Screening of Chronic Kidney Disease in Primary Health: Comparison of the Urine Dipstick Albumin-to-Creatinine Ratio and Dipstick Proteinuria
Abstract
Background: Chronic kidney disease (CKD) needs to be detected early in order to prevent a poor outcome in the general population. A semiquantitative evaluation based on a dipstick has become available to detect the urinary albumin-to-creatinine ratio (ACR) and proteinuria simultaneously in spot urine samples. The aim of this study was to compare dipstick ACR with proteinuria for CKD screening in a primary healthcare setting.
Methods: This cross-sectional study analyzed 88,479 subjects who underwent a health check up at 16 health promotion centers in 13 Korean cities. Dipstick ACR and proteinuria was measured using the automated urine test strip analyzer UC-3500 (Sysmex, Kobe, Japan). CKD definition and risk categories according to the 2012 Kidney Disease: Improving Global Outcomes guidelines were created using a combination of eGFR and albuminuria. Albuminuria was defined using dipstick ACR ≥ 30 mg/g and dipstick proteinuria as ≥ trace or protein-to-creatinine ratio (PCR) ≥ 150 mg/g.
Results: The prevalence of CKD using dipstick ACR, proteinuria, and PCR were 16.3%, 12.7%, and 11.9%, respectively. The concordance rates between the dipstick ACR- and proteinuria- or PCR-based CKD risk categories were 88.76% (κ = 0.567) and 92.06% (κ = 0.683), respectively. On being grouped according to dipstick proteinuria, CKD risk categories would be underestimated than be overestimated. 37.2% and 37.6% of the subjects with ≥ moderately increased CKD risk using ACR-based category were reclassified into lower risk CKD using dipstick proteinuria (≥ trace) and PCR, respectively.
Conclusion: The CKD risk category using dipstick proteinuria was underestimated compared to the ACR-based CKD risk category. These data suggest that screening of CKD using dipstick ACR is recommended in primary healthcare settings.
Keywords
Chronic kidney disease, Dipstick albumin-to-creatinine ratio, Protein-to-creatinine ratio, Dipstick proteinuria, Spot urine
Introduction
Chronic kidney disease (CKD) is being increasingly recognized as a major global health problem [1]. CKD is considered a significant risk factor for not only end-stage kidney disease but also cardiovascular disease and premature death [2,3]. It needs to be detected early in order to prevent a poor outcome, but it is usually asymptomatic in its early stages. It can be initially suspected based on routine laboratory tests such as the estimated glomerular filtration rate (eGFR) or the presence of kidney damage markers such as proteinuria or albuminuria.
The 2012 KDIGO guidelines recommend that CKD can be classified based on cause, the GFR category, and the albuminuria category. And it was grouped the eGFR and the albuminuria categories with a similar relative risk for adverse outcomes into three risk categories: Moderately increased risk, high risk, and very high risk [4]. Albumin is the principle component of urinary protein in most kidney disease, and it is the most sensitive marker for kidney damage and CKD [5]. The early detection of urinary albumin is a key prognostic biomarker for CKD. However, dipstick proteinuria is more widely used than albuminuria to screen the risk of CKD due to it being a low-cost, simple, and rapid measurement technique suitable for use in primary care [6,7]. In the Korean National Health Screening Program (NHSP), dipstick proteinuria and eGFR have been used for screening of CKD. If there is a positive proteinuria on dipstick or eGFR < 60 mL/min/1.73 m2, NHSP classified it as abnormal and recommend a nephrology referral.
Albuminuria and proteinuria can be detected using spot urine samples via direct measurements of urinary albumin or protein concentrations. And the urine dipstick test can be used to detect albuminuria, semiquantitatively as albumin-to-creatinine ratio (ACR) in spot urine [8]. There is a strong positive correlation between the urinary albumin levels measured in a dipstick analysis and immunonephelometry assay [9]. Recently, the urine test strip was designed to screen for urinary ACR, which is included as an additional examination in urinalysis [10]. Few studies have evaluated the urinary dipstick ACR and dipstick proteinuria simultaneously using spot urine samples for CKD screening. The aim of this study was to compare urinary dipstick ACR with dipstick proteinuria for CKD screening in a general population.
Materials and Methods
Study subjects
This study had a cross-sectional design. The study subjects were recruited from the participants who underwent a health checkup including urinary ACR and proteinuria using a dipstick at 16 health promotion centers in 13 Korean cities between January 2018 and September 2019. The 16 health-promotion centers belongs to Korea Association of Health Promotion, and comprise 3 centers in Seoul, 2 in Daegu, and 1 in each of Busan, Ulsan, Changwon, Incheon, Jeonju, Kwangju, Daejeon, Suwon, Chuncheon, Chungju, and Jeju. These health-promotion centers perform more than 10% of the health checkups that are provided by the National Health Insurance Service (NHIS) in Korea. The NHIS provides a health checkup to Koreans biannually. Their medical records were also reviewed. Demographics and clinical characteristics such as age, sex, body mass index (BMI), and blood pressure were gathered. After excluding 183 subjects who were younger than 18 years from among the initial 88,661 health examinees, 88,478 subjects (48,265 males and 40,213 females) were included in this study. The study protocol was reviewed and approved by the Korea Association of Health Promotion review board (Approval no: 130750-202005-HR-007).
Clinical and laboratory measurements
Blood samples were collected from the antecubital vein of each subject in a sitting position after fasting for > 8 hours, and random spot urine samples were also obtained from the subjects. The blood samples were centrifuged and the serums were stored in refrigerator before analysis. Those were measured in a laboratory at each health promotion center. The biochemical measurements, such as blood glucose, lipids and serum creatinine, were measured using the Hitachi 7600 analyzer (Hitach, Tokyo, Japan).The ACR and urinalysis were completed within 2 hours of urine collection. The model of analysers and methodologies used for laboratory analyses were the same in all center laboratories.
ACR and proteinuria were measured using urine test strip analyzer UC-3500 (Sysmex, Kobe, Japan). Test strips (Meditape UC-11A, Sysmex, Kobe, Japan) were used in this study. The test strip uses the following three pads (two for albumin and third for creatinine): A sensitive pad containing 10 μg of tetrabromphenol blue to measure albumin up to 150 mg/L, a less-sensitive pad that contains 15 μg of tetrabromphenol blue for measuring albumin concentrations above 10 g/L, and a creatinine-pad based on the Benedict-Behre method with a measuring range from 10 to 300 mg/dL. The ACR is automatically computed according to the settings in the analyzer. The semiquantitative ACR is reported as "normal" when below 30 mg/g, "moderately increased albuminuria" when the value is between 30 and 300 mg/g, and "severely increased albuminuria" when above 300 mg/g; additionally, the analyzer semiquantitatively reports the albumin level as 10, 30, 80 or 150 mg/L, and the creatinine level as 10, 50, 100, 200 or 300 mg/dL. UC-CONTROL-L and UC-CONTROL-H (Sysmex, Kobe, Japan) was used for quality controlling urine test strip analyzer UC-3500. Internal quality control measures and calibration were performed one run a day. Devices in the all center laboratories participate in the proficiency tests programs by the Korean Association of Quality Assessment Service. In addition, laboratories in all centers have performed validation studies of ACR by standard method on the introduction of the method into the laboratories. The sensitivity/specificity for protein and albumin was 94.2/88.2% and 81.8/89.2%, respectively. The urinary protein was detected by protein error of a pH indicator. Dipstick proteinuria is reported as trace, 1+, 2+, or 3+, which corresponds to 15, 30, 100, or 300 mg/dL, respectively. The protein-to-creatinine ratio (PCR) was calculated based on paired spot urine protein and creatinine results obtained from dipstick.
The serum creatinine concentration was measured with the Jaffe rate-blanked colorimetric method using the Hitachi Automatic Analyzer 7600 (Hitachi, Tokyo, Japan). The eGFR was calculated using the following equation from the Modification of Diet in Renal Disease study (MDRD): eGFR (mL/min/1.73 m2) = 175x (serum creatinine)-1.154 × (age)-0.203 × (0.742 if female) [11]. Albuminuria was defined using dipstick ACR ≥ 30 mg/g in this study. In accordance with the KDIGO staging system, we used five eGFR categories (G1, G2, G3a, G3b, and G4/5, corresponding to eGFR ≥ 90, 60-89, 45-59, 30-44, and < 30 mL/min/1.73 m2, respectively), three albuminuria categories (A1, ACR < 30 mg/g; A2, ACR 30-300 mg/g; A3, ACR > 300 mg/g) and approximately equivalent three proteinuria categories (P1,negative or PCR < 150 mg/g; P2, trace or PCR = 150-500 mg/g; and P3, ≥ 1+ or > 500 mg/g) [12].
CKD was defined as ACR ≥ 30 mg/g and/or eGFR < 60 mL/min/1.73 m2. Moreover, CKD was also defined as PCR ≥ 150 mg/g (trace) and/or eGFR < 60 mL/min/1.73 m2. In subjects with CKD, the eGFR and albuminuria categories were grouped based on similar relative risks for adverse outcomes into the following three risk categories: Moderately increased risk, G3a-A1 or G1/2-A2; high risk, G3b-A1, G3a-A2, or G1/2-A3; and very high risk, G4/5-A1, G3b-5-A2, or G3a-5-A3 [4]. CKD was also classified according to the conventional staging system as follows: Stage 1, G1-A2/3; stage 2, G2-A2/3; stage 3a, G3a-A1-3; stage 3b, G3b-A1-3; and stage 4/5, G4/5-A1-3.
Statistical analysis
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). Data are presented as mean ± standard deviation and frequency (percentage) values. A univariate analysis was performed to assess differences between the three ACR categories or PCR categories using ANOVA and χ2 tests. The differences between groups were analyzed by using Scheffe's multiple-comparisons test. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the receiver operating characteristics curve (AUC) for ACR ≥ 30 mg/g, PCR ≥ 150 mg/g, and urine protein dipstick positivity ≥ trace (or ≥ 1+) for ≥ moderately increased risk and ≥ high risk were evaluated using receiver operating characteristics analysis.
Results
Demographic and clinical characteristics of the study subjects according to urinary ACR and proteinuria
The mean age of the subjects was 55.2 years (age range = 18-97 years), 54.6% of them were male, and their mean eGFR was 78.55 mL/min/1.73 m2. Subjects with albuminuria (ACR ≥ 30 mg/g) or proteinuria (PCR ≥ 150 mg/g) were older and had a higher prevalence of comorbidities such as obesity, hypertension, diabetes, and dyslipidemia compared to subjects without albuminuria (ACR < 30 mg/g) or proteinuria (PCR < 150 mg/g) (Table 1).
Distribution of albuminuria and proteinuria among study subjects
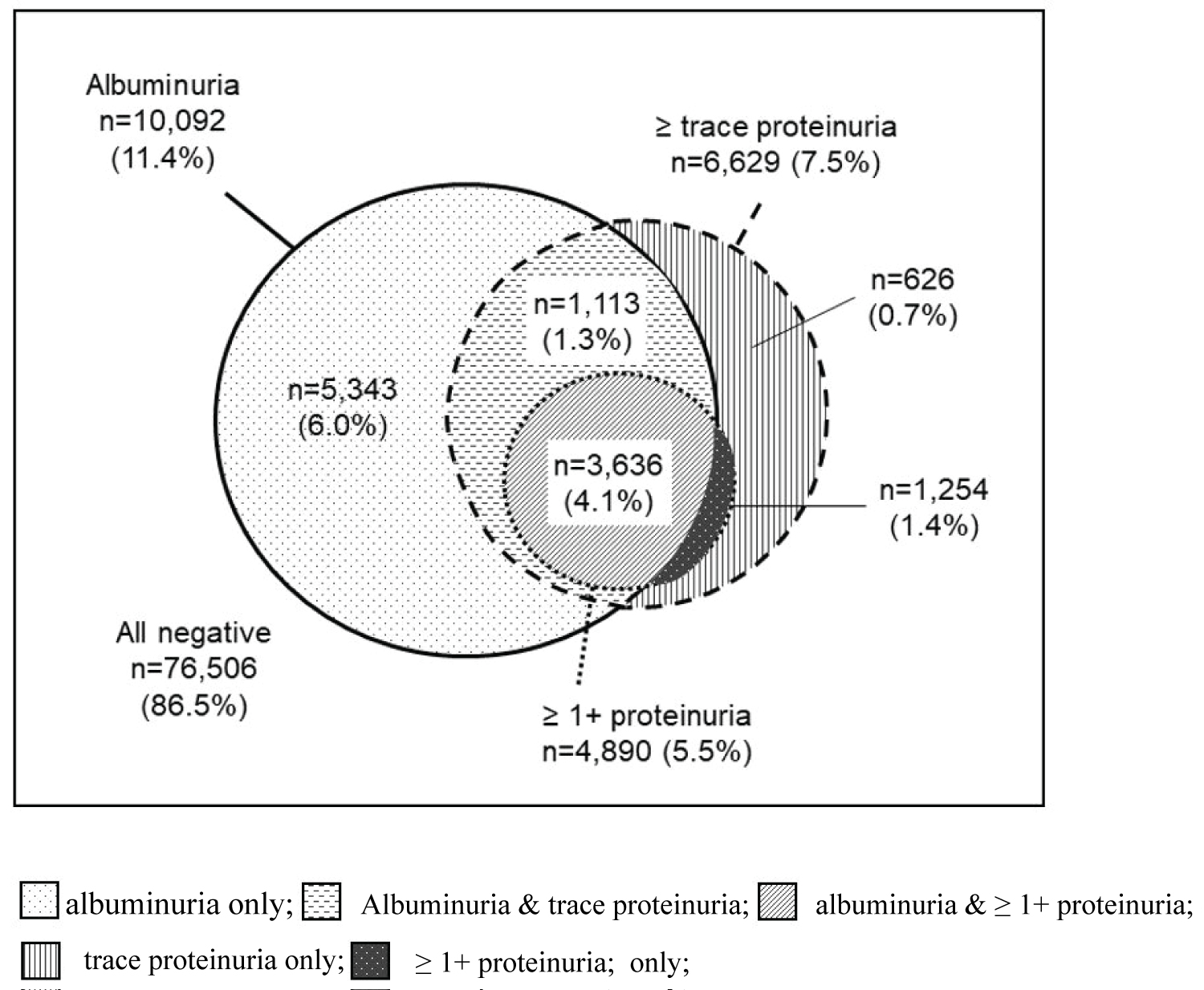
The distribution of albuminuria (ACR ≥ 30 mg/g), ≥ trace proteinuria, and ≥ 1+ proteinuria among the study subjects is shown in Figure 1. Six percent of all of the study subjects had albuminuria without proteinuria. More than a quarter of the subjects with proteinuria (28.3% of subjects with ≥ trace proteinuria and 25.6% of subjects with ≥ 1+ proteinuria) did not have albuminuria.
Classification of study subjects by eGFR and albuminuria or proteinuria categories
The category distributions based on eGFR and albuminuria or proteinuria are presented in Table 2. The total prevalence of albuminuria with ACR ≥ 30 mg/g was 11.4%, comprising 9.5% with ACR = 30-300 mg/g and 1.9% with ACR > 300 mg/g. The total prevalence of proteinuria with PCR ≥ 150 mg/g was 6.6% (7.5% with ≥ trace proteinuria), comprising 5.2% with PCR = 150-500 mg/g, (2.0% with trace proteinuria,) and 1.9% with PCR > 500 mg/g, (1.4% with ≥ 1+ proteinuria). The total prevalence of decreased GFR with an eGFR < 60 mL/min/1.73 m2 was 6.1%, comprising 5.6%, 0.42%, and 0.079% at G3a, G3b, and G4/5, respectively. The prevalence of CKD by conventional staging system was 16.5%, comprising 2.6%, 7.7%, 5.7%, 0.42%, and 0.079% at stages 1, 2, 3a, 3b, and 4/5, respectively.
Prevalence of CKD by risk category and agreement of CKD categories between ACR- and proteinuria-based classifications
Prevalence of CKD by risk category and agreement of CKD categories between ACR- and proteinuria-based classifications are presented in Table 3. CKD patients were categorized into each risk category based on eGFR value and severity of albuminuria according to 2012 KDIGO classification. CKD was grouped into risk categories based on the eGFR and ACR or dipstick proteinuria categories. The CKD risk categories were compared using albuminuria (ACR ≥ 30 mg/g) or proteinuria (≥ trace and ≥ 1+ or PCR ≥ 150 mg/g). The concordance rates between the ACR-based and protein-dipstick (≥ trace and ≥ 1+)- or PCR-based CKD risk categories were 88.76% (κ = 0.567), 90.48% (κ = 0.607), and 92.06% (κ = 0.683), respectively. The prevalence of ≥ moderately increased risk according to ACR-based, dipstick-proteinuria (≥ trace)-based, and PCR-based classifications were 16.37% (moderately increased risk, 13.44%; high risk, 2.49%; very high risk, 0.44%), 12.71% (moderately increased risk,6.78%; high risk, 5.17%; very high risk, 0.76%), and 11.96% (moderately increased risk, 9.71%; high risk, 1.88%; very high risk, 0.37%), respectively.
Performance of urine protein reagent strip in classification of CKD risk compared to urinary ACR strip
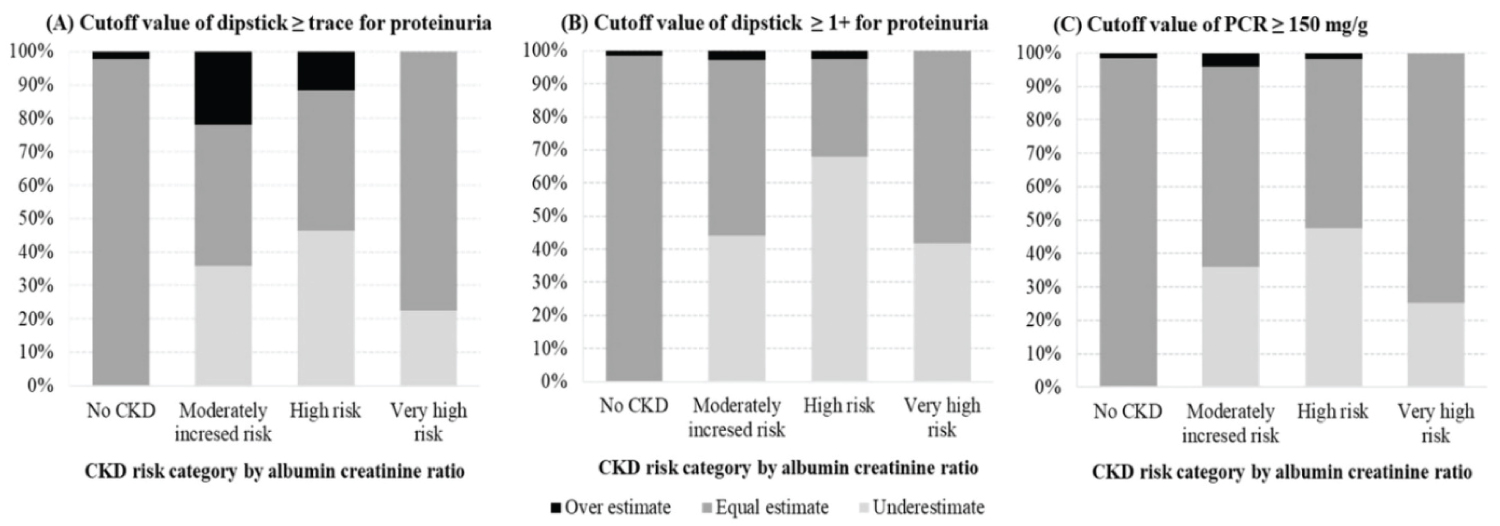
If ACR-based CKD risk categories were grouped using dipstick proteinuria, subjects with ≥ moderately increased risk were underestimated into lower risk categories. Among 14,149 subjects with ≥ moderately increased risk by ACR-based category, 5,262 (37.2%), 6,748 (47.7%), and 5,318 (37.6%) subjects were reclassified into lower risk CKD by dipstick-proteinuria (≥ trace)-, dipstick-proteinuria (≥ 1+)- based and PCR-based categories, respectively, (Table 3) (Figure 2).
Discussion
This study found that the prevalence of CKD estimated using the dipstick ACR was higher than that estimated using dipstick proteinuria. If dipstick proteinuria is utilized, CKD risk categories would be underestimated. One-third of the subjects with ≥ moderately increased CKD risk might be reclassified into the low risk categories on screening using dipstick proteinuria in primary health.
The prevalence of CKD worldwide reportedly ranges from 8.0% to 14% across different countries: 8.2% in subjects aged ≥ 20 years [13] and 13.7% in those aged ≥ 35 years from the Korean National Health and Nutrition Examination Survey [14], 13.1% in subjects aged ≥ 20 years in the United States [15], 12.5% in subjects aged ≥ 18 years in Canada [16], 10.8% in subjects aged ≥ 18 years in China [17], and 13.2% in subjects aged ≥ 20 years in Japan [18]. These variations in the prevalence estimates of CKD may have been due to differences in the study sampling designs, definitions and classifications of CKD, assay methods for serum creatinine and urine albumin concentrations, and the formulae used to calculate eGFR. In the present study, the prevalence of CKD in adults aged ≥ 18 years was 16.3% when CKD was defined as a urinary dipstick ACR of ≥ 30 mg/g or eGFR < 60 mL/min/1.73 m2. Meanwhile, the prevalence of CKD was 12.7% or 11.9% when CKD was defined as the presence of urinary protein (protein dipstick (≥ trace)/PCR (≥ 150 mg/g)) or eGFR < 60 mL/min/1.73 m2. A previous study involving a Korean population found a lower prevalence of 8.2% [13]. However, the mean age of the subjects included in that study was 46 years, which is younger than the mean age of 55 years in the present study. Another Korean study [14] found a CKD prevalence of 13.7% in adults aged ≥ 35 years, which is comparable to our findings. Another difference between previous studies and the present one is in the methods used to detect albuminuria: While the other studies measured the urinary albumin concentration using a turbidimetric immunoassay, we detected the ACR semiquantitatively using a dipstick albuminuria test.
We found that the prevalence of albuminuria estimated using dipstick ACR was higher than that estimated using dipstick proteinuria in this study. Six percent of all of the study subjects had albuminuria without proteinuria. Moreover, more than one-fourth of the study subjects with proteinuria (28.3% of subjects with ≥ trace proteinuria and 25.6% of subjects with ≥ 1+ proteinuria) did not have albuminuria in this study. In CKD with a normal or mildly decreased eGFR (60-89 mL/min/1.73 m2), albuminuria is more predictive of an adverse kidney outcome and all-cause mortality than eGFR [19]. If albuminuria was overlooked as an initial screening tool for CKD detection, stage 1 and 2 CKD, which together constitute 63% of all cases of CKD, would have been missed in our study. This finding is particularly concern in Korea, where stage 1 and 2 CKD are more prevalent in the general population. This finding suggested that if albuminuria was overlooked in the initial screening for CKD, considerable portion of CKD would be missed in the primary care. Meanwhile, a protein dipstick may react to nonalbumin proteins such as alpha 1-globulin, beta 2-globulin, gamma-globulin, and Bence-Jones protein, which supports that low-grade proteinuria may not always indicate albuminuria [20]. Konta, et al. [21] reported that the threshold of ACR for albuminuria might be lower than those for trace and 1+ proteinuria if albuminuria was screened by proteinuria. Those authors reported that the median urinary ACR was 43 mg/g for trace proteinuria and 81 mg/g for 1+ proteinuria. This could be one of the main reasons for the prevalence of albuminuria being higher than that of proteinuria in our study subjects.
When the CKD risk categories were grouped according to dipstick proteinuria without considering other variables such as cause of CKD or other risk factors and comorbid condition, the ACR-based CKD risk category tended to be classified into lower risk categories. More than 30% of our study subjects with ≥ moderately increased risk according to the ACR-based category were reclassified into low risk CKD when using dipstick proteinuria-based categories. This means that if urine protein dipstick tests were used as an initial screening tool for CKD, more than 30% of ≥ moderately increased risk cases would be missed.
Manns, et al. [22] reported that CKD screening in the general population using urine albumin measurements is not cost-effective. Meanwhile, Salinas, et al. [23] demonstrated that the ACR strip test can replace quantitative technologies. The semiquantitative ACR strip test using spot urine has several advantages, in being cost-effective, technically simple and rapid to apply and included in highly-established urinalysis methods. These aspects should be considered along with the actual diagnostic performance at primary care.
Study strengths and limitations
This study has some strengths including the enrollment of a large number of subjects of 88,479. This study shows rationale of the affordable semiquantitative method for identifying albuminuria which has potential clinical utility in primary care. Moreover, this study involves unexposed asymptomatic CKD which is an important area of public health concern in general population.
Our study has some limitations. First, we calculated the eGFR using the MDRD equation. The prevalence of CKD could be overestimated when using the MDRD equation, which may classify more subjects into CKD compared to using the CKD Epidemiology Collaboration (CKD-EPI) equation. Second, the serum creatinine, urine dipstick ACR and dipstick proteinuria were measured once only, and so transient albuminuria or acute kidney injury could not be excluded. CKD should be diagnosed over a period of > 3 months as definition by KDIGO. However, we couldn't follow the duration of abnormalities of kidney function due to the cross-sectional study design. Third, CKD risk categories were classified without real outcome in this study. However, we just intended to categorize CKD risk without considering other variables such as cause of CKD or other risk factors and comorbid condition in general population. Lastly, we used the dipstick ACR as the reference standard in spot urine samples instead of quantitative measurements of albumin excretion. However, although the 24-hour collection has been the gold standard, alternative methods for detecting albumin or protein excretion such as ACR or PCR correct for variations in urinary concentration due to hydration as well as provide more convenience than timed urine collections. Moreover, semiquantitative method to urinary albumin in a spot urine sample and reported as ACR showed strong correlation with quantitative assay [9,10]. In addition, laboratories in all centers have performed validation studies of ACR by standard method on the introduction of the method into the laboratories. In addition, the spot urine ACR is a very affordable test as it is included as an additional exam in the urinalysis strip, which can necessarily improve delivery of clinical care or estimated rates of future complications.
In conclusion, urine dipstick proteinuria had a lower sensitivity and specificity for screening CKD compared to the dipstick ACR in primary health. The CKD risk category using urine dipstick proteinuria was underestimated compared to the ACR-based CKD risk category. This finding is particularly concern in screening early stages of CKD, which are mostly asymptomatic and are more prevalent in primary care. In addition, the dipstick ACR test has advantages of being a cost-effective and convenient examination. These points together with the diagnostic performance should be considered at national health screening, which suggests that ACR-based CKD screening is needed in primary health.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval
This study was approved by the Institutional Review Board of the Korea Association of Health Promotion (approval no. 130750-202005-HR-007).
Contribution
All of the authors participated in designing this study. SC performed data collection. SK undertook the statistical analyses. EN and SK analyzed and interpreted the data. EN wrote the first draft of the manuscript, which was reviewed by all of the other authors, who also provided further contributions and suggestions.
Acknowledgements
The authors thank the Central Data Center at Korea Association of Health Promotion for collecting health information data.
References
- Jha V, Garcia-Garcia G, Iseki K, et al. (2013) Chronic kidney disease: Global dimension and perspectives. Lancet 382: 260-272.
- Go A, Chertow GM, Fan D, et al. (2004) Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296-1305.
- Levey A, Coresh J (2012) Chronic kidney disease. Lancet 379: 165-180.
- Levin A, Stevens PE, Bilous RW, et al. (2013) Kidney disease: Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Inter Suppl 3: 1-150.
- Salinas M, Lopez-Garrigos M, Flores E, et al. (2016) Indications for laboratory tests in primary care: Assessment of the most frequent indications and requests with blank clinical information. Biochem Med 26: 431-435.
- Tanihara S, Hayakawa T, Oki I, et al. (2005) Proteinuria is a prognostic marker for cardiovascular mortality: NIPPON DATA 80, 1980-1999. J Epidemiol 15: 146-153.
- White S, Yu R, Craig JC, et al. (2011) Diagnostic accuracy of urine dipsticks for detection of albuminuria in the general community. Am J Kidney Dis 58: 19-28.
- Tugirimana P, Delanghe JR (2009) Development of an affordable dye-stained microalbuminuria screening test. Nephrol Dial Transplant 24: 1485-1490.
- Delanghe J, Himpe J, De Cock N, et al. (2017) Sensitive albuminuria analysis using dye-binding based test strips. Clin Chim Acta 471: 107-112.
- Oyaert M, Delanghe JR (2019) Semiquantitative, fully automated urine test strip analysis. J Clin Lab Anal 33: e22870.
- Lamb E, Tomson CR, Roderick PJ (2005) Estimating kidney function in adults using formulae. Ann Clin Biochem 42: 321-345.
- Levin A, Stevens PE (2014) Summary of KDIGO 2012 CKD guideline: Behind the scenes, need for guidance, and a framework for moving forward. Kidney Int 85: 49-61.
- Park J, Baek H, Jung HH (2016) Prevalence of chronic kidney disease in Korea: The Korean National Health and Nutritional Examination Survey 2011-2013. J Korean Med Sci 31: 915-923.
- Kim S, Lim CS, Han DC, et al. (2009) The prevalence of chronic kidney disease (CKD) and the associated factors to CKD in urban Korea: A population-based cross-sectional epidemiologic study. J Korean Med Sci 24: S11-S21.
- Coresh J, Selvin E, Stevens LA, et al. (2007) Prevalence of chronic kidney disease in the United States. JAMA 298: 2038-2047.
- Arora P, Vasa P, Brenner D, et al. (2013) Prevalence estimates of chronic kidney disease in Canada: Results of a nationally representative survey. CMAJ 185: E417-E423.
- Zhang L, Wang F, Wang L, et al. (2012) Prevalence of chronic kidney disease in China: A cross-sectional survey. Lancet 379: 815-822.
- Imai E, Horio M, Watanabe T, et al. (2009) Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol 13: 621-630.
- Park J, Baek H, Kim BR, et al. (2017) Comparison of urine dipstick and albumin: Creatinine ratio for chronic kidney disease screening: A population-based study. PLoS One 12: e0171106.
- Sato H, Konta T, Ichikawa K, et al. (2016) Comparison of the predictive ability of albuminuria and dipstick proteinuria for mortality in the Japanese population: The Yamagata (Takahata) study. Clin Exp Nephrol 20: 611-617.
- Konta T, Hao Z, Takasaki S, et al. (2007) Clinical utility of trace proteinuria for microalbuminuria screening in the general population. Clin Exp Nephrol 11: 51-55.
- Manns B, Hemmelgarn B, Tonelli M, et al. (2010) Population based screening for chronic kidney disease: Cost effectiveness study. BMJ 341: c5869.
- Salinas M, López-Garrigós M, Flores E, et al. (2018) Urinary albumin: A risk marker under-requested in primary care in Spain. Ann Clin Biochem 55: 281-286.
Corresponding Author
Dr. Eun-Hee Nah, Department of Laboratory Medicine and Health Promotion Research Institute, Korea Association of Health Promotion, 396, Gonghang-daero, Gangseo-Gu, Seoul 07649, Korea, Tel: +82-2-2600-0107, Fax: +82-2-2690-4915
Copyright
© 2021 Nah EH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.