic (5 weeks) toxicity of Nitrate has been investigated using groundwater crustaceans (Niphargopsis casparyi, Pratz 1866 and Proasellus slavus, Remy 1948) and surface water amphipods (Gammarus fossarum, Koch 1835) as comparison. Already at the German drinking water law threshold value of 50 mg/l Nitrate N. casparyi responded within 24 h with a decrease in locomotor activity and at 100 mg/l Nitrate, 37.5% of the animals died within 24 h of exposure. G. fossarum showed drastic behavioral effects only at 100 mg/l Nitrate, but did survive the 24 h exposure. Chronic exposure to Nitrate revealed significant differences in behavior for G. fossarum especially in their ventilation activity at 50 and 100 mg/l Nitrate and lower feeding activity at 100 mg/l Nitrate. N. casparyi showed lower ventilation rates at 100 mg/l Nitrate. Mortality of both groundwater species was lower than that of G. fossarum, however this might partly be due to problems in aeration and potential cannibalism in G. fossarum in the chronic laboratory exposures, esp. at 18 ℃.
In conclusion, in acute tests N. casparyi was the most sensitive to Nitrate. For chronic exposures of 5 weeks G. fossarum showed severe effects at 50 and 100 mg/l and N. casparyi at 100 mg/l, however, more chronic direct comparative toxicity studies and even longer exposures are needed to finally determine which species is the most sensitive towards Nitrate.
Keywords
Toxicity data, Nitrate, Gammarus fossarum, Niphargopsis casparyi, Proasellus slavus
Introduction
Groundwater is classified as protected property, mainly as resource for drinking water. Worldwide about 300 Mio people draw drinking water from groundwater resources; e.g. in Uttar Pradesh, India 85% [1] and in Germany about 70% of the drinking water comes from ground- and spring water [2]. The threshold value for Nitrate (50 mg/l) has been aligned with the German drinking water directive (98/83/EG). However, some countries have stronger regulations, e.g. Nitrate levels in the USA drinking water should be below 45 mg/l, in Switzerland Nitrate may only reach 25 mg/l in groundwater bodies [3]. Globally groundwater Nitrate levels vary between 0.08 and 487 mg/l Nitrate, their values correlate positively with fertilizer application on soils; and 28% of the public wells in India and 13% of those in Saudi Arabia exceed 50 mg/l Nitrate [3].
Nitrate concentrations in many European countries have gradually increased over the past few decades, e.g. by 0.7 mg/l Nitrate in UK rivers [3]. In 2018, the European Court doomed Germany for violation of the European Nitrate Guideline (91/676/EG), as 28% of the monitoring sites exceeded the nitrate threshold until 2014 [4]. A recent EU fertilizing regulation (EU 2019/1009) of 25.06.2019 aims to harmonize, regulate and improve the standards and use of fertilizers in the European Union in order to reduce pollution with nutrients and toxins.
Nitrate is frequently being used in agriculture, either as inorganic or as natural organic fertilizer such as manure or sewage sludge. Nitrogen pollution and esp. Nitrate is causing the most major environmental issue throughout the world [1]. Germany and 5 other European countries show the highest amounts of Nitrogen/ha in the European Union [5]. The application of sewage sludge as fertilizer implies problems in both hygiene and toxicity; therefore bans are under discussion throughout Europe [6]. In Germany, the most recent Nitrate-report [7] summarizes the results of the occurrence of Nitrate in the environment and the effects of countermeasures: About 50% of the Nitrate found in surface waters comes from agriculture, via leaching, erosion and drainage. For example, about 28% of the German groundwater sampling sites exceeded the European threshold value of 50 mg/l Nitrate in 2014, being the 2nd highest value after Malta. Since 2008, generally significant decrease in groundwater pollution with Nitrate could not be noticed, the sites with > 25 mg/l Nitrate remained as high as 35% [4]. This is the reason why in 2016 the European court (EUGH) started a case against Germany violating the EU Nitrate Guideline (91/676/EU), demanding rapid and more effective countermeasures to reduce Nitrate pollution [4]. It is important to strengthen the fertilizer regulations, restricting the application and use of controversial Nitrate sources such as sewage sludge and lifestock waste and to establish good practice training for the stewardship of nutrients combined with serious penalties in case of infringement. Several countermeasures seem to be very effective in reducing Nitrate pollution of groundwater, such as transition of crop farming into pastures and enhancement of ecological farming. A recent EU fertilizing regulation (EU 2019/1009) aims to harmonize, regulate and improve the standards and use of fertilizers in the EU in order to reduce pollution with nutrients and toxins. As Nitrate can remain long time in deeper soil horizons, current and future reduction of Nitrate pollution on agricultural land might only show improvements after several decades in deeper groundwater.
Moreover, climate change might enhance the Nitrate problematic even more [8]. The demand of clean drinking water will increase due to longer drought periods for both human and agriculture usage (e.g. for irrigation of potatoes and vegetables) as well as for industry (e.g. cooling) [5]. The increased water demand by humans resulting in rising groundwater withdrawal might lead to dropping water storage [7]. Decreasing precipitation in winter might lead to declining groundwater regeneration and decreasing precipitation in summer might result in lower spring yield and stream discharge [7]. Apart from the effects of climate change on groundwater quantity there might be effects on water quality too, resulting in an increase of DOC, metals and salinization. On the other hand, punctual heavy rainfall events are increasing leading to rising leaching of Nitrate from soils into surface water and groundwater [9].
Nitrate is a good water-soluble ion, which might be transformed into Nitrite in the guts, which is regarded as toxic for babies as they have a less acid gut milieu. Nitrite in the blood cycle might oxidate haemoglobin to methaemoglobin, the latter being less able to bind oxygen, thus leading to an irreversible critical shortage in oxygen for the body functions. Nitrate and Nitrite are also toxic for aquatic invertebrates, which has been shown in a few studies: acute LC50-48h values for different invertebrates revealed E. toletanus and Echinogammarus echinosetosus to be more sensitive towards NO3 compared to Daphnia magna (E. t.: 180 mg/l; E.e.: 107 mg/l; D. magna neonates: 465 mg/l), whereby Cheumatopsyche pettiti and Hydropsyche occidentalis were the most sensitive species studied (6.7, resp. 4.5 mg/l) [10]. Already 44 mg/l Nitrate might affect survival rates of surface water invertebrates such as Echinogammarus echinosetosus and Eulimnogammarus toletanus [10]. Di Lorenzo and Galassi [11] found in a field study that up to 150 mg/l Nitrate the biodiversity of groundwater invertebrates appeared unaffected. The LC50-96h for Nitrite-N was 2.59 mg/l for E. echinosetosus and 2.09 mg/l for E. toletanus [12]. No toxicity data for Nitrate/Nitrite are currently available for groundwater invertebrates.
During the past 20 years groundwater has received increasing attention as ecosystem, providing biodiversity and specific ecosystem functions and services. This new focus has been implemented in groundwater legislation, too, e.g. in Australia [13] and European Union [14]. However, studies of groundwater ecosystems as subject of biodiversity and protection are still rare, especially the risk assessment, which still relies on standard test species data from surface waters [15]. Apart from very few comparative toxicity studies regarding the sensitivity towards chemicals [16], there is a huge lack of toxicity data on different macro- and meiofauna in groundwater as basis to decide whether the standard risk assessment for surface water can be applied to groundwater or not [17]. In the BMBF project GroundCare we performed different toxicity tests with Niphargopsis casparyi, and Proasellus slavus in direct comparison to Gammarus fossarum on selected toxicants such as copper [18] and Bisphenol A [19].
The aim of the current study was to provide toxicity data on Nitrate for groundwater macrocrustaceans in direct comparison to their surface water relatives, aiming at a decision which species should be used for risk assessment in groundwater ecosystems, which is currently based on surface water species used in standard toxicological testing. It was anticipated that groundwater crustaceans might be more sensitive towards Nitrate than surface water species.
Materials & Methods
Test species
Gammarus fossarum (Koch 1835) is a widespread surface water amphipod in small headwater streams, where it occurs throughout the year in high abundances in the aquatic invertebrate biocoenosis. Generally, about 100 Gammarus species inhabit aquatic ecosystems of the Northern Hemisphere [20]. G. fossarum play an important role in the aquatic food web, being prey for fish, waterbirds and large invertebrates and being omnivorous, i.e. feeding on small chironomids, fine particulate organic matter and decomposing leaves [21]. Gammarids have a long life cycle of 1-2 years with moltings every 2 weeks spending their whole life in water; hence they need permanent water flow [22]. As gammarids reproduce several times and almost during the whole year, different life stages can be found throughout the year easily and in large amounts for laboratory toxicity testing [20]. Compared to the relative species G. pulex, G. fossarum needs rapid water flow with high oxygenation and occurs in higher altitudes (> 100 m above sea level) [23]. G. fossarum is used as bioindicator for the saprobic system (SI 1.6) of the bioassessment in stream ecosystems [20]. The animals for the experiments were taken from the laboratory culture (15 L filtered water from Lake Constance, 10 ℃, darkness, and leached alder leaves as food source, stones as substrate, aeration).
Both groundwater macrocrustacean species used in this study are adapted to the typical conditions in subterraneous environments, such as lack of light and space, very low water exchange, oxygen and nutrients and food. Groundwater ecosystems depend on inputs of organic matter and water from "above the surface". Stygobiont species show the following adaptations: Neither eyes nor pigmentation, slow metabolism and long life cycles (up to 10 years for amphipods) combined with low reproduction rates and restricted abilities to distribute geographically. Moreover, they tolerate low oxygen levels and are used to stabile environmental conditions, such as constant low temperatures [17].
Niphargopsis casparyi (Pratz 1866) is a rare ecotone stygobiont (= true groundwater species) amphipod found in the Danube countries as well as in France, Southern Germany and Switzerland, inhabiting the Rhône and Rhine valleys (https://fauna-eu.org). Originally the species was taxonomically classified as Niphargus (Pratz 1866), however later Chevreux (1922) distinguished a sub-genus nested in Niphargus and named it Niphargopsis. The rare findings in Southern Germany led to the fact that N. casparyi appears on the Red List of endangered species, e.g. in Bavaria (www.lfu.bayern.de), however this might partly be due to the lack of research in groundwater ecology and data about stygobiont species. N. casparyi was collected from the Rhine valley together with P. slavus, at a depth of 20-50 m in a groundwater pipe (47°48'45.1''N, 7°32'505''E).
Proasellus slavus (Remy 1948) is a stygobiont groundwater isopod, typical for the pliocene Danube watershed and predominant distribution in Southern and Eastern Europe, incl. Poland (https://fauna-eu.org). In the upper Rhine valley is has already been found in 1996 [24]. This morphospecies seems to contain several cryptic DNA-based subspecies [25]. In contrast to the larger and actively swimming N. casparyi (> 1 cm), P. slavus remains smaller than 1 cm, is less active and moves by slow walking. It can inhabit dense substrates as it tolerates low oxygen levels. P. slavus co-occurred with N. casparyi and was also collected from the Rhine valley, at a depth of 20-50 m in a groundwater pipe (47°48'45.1''N, 7°32'505''E).
Experimental setup
As the aim of this study was to directly compare the sensitivity of the three test species, they were exposed to the same test conditions and water, to be able to assign the observed effects to Nitrate concentrations only and not to differences in water quality or test design. However, a few differences in the set-up were necessary in the chronic tests such as oxygenation of G. fossarum and provision of appropriate food (alder leaves to G. fossarum; fine (FPOM) detritus and ash leaves to N. casparyi and P. slavus). Filtered water from Lake Constance (drinking water quality) was used for all tests as G. fossarum could successfully be reared in this water for several years and the groundwater species showed no adverse effects during acclimation to this water. Lake Constance is part of the Rhine valley water system, where the groundwater species had been collected in groundwater horizons.
The acute tests were performed with G. fossarum and Niphargopsis casparyi in a thermostat (10 ℃) in darkness using the Multispecies Freshwater Biomonitor© (MFB) for 24 h in both 50 and 100 mg/l Nitrate and control treatments with filtered (100 µm) water from Lake Constance. Eight gammarids (7-9 mm of size) were randomly taken from the laboratory culture in filtered water from Lake Constance, placed individually in the 8 test chambers (2 cm diameter, 5 cm length) and exposed to filtered water from Lake Constance for 2 hours. Afterwards 4 randomly chosen test chambers were exposed in 50 mg/l Nitrate solution prepared with Lake water, whereas the remaining 4 chambers were exposed in Lake water only for 24 h. This experiment was performed twice. Survival and behavior (e.g. locomotion, gill ventilation) were recorded quantitatively in real time. At the end of the acute experiments survival was assessed by visual inspection. The niphargids had been sampled at the field site and acclimated to the laboratory conditions and filtered water from Lake Constance in the thermostat for 2 weeks prior to the experiment. The experiment was performed as described for the gammarids, however the water was not aerated during the continuous recordings in the MFB. The concentration of 50 mg/l was chosen as lowest test concentration as this is the legal limit for drinking water according to German law (Table 1).
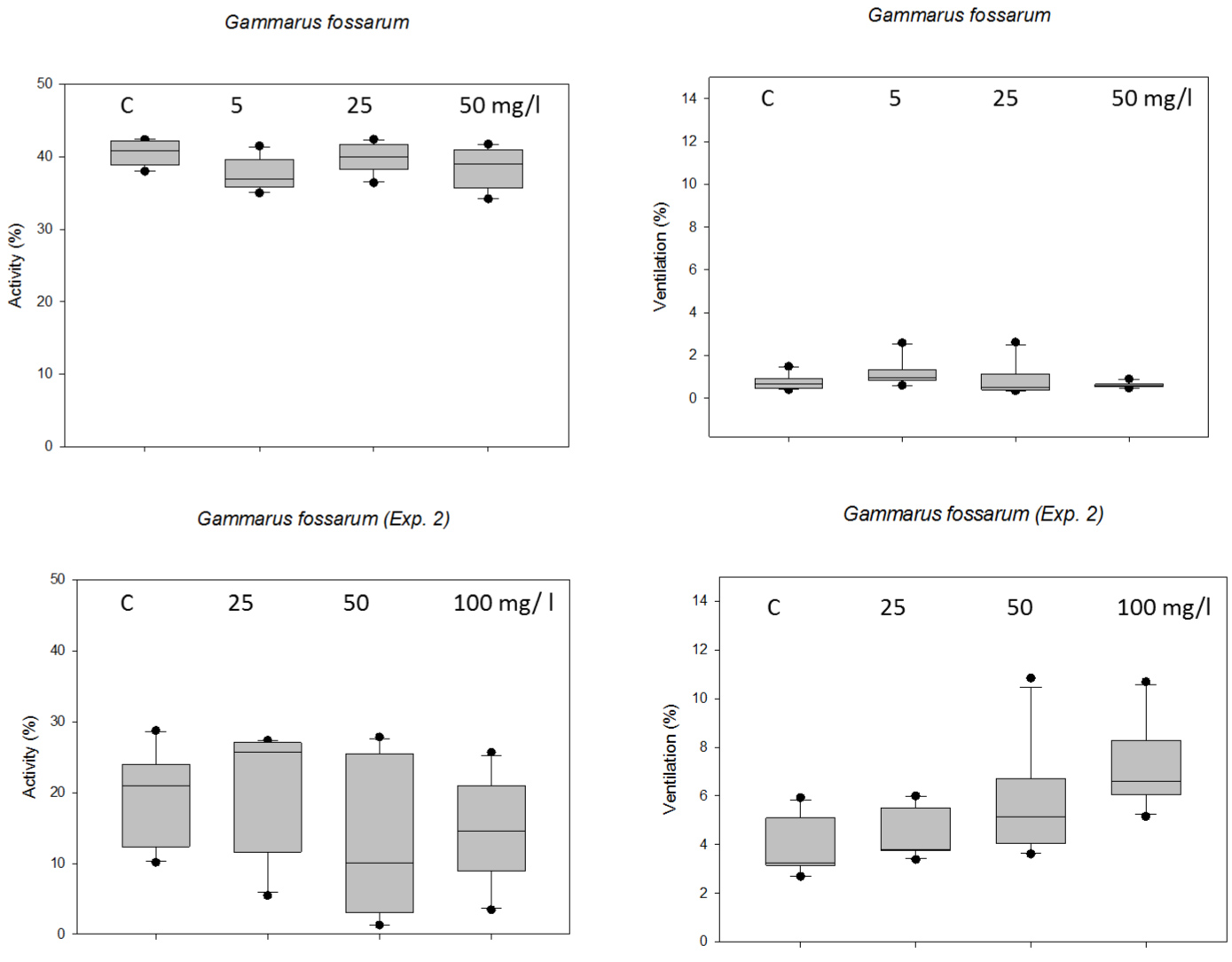
In the chronic toxicity tests, the three species were tested for 5 weeks, exposed in a dark thermostat (10 ℃ or 18 ℃) in a static water volume of 150 ml in glass beakers sealed with parafilm. Each of the 5 replicate beakers contained 5 specimens and a previously leached alder leaf (3 cm diameter) as food for G. fossarum, or a suspension of fine detritus from the sampling site (N. casparyi, P. slavus), ash leaves and pebbels. (9 g) as substrate for hiding. Before use, the pebbles had been incinerated for 1 hour at 500 ℃ to remove all organic matter. The alder leaves had been leached for 1 week in water from Lake Constance before use. The following concentration levels were tested in 5 replicates: 0, 25, 50 and 100 mg/l and solutions were prepared from KNO3 with filtered water from Lake Constance (drinking water quality). The beakers were aerated continuously by individual aquarium pumps for the experiments with G. fossarum in order to insure 100% oxygen saturation levels. Two chronic toxicity tests were performed, one at 18 ℃ (control, 5, 25, 50 mg/l Nitrate) and one at 10 ℃ (control, 25, 50, 100 mg/l Nitrate), the latter in order to better compare with the tests using groundwater species, which are adapted to low temperatures. For the groundwater species, only 3 replicates with each 3 specimens could be used in the chronic exposures to 50 and 100 mg/l Nitrate as not enough specimens could be sampled in the field.
Twice a week 8 randomly chosen animals per Nitrate concentration were selected randomly from the replicate beakers for quantitative behavior recordings in the Multispecies Freshwater Biomonitor© (MFB) for a duration of 2 hours in aerated water from Lake Constance under the same conditions as described above in the dark thermostat. Once a week, the water in the beakers was changed and both survival and feeding rates were determined. In the beginning and at the end of the experiments the leaves were weighed (dry weight after 3 days drying at room temperature), during the experiments the feeding rates were determined as leaf loss both by estimate and by image processing software (ImageJ). Nitrate levels were checked weekly before renewal of the water using a photometric test kit (Caldur, Spectro3) in order to get an idea of potential Nitrate losses. The mean Nitrate concentrations and SDs (N: 5) were for the control 1.7 (0.9), for 5 mg/l 4.4 (0.4), for 25 mg/l 26.9 (4.2), for 50 mg/l 46.5 (3.2) and 119.7 (6.5) for the resp. nominal concentration level of 100 mg/l.
As the groundwater crustaceans were collected from the field and their numbers were restricted only 3 replicates were performed. In each replicate beaker 3-5 specimens were placed randomly and 1 drop of detritus suspension from the sampling site was added, pebbles and additionally a piece of leached ash leaf (0.25 cm2). Ash seems to be the preferred food source for groundwater macrocrustaceans (N. Rütz, pers. communication). As groundwater species have a slower metabolism and the number of specimens was lower, a smaller leaf was added in order to avoid excess organic matter as potential source for uncontrolled fungal growth in the beakers. The beakers were not aerated to better mimic natural groundwater conditions.
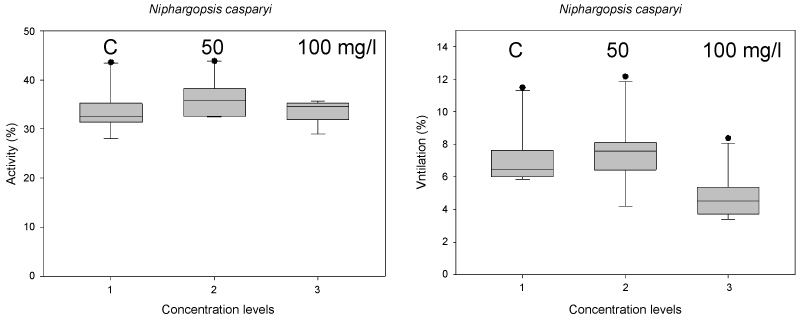
Twice a week 4 randomly chosen N. casparyi were used for the MFB online recordings of their behavior in control water as described above. P. slavus was not recorded in the MFB as it showed generally very low locomotory activity and no gill ventliation, providing not enough data for statistical comparisons. Moreover, P. slavus was smaller and more sensitive to handling with the pipette compared to the amphipods. Once a week survival and feeding was monitored in every beaker before the water was renewed and Nitrate concentration were measured as described above. The Nitrate levels and SDs (N: 3) were 2.3 (0.9) and 44.3 (3.1).
Data analysis
Data were generated by the MFB-software, exported to Excel and then treated in Sigma Plot 12 for both graphical presentation and statistical testing using non-parametric tests for comparisons of either two or multiple groups for time-dependent data and repeated measurements (RM), such as one way ANOVA on Ranks (chronic tests) and RM one way ANOVA on Ranks (acute tests). Feeding data were analyzed with the freely available software package ImageJ.
Results
Acute toxicity tests
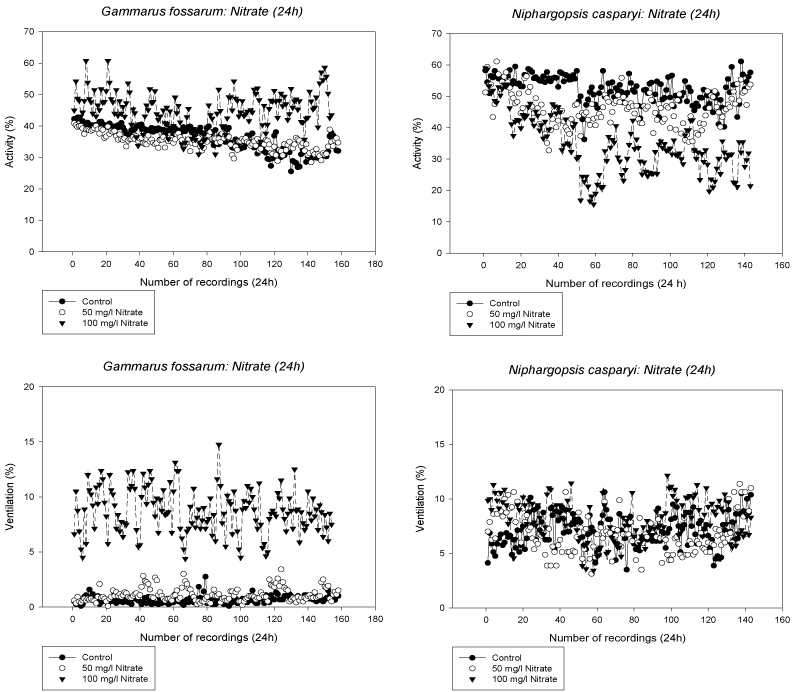
G. fossarum showed a variable locomotory activity of generally higher levels in the 100 mg/l Nitrate group, compared to the 50 mg/l Nitrate treatment group, the latter did not differ significantly from the control throughout the whole exposure time (p < 0.05, RM ANOVA on Ranks for 100 mg/l vs. c, 50 mg/l) (Figure 1). Gill ventilation activity of G. fossarum was significantly higher at 100 mg/l Nitrate compared to the control and 50 mg/l groups respectively (p < 0.001, RM ANOVA on Ranks for 100 mg/l vs. c, 50 mg/l). All animals survived all treatments to 100%.
During the first 2 hours of behavioral measurements (i.e. 12 recordings) N. casparyi was highly active in all treatments (N: 2 × 4, R: 2), after the change of control water to the Nitrate solutions the locomotory activity of the niphargids decreased immediately in both 50 and 100 mg/l Nitrate. Another drastic decrease in locomotion occurred in the 100 mg/l Nitrate treatment after 5 hours (ca. 50 recordings) of exposure, thereafter niphargids remained less active compared to the control and the 50 mg/l treatment group ((p < 0.05, RM ANOVA on Ranks for all groups). Ventilation activity was recorded at low activity levels in all treatments without any statistical difference. During the exposure 37.5% of the animals died at 100 mg/l Nitrate, whereas all animals survived the control and the 50 mg/l Nitrate treatment (Figure 1).
Chronic toxicity tests
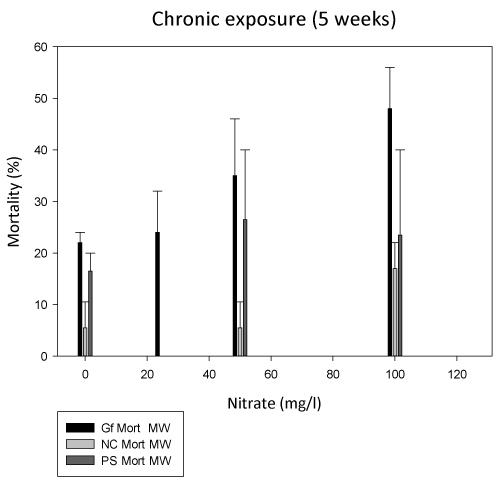
Mortality: The trends in mortality are shown in Figure 2. In spite of the artificial conditions in the laboratory exposure for a long duration of 5 weeks, mortality never reached 50% in the experiments. The highest mortality was reached in a control during the experiments with G. fossarum at 18 ℃, probably due to some problems with the individual aeration in some beakers. In the subsequent experiment at 10 ℃, mortality of G. fossarum in the control reached only 24%. "Mortality" was determined as loss of specimens, hence included potential cannibalism, too. Apart from the high control mortality at 18 ℃, mortality of G. fossarum in the two experiments at both temperatures did not differ regarding the Nitrate exposure groups. When including 100 mg/l Nitrate the slope indicates a concentration-dependent increase in mortality of the animals (RM ANOVA on Ranks, p < 0.025).
Niphargus casparyi showed 5.5% control mortality and only slightly elevated mortality at 100 mg/l Nitrate. The same was true for Proasellus slavus, which was more sensitive to handling compared to N. casparyi, thus showed higher control mortality and no Nitrate dependent effects (Figure 2).
Feeding
Neither N. casparyi nor P. slavus fed from the ash leaves in any treatment group.
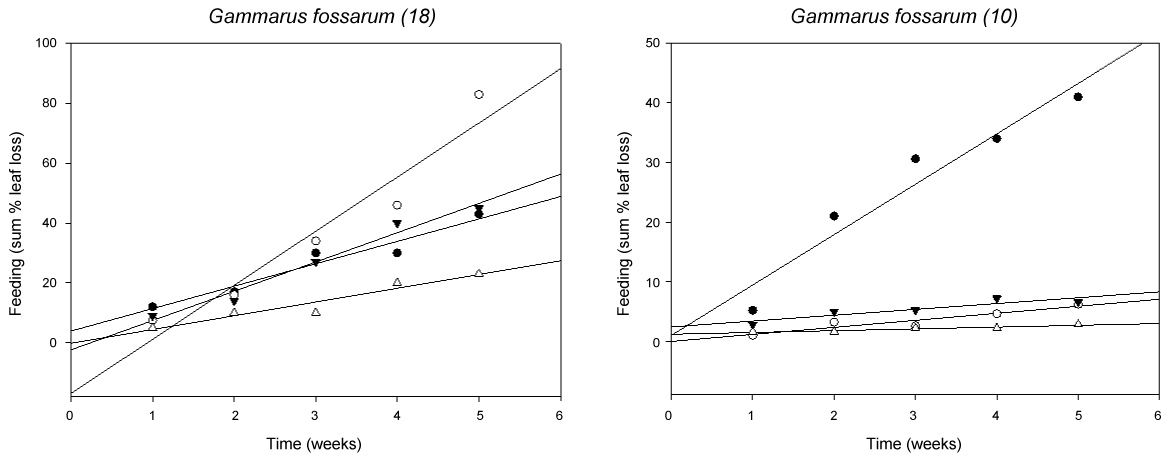
Feeding activity by G. fossarum was calculated as cumulative % leaf loss by estimation (0, 5, 25, 50, 75, 100%) in different classes and presented as means of the 5 replicate beakers per treatment group in (Figure 3).
The alder leaves were eaten to a similar extent throughout the whole exposure time as similar slopes indicate, except for a strong increase in feeding in week 5 in the 5 mg/l Nitrate treatment (Figure 3 left). The ANOVA on Ranks did not reveal any significant concentration-dependent difference in feeding activity of G. fossarum at 18 ℃. In the experiment at 10 ℃, however, feeding activity of G. fossarum was significantly lower in all Nitrate treatments (below 10% of the alder leaf) (p < 0.05, RM ANOVA on Ranks), and only feeding in the control was higher and similar to that at 18 ℃ (Figure 3 right).
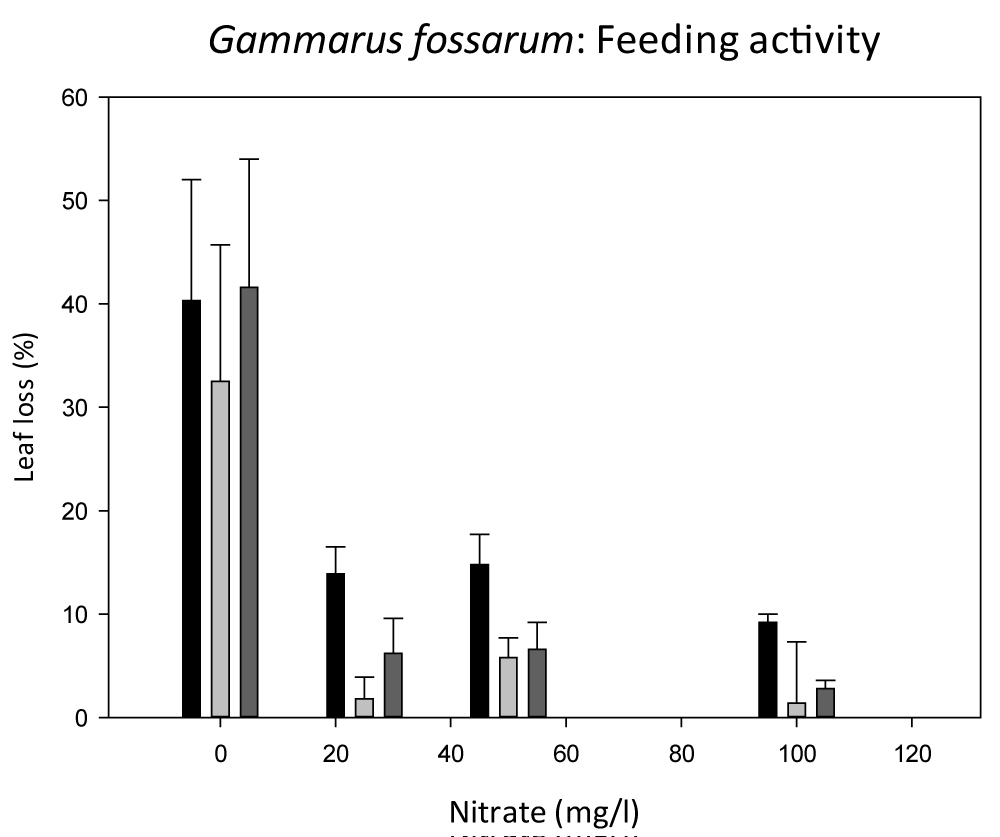
When the weekly leaf loss during 5 week's exposure was compared regarding a potential effect of Nitrate an almost significant difference between 50 and 100 mg/l Nitrate was found when the method of estimation was used (p = 0.08), with Image J analysis this trend became more significant (p = 0.04). Comparing the different feeding analysis methods at the end of the experiment (Figure 4) all methods showed a concentration-dependent decrease in feeding activity, whereof the weighing of air-dried leaves before and after the experiment was the most exact method. Weighing produced the highest feeding rates, followed by estimation and the lowest feeding rates were generated with ImageJ analysis (being significantly different from weighing: p < 0.05, RM AVOVA on Ranks) (Figure 4).
Behavior
Gammarus fossarum: Comparing both chronic toxicity tests with G. fossarum generally their locomotory activity was higher at 18 ℃ than at lower temperature (Figure 5 top versus bottom). At 5 mg/l Nitrate the animals were significantly less active (p = 0.012) and showed more ventilation (p = 0.027) in the high temperature experiment (ANOVA on Ranks). At low temperature, significant differences in ventilation could be seen at 100 mg/l Nitrate compared to the control and lower Nitrate concentrations (p < 0.001, ANOVA on Ranks), whereas locomotion was very variable and generally low in all treatments (Figure 5).
Niphargopsis casparyi: The locomotory activity of N. casparyi was generally high troughout the whole exposure period and independent of the Nitrate levels (ANOVA on Ranks, ns). Ventilation activity, however, was significantly reduced at 100 mg/l Nitrate (ANOVA on Ranks, p < 0.005) (Figure 6).
Discussion
During the acute toxicity studies of 24 h continuous recording in the biomonitor G. fossarum showed behavioral effects only at 100 mg/l Nitrate, consisting of an increase in both locomotion and ventilation. All animals survived. This finding from the laboratory study could be verified in a field study. During a 24 h exposure of G. fossarum in an in situ groundwater pipe with continuous recording of survival and behavior in the Multispecies Freshwater Biomonitor© (MFB) the amphipods directly responded to irrigation and elevated Nitrate (up to 300 mg/l Nitrate), which was washed out, showing decreasing locomotory activity [26]. N. casparyi showed decreasing locomotion at 50 mg/l (trend) and 100 mg/l Nitrate and elevated mortality at 100 mg/l Nitrate (37%). It might be concluded that the groundwater species N. casparyi is more sensitive towards Nitrate in short term exposures in the laboratory compared to the surface water gammarid. Nordin, et al. [27] showed that the acute toxicity of Nitrate recorded as mortality in the invasive surface water crustacean species E. echinosetosus (62.5 mg/l Nitrate) and the trichopteran Hydropsyche occidentalis (97.4 mg/l) were above the threshold values for drinking water (45 mg/l Nitrate) in British Columbia. LC50-48h values for different invertebrates revealed the surface water crustacean species E. toletanus (E.t.) and Echinogammarus echinosetosus (E.e.) to be more sensitive towards NO3 compared to Daphnia magna (E. t.: 180 mg/l; E.e.: 107 mg/l; D. magna neonates: 465 mg/l), whereby the trichopteran such as Cheumatopsyche pettiti was the most sensitive species studied: their LC0.01-120h for the early and the last larval instars were for 29.5 mg/l resp. 42.2 mg/l, showing early life stages to be more sensitive than older larval stages.
This reveals that 1% of the population of early insect instars of hydropsychids might not be protected by the current drinking water limits. The LC50-120h for Nitrate were 321.6 mg/l (E. toletanus) and 247.3 mg/l (E. echinosetosus), before death gammarids showed abnormal locomotion [10]. The LC50-96h for Nitrite-N was 2.59 mg/l for E. echinosetosus and 2.09 mg/l for E. toletanus [28]. During a 7 day long in situ exposure of Niphargopsis casparyi in a groundwater pipe continuous online monitoring with the Multispecies Freshwater Biomonitor showed decreasing locomotory activity of N. casparyi to elevated Nitrate (1st exp.: 94 mg/l Nitrate) and increased mortality due to elevated Nitrite (2nd exp.: 0.42 mg/l Nitrite) combined with elevated Sulfamethoxazole (0.07 mg/l) in the 2nd experiment performed as artificial irrigation after application of pig manure fertilizer spiked with the antibiotic [26]. These field experiments represented pulses of realistic applications of organic fertilizer and N-depots in the soils being washed out during rainfall events, hence symbolizing acute exposures leading to adverse effects on both behavior and survival in N. casparyi.
During the chronic tests of 5 weeks' duration, G. fossarum survived less well than N. casparyi and P. slavus in the laboratory exposures, while all species showed a concentration-dependend increase in mortality under Nitrate exposure. Moreover, G. fossarum showed the following effects of Nitrate: decreased feeding (>= 25 mg/l Nitrate), lower locomotion (100 mg/l Nitrate) and higher ventilation (>= 50 mg/l Nitrate). This means, that the threshold value of 50 mg/l Nitrate in groundwater might not be safe for G. fossarum during a continuous exposure period of 5 weeks. However, Nitrate levels might remain high during much longer periods, as shown by the long-lasting monitoring in the past years at sites in Germany, i.e. more significant time span of the life cycle of the crustaceans are under Nitrate pressure. The few literature studies about chronic Nitrate exposures using surface water invertebrates showed the most sensitive species being Delatidium sp. (Ephemeroptera) with an NOEC of 20.3 mg/l Nitrate-N (89.32 mg/l Nitrate) and Ceriodaphnia dubia 17 mg/l Nitrate-N (74.8 mg/l Nitrate) [29]. Camargo, et al. [10] listed several toxicity studies with Nitrate, e.g. 7 day tests with Ceriodaphnia dubia resulted in very variable LOEC (mortality) values ranging from 62.04 to 497.2 mg/l Nitrate. Comparing these values with the present study, G. fossarum proved to be at least as sensitive as the mayfly and the daphnids.
The groundwater amphipod N. casparyi showed no effects on locomotion but less ventilation at 100 mg/l Nitrate. Under chronic exposures both groundwater crustaceans were more robust regarding survival at high Nitrate values. Groundwater species have a slower metabolism and show less locomotory activity, i.e. they might be able to spare energy resources, which they can allocate for detoxification processes. Due to the fact that groundwater species show slower growth and metabolism they might demand even longer exposure periods than 5 weeks to show chronic persistent effects [30]. Di Lorenzo and Galassi [11] found in a field study that up to 150 mg/l Nitrate the biodiversity of groundwater invertebrates appeared unaffected.
However, acute exposures to Nitrate revealed a higher sensitivity of N. casparyi compared to G. fossarum, which might indicate different mechanisms behind acute and chronic toxicity expression. Acute salt stress in the otherwise usually very constant groundwater conditions might provoke immediate osmoregulative stress, whereas G. fossarum, used to varying surface water levels and temperatures as well as different salt emissions from the environment might be better adapted to tolerate higher variations of these parameters. On the other hand, seawater crustaceans were more tolerant to Nitrate due to an anticipated ameliorating effect of salinity, i.e. the presence of other ions such as sodium, calcium, chlorine, etc., which might either bind or outcompete Nitrate at receptor sites [3,10].
The toxic effects of Nitrate have been summarized for humans and other mammals [3], revealing acute effects such as changes in heart rate, blood pressure, hypotension and methemoglobin formation via transformation of Nitrate into Nitrite. In aquatic invertebrates these effects might lead to increased gill ventilation (respiration), as has been observed in the surface water G. fossarum, but not in the groundwater N. casparyi in the present study. Reported chronic effects include increased aggressive behavior (in rats), different types of cancers due to nitrosamine formation in the stomach and hypothyroidism [3]. In our study G. fossarum showed higher cannibalism at high Nitrate levels at 18 ℃ compared to 10 ℃, which might be due to either elevated general activity at higher temperatures and/or increased agressivity at higher Nitrate levels. Nitrate is taken up in the intestinal tract of humans via active transport (sodium iodide symporter (NIS)), whereas Nitrite is taken up via diffusion across the gastric mucosa. About 80% of Nitrite in humans is being transformed by in vivo reduction of Nitrate [3]. The observed chronic toxic effects of high Nitrate concentrations might also partly be due to transformation into Nitrite in their bodies.
Comparing both groundwater macrocrustaceans N. casparyi was larger, more easy to handle, provided good signals in the MFB biomonitor due to higher inherent activity levels compared to P. slavus. Moreover, N. casparyi could be maintained easily in the laboratory in a thermostat at 10 ℃ with filtered water from Lake Constance without aeration in darkness for 12 months. Therefore, we recommend to use N. casparyi or close relative Niphargus spp. to study ecotoxicity and monitor groundwater in future. However, further studies are needed to compare directly both amphipod species (G. fossarum versus Niphargus spp.) regarding their sensitivity towards pesticides and pharmaceuticals, relevant for groundwater pollution. Only direct comparisons under similar test conditions will allow to decide the question which species are more sensitive to pollutants relevant for groundwater.
In conclusion, the present studies show that the German drinking water limit of 50 mg/l Nitrate might not be safe to protect the tested Crustacean species, leading to behavioral and also to increased mortality both under acute and long-term continuous exposure. Several authors already proposed lower limits such as 10 mg/l Nitrate [10,30], where by Camargo, et al. [10] even suggested 2 mg/l Nitrate to protect also the most sensitive species and their most sensitive life stages.
Acknowledgements
Natalie Badouin and Marlene Randl are acknowledged for the skillful support in the performance of the toxicity tests. The Niphargopsis casparyi was taxonomically determined by Cene Fiser, University of Ljubljana, Slovenia. The study was co-financed by the German Federal Ministry of Education and Research (BMBF) as part of the funding measure "Regional Water Resource Management for Sustainable Protection of Waters in Germany" (ReWaM) on the project GroundCare (funding code 033W037H).
References
- Agarwal M, Singh M, Hussain J (2019) Assessment of groundwater quality with specific emphasis on Nitrate contamination in parts of the Gautaum Budh Nagar District, Uttar Pradesh, India. Acta Geochim 38: 703-717.
- Umweltbundesamt (2016) Trinkwasser.
- ATSDR (2017) Toxicological profile for Nitrate and Nitrite. Agency for Toxic Substances and Disease Registry (ATSDR), US Department of Health and Human Services. Atlanta, Georgia 273: 30329-4027.
- Merlot J (2018) Nitrat im grundwasser-Darum geht es in der gülleklage. Spiegel Online.
- Eulenstein F, Pickert J, Cremer N, et al. (2018) Stickstoffeintrag in Oberflächengewässer und Grundwasser in Deutschland auf Basis bundesweiter Auswertungen. Korrespondenz Wasserwirtschaft, Nr. 6.
- Rastetter N, A Gerhardt (2017) Toxic potential of different types of sewage sludge as fertiliser in agriculture: ecotoxicological effects on aquatic and soil indicator species. Journal of Soil and Sediments 17: 106-121.
- Grimm F, Fischer D, Keppner L (2017) Nitratbericht 2016, (in German), Bundesmin. f. Ernährung und Landwirtschaft und Bundesmin. F. Umwelt, Naturschutz, Bau und Reaktorsicherheit (Hrsg.), 99.
- Riedel T, Lersch SS, Aus der Beek T (2019) Umgang mit Zielkonflikten bei der Anpassung der Wasserwirtschaft an den Klimawandel. Abschlussbericht. (erstellt von IWW Rheinisch-Westfälisches Institut für Wasserforschung, gGmbH), Bund/Länder-Arbeitsgemeinschaft Wasser, LAWA, (Hrsg.), Erfurt, 55.
- RaulGarzon-Vidueira, RaquelRial-Otero, M LuzGarcia, et al. (2020) Identification of nitrates origin in Limia river basin and pollution-determinant factors. Agriculture, Ecosystems & Environment 290: 1106775.
- Camargo JA, Alonso A, Salamanca A (2005) Nitrate toxicity to aquatic animals: a review with new data for freshwater invertebrates. Chemosphere 58: 1255-1267.
- Di Lorenzo T, DMP Galassi (2013) Agricultural impact in Mediterranean alluvial aquifers: do groundwater communities respond? Fund. Appl Limnol 182: 271-282.
- AlonsoA, JA Camargo (2006) Toxicity of nitrite to three species of freshwater invertebrates. Environm Toxicol 21: 90-94.
- EPA (Environmental Protection Agency) (2003) Guidance for the assessment of environmental factors: consideration of subterranean fauna in groundwater and caves during environmental impact assessment in Western Australia. EPA, Perth, Australia.
- EU (European Union) (2006) Grundwasser Richtlinie 2006/118/EU des Europäischen Parlaments und des Rates. Amtsblatt der Europäischen Union L 19: 372.
- Schäfers C (2017) Hazard assessment of active substances in groundwater -what do we want to protect (in German). Zbl Geol Paläont, Teil 1, Heft 1: 5-11.
- Castano-Sanchez A, Hose GC, Reboleira ASPS (2020) Ecotoxicological effects of anthropogenic stressors in subterranean organisms: a review. Chemosphere 244: 125422.
- Mösslacher F, J Notenboom (1999) Groundwater Biomonitoring. In: Gerhardt A, Biomonitoring of polluted water. Trans Tech Publications, Environmental Research Forum 9: 119-140.
- Grimm C, A Gerhardt (2018) Sensitivity towards copper: comparison of stygal and surface water species' biomonitoring performance in water quality surveillance. Intern J Sci Res Environm Sci Toxicology 3: 15.
- Gerhardt A (2019) Plastic additive Bisphenol A: toxicity in surface- and groundwater crustaceans. Journal of Toxicology and Risk Assessment 5: 1-10.
- Gerhardt A (2010) Gammarus spp. Rückgang oder Fehlen von Bachflohkrebsen (Gammarus spp.) in Bächen. Aquaplus (Hrsg.), Zug, im Auftrag des Dept. Bau, Verkehr, Umwelt, Kanton Aargau, 56.
- Garmendia-Tolosa AJ, B Axelsson (1993) Gammarus, their biology, sensitivity and significance as test organisms. Swedish Environmental Research Institute, Report B-1095, Stockholm, 88.
- Mccahon CP, D Pascoe (1988) Use of Gammarus pulex (L.) in safety evaluation tests: Culture and selection of a sensitive life stage. Ecotoxicol Environm Safety 15: 245-252.
- Hübner G (2007) Okologisch faunistische Fliessgewässerbewertung am Beispiel der salzbelasteten unteren Werra und ausgewählter Zuflüsse. Okologie und Umweltsicherung 27: 1-303.
- Titizer T, F Krebs (1996) Okosystemforschung: Der Rhein und seine Auen: Eine Bilanz. Bundesanstalt für Gewässerkunde, 262.
- Culver DC, T Pipan (2019) The biology of caves and other subterranean habitats. Oxford Univ Press, Oxford, United Kingdom.
- Gerhardt A, Badouin N, Weiler M (2020) In situ online biomonitoring of groundwater quality with freshwater amphipods exposed to organic fertilizer and rainfall events. Research Trends: Current Topics in Toxicology (in press).
- Nordin RN, Pommen LW, Meays CL (2009) Water quality guidelines for Nitrogen (Nitrate, Nitrite, Ammonia). Report for Ministry Environment, British Columbia, 29.
- Camargo JA, A Alonso (2006) Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: a global assessment. Environment International 32: 831-849.
- Hickey CW (2013) Updating Nitrate toxicity effects on freshwater aquatic species. Ministry Building, Innovation & Employment, NIWA report, HAM 2013-009, ELF 13207: 39.
- OERTEL A, STRAUB R (2009) Biological underwater research by the Blautopf working group in the Blautopf cave (Cat.No. 7524/30b) from 1997-2009. 118-124.
Corresponding Author
Gerhardt A, Lim Co International GmbH, Wollmatinger Str. 22, D-78467 Konstanz, Germany.
Copyright
© 2020 Gerhardt A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.