Primary Sjögren's Syndrome and the Therapeutic Response to Rituximab: Case Report
Abstract
The Sjögren's syndrome (SS) is a multisystemic, progressive and chronic autoimmune disease that affects primarily the exocrine glands and is characterized by the sicca syndrome. It can be a primary autoimmune dysfunction (pSS) or secondary to another autoimmune disorder (sSS). The physiopathology is resumed as the lymphocytic infiltration and B cell hyperactivity around the epithelium of the affected tissues. The etiology is still unknown, although genetic and environmental factors are believed to play a very important role in the manifestation of pSS. The diagnosis and classification are made based on pre-established criteria that consider the clinical manifestations, biological markers and cytopathological alterations. The treatment is widely based on the symptoms and clinic presented by the patient, for severe extraglandular manifestations, B-cell-targeted therapies have been showing great results especially in cryoglobulinemia, vasculitis, severe parotid swelling, inflammatory arthritis, pulmonary disease and peripheral neuropathy. We present a case of a 49-years-old female, seven years diagnosed with primary Sjogren's syndrome, with severe pulmonary and cutaneous manifestations who was referred to immunosuppressive treatment with rituximab. The aim of this study is to describe the response to the therapy, comparing clinical and biological parameters before and up to 5 months after the first rituximab infusion.
Keywords
Primary Sjögren's syndrome, Autoimmune disease, Rituximab
Introduction
Characterized by the sicca complex, from the Latin sicca meaning dryness, the Sjögrens's syndrome (SS) is a multisystemic, progressive and chronic autoimmune disease, that occurs predominantly in women (9:1), between their fourth and fifth decade [1,2]. It can occur as a primary autoimmune dysfunction (pSS) or secondary to another autoimmune disorder such as sytemic lupus erythematosus (SLE), being so called secondary Sjögren's syndrome (sSS) [2].
The SS was primarily reported in 1933 by the Swiss ophthalmologist Henrik Sjögren, who identified and related the symptoms of keratoconjunctivitis sicca, xerostomia and polyarthrite in a specific group of women and linked to a distinct pathology, which was named in his honor [3]. The etiology is still unknown, although genetic and environmental factors are believed to play a very important role in the manifestation of pSS [4].
The clinical manifestations are separated into two categories, glandular (xerostomia, xerophtalmia and parotid hypertrophy) and extraglandular (arthralgia, neuropathy, fatigue, pulmonary symptoms among others) [4]. The wide clinical spectrum is the result of two main immunological mechanisms: the lymphocytic infiltration in the epithelium of the affected tissues and the B cell hyperactivity [5,6].
The treatment for SS is mainly based on the symptoms and clinic presented by the patient [4]. For severe extraglandular manifestations, B-cell-targeted therapies have been showing great results [7]. Rituximab is a monoclonal antibody that binds to the CD20 surface marker expressed on B-cells and its precursors, inducing cell depletion through three main mechanisms: antibody-dependent cell mediated cytotoxicity, complement-dependent cytotoxicity and apoptosis [8].
Originally developed to remove malignant B-cell, rituximab have been recently included in the treatment recommendations list for SS [7]. Although not suggested for treating dryness, it may be considered as a therapeutic option for vasculitis, pulmonary disease and peripheral neuropathy among others [9,10].
In this study, we present a case of a 49-years-old female who, since the disease started manifesting, has presented extraglandular complications that evolved to a pulmonary failure being then referred for rituximab therapy. The aim of this report is to describe the clinic and physiologic alterations in this primary Sjögren's syndrome case, and the therapeutic response to immunosuppressive treatment with rituximab, through a retrospective analysis of the clinical manifestations and pSS biomarkers: Complement C3 and C4, Erythrocyte sedimentation rate (ESR), C-Reactive protein (CRP), Rheumatoid factor (RF) and Immunoglobulin (IgG, IgA and IgM).
Case Presentation
A.M.T.B., female, 49-years-old, diagnosed with primary Sjögren syndrome in 2010 after presenting a chronic urticaria and light signs of xerostomia. She was positive for anti-SSA, anti-SSB and positive antinuclear antibody (ANA Hep-2) at fine speckled pattern 1:160. She had low C3 and C4 complement, elevated C-reactive protein (CRP) and normal levels for erythrocyte sedimentation rate (ESR) as shown in Table 1. The pSS was then confirmed by salivary gland 99mTc-pertechnetate scintigraphy that presented loss of glandular function due to inflammatory activity.
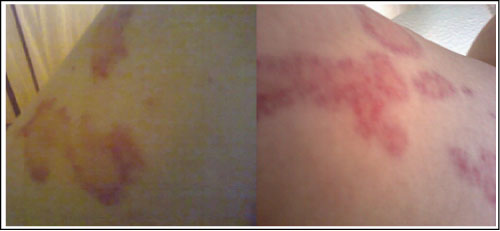
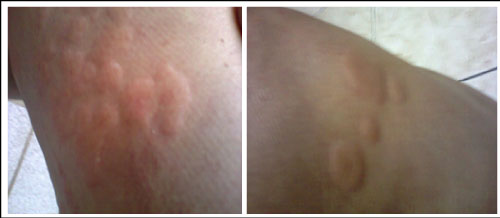
The inflammatory manifestations were controlled by the administration of oral prednisone and hydroxychloroquine, the second one being replaced by azathioprine two years later due to the cardiotoxicity of the drug. From the outset, the patient showed systemic complications with cutaneous vasculitis (Figure 1) and urticaria (Figure 2). In early 2015, she started presenting pulmonary manifestations, leading to a progressive loss of respiratory capacity, highly decreasing her quality of life. She was then treated with budesonide, formoterol fumarate dehydrate and glycopyrronium bromide.
In early 2017, the complaints of dyspnea and vasculitis increased, followed by a severe anemia detected on the 27th of February, as shown in Table 1. The RBC, Hb and Hct levels did not respond to consecutive blood transfusions (Table 2) which were followed by a respiratory failure. She was then referred to intensive care unit (ICU) for treatment and evaluation.
Computed tomography (CT) scans were performed on chest, head and abdomen in search of an internal hemorrhage. The abdominal and cranial CT did not show any abnormality. However, the thoracic scan revealed bilateral pleural effusion, atelectasis and increased attenuation areas into both of lungs displayed as ground glass opacification.
The physicians concluded a hemorrhage in the pleural spaces due to the disease high activity in the lungs and the patient was treated for pneumonia. Once stabilized, she was referred for immunosuppressive therapy with rituximab under the following protocol: 500 mg of rituximab at week 1 and 2 by intravenous infusion (IV) with initial rate of 50 mg/hour increased by 50 mg/hour every 30 minutes to the maximum of 400 mg/hour. It was also administrated methylprednisolone 100 mg (IV) and diphenhydramine hydrochloride 50 mg (IV), sixty minutes before the infusion of rituximab. The patient did not experience any side effects from the infusion. She was discharged after being hospitalized for fifty eight days.
One month after the administration of the rituximab, she could breathe without mechanical ventilation and there were no episodes of dyspnea. During the following five months there were no signs of any urticaria or cutaneous vasculitis, and she started regaining her quality of life. The last CT Scan performed on chest in September showed both lungs to be completely free from any pleural effusion and showed a significant decrease of the atelectasis. Remaining, in a considerably reduced scale, were areas with ground-glass attenuation into both lungs (Table 3).
Discussion
Sjogren's syndrome is usually diagnosed in the presence of symptomatic sicca syndrome (xerostomia and xerophthalmia) [2]. Although our patient did not have any ocular signs, she presented dry mouth associated with cutaneous manifestations and the diagnosis was made considering the presence of auto antibodies ASSA, ASSB, ANA, rheumatoid factor, hypocomplementaemia and the scintigraphy showing immune activity with loss of glandular function.
According to the classification criteria of the American-European Consensus Group (AECG) from 2002, the diagnosis of SS requires at least four of the following, in addition to the presence of either number 5 or 6: 1) Ocular symptoms - dry eyes for over 3 months, foreign-body sensation or use of tear substitutes more than 3 times daily; 2) Oral symptoms - dry mouth, recurrently swollen salivary glands, frequent use of liquids to help swallowing; 3) Ocular signs - Schirmer test (< 5 mm in 5 min), positive vital dye staining results; 4) Oral signs - Abnormal salivary scintigraphy, parotid sialography and sialometry findings; 5) Positive minor salivary gland biopsy; 6) Positive anti-SSA or anti-SSB antibody [11].
The second main criteria, published in 2012 by the Sjögren's International Collaborative Clinical Alliance (SICCA), approved by the American College of Rheumatology (ACR), proposes that the diagnosis can be determined once meeting at least two of the following criterions; 1) Positive anti-SSA and/or anti-SSB or positive rheumatoid factor and ANA titer ≥ 1:320; 2) Labial salivary gland biopsy presenting focal lymphocytic sialadenitis with a focus score ≥ 1 focus/4 mm2; 3) Keratoconjunctivitis sicca with ocular staining score ≥ 3 [12].
If either criterion were precisely followed our patient would not have the sufficient score to classify as Sjögren's syndrome. Although labial salivary gland biopsy was not performed due to the invasive nature of the procedure, some new studies have recommended the scintigraphy test 99mTc-pertechnetate [13,14] as this methodology has been reported as sufficient for the assessment of the status of the salivary glands in the majority of patients with SS [14]. Both criteria offer a great diagnostic guideline, yet they continue to be refined as they are not universally reliable due to the particular set of manifestations each patient present [15].
Analyzing the SS biomarkers through the years (Table 1 and Table 2), our patient has shown little variability within the immunoglobulins, a persistent elevated CPR, slight alterations in the ESR and a very strong hypocomplementaemia. The rheumatoid factor is an auto antibody that reacts to the Fc portion of polyclonal IgG that is also present in other autoimmune diseases [16] and it is an important diagnosis marker for SS [12]. In this case the FR did not suffer alterations since the patient started immunosuppressive therapy.
Looking at the immunoglobulin levels (Table 2) we only observe a few slights alterations within the IgG. Monitoring the immunoglobulins are important to assist the evaluation of the disease activity as it is one of the criteria for rating the biological domain in the EULAR Sjögren's syndrome disease activity index (ESSDAI), guide developed by the European League against Rheumatism (EULAR), that classifies risk and prognosis. Hypergammaglobulinemia or high IgG level (1600-2000 mg/dL) is classified as a low rating, and IgG level > 2000 mg/dL is classified as moderate rating for this domain [17].
The ESR and CRP are very responsive to inflammatory process. However, CRP is a better indicator of the current inflammation for being more stable and for not being affected by other serum components [18]. Neither of them are known to have a prognosis role in autoimmune diseases, although ESR is mentioned as a useful biological marker to identify patients with increased B-cell hyperactivity, being considered relevant when > 50 mm/hour [19,20].
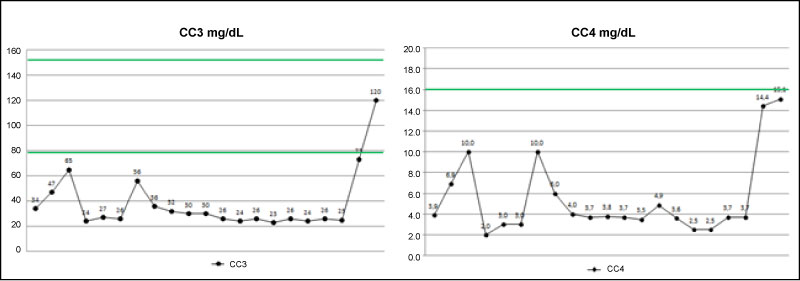
According to the literature, complement is considered to be a great marker of prognosis in SS and it is also evaluated in the biological domain of the ESSDAI [17]. Low levels of components C3 and/or C4 occur as a result of the increased consumption due to the elevated activity of the immunological system, and are associated with unfavorable outcomes such as severe extraglandular manifestations, vasculitis and lymphoma [21,22]. Our patient has shown very low CC3 and CC4 levels through the years, however, we observe a very positive response in the last two serum analyses, which were made 1 and 4 months after the infusion of rituximab (Figure 3). This elevation in the complement components corroborates with the remission of the symptoms which indicates decrease in the disease activity and treatment effectiveness.
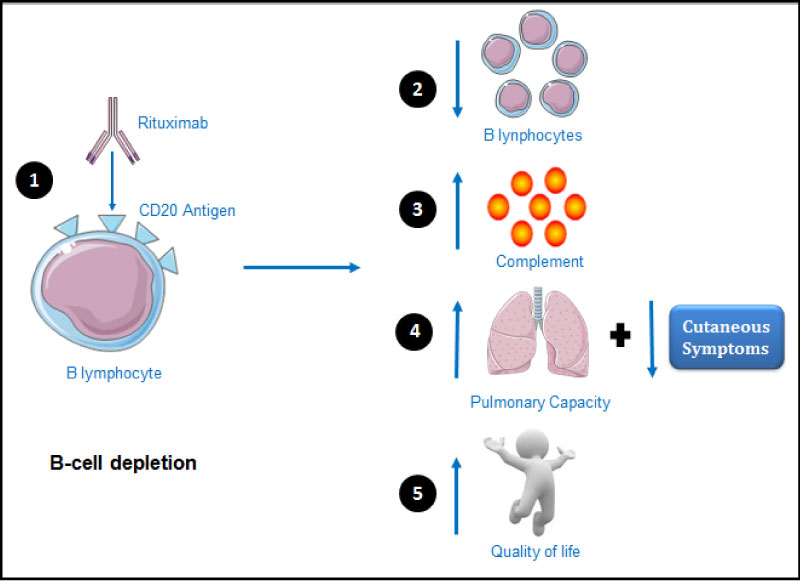
The efficacy of the rituximab observed in this case, as illustrated in Figure 4, is believed to be due to the decrease in the rate of new plasma cell synthesis and the interruption of the role of B cells as antigen-presenting to T cells, as it does not reduce plasma cells because they do not express CD20 [8,23]. B cells originate from hematopoietic stem-cell precursors in the bone marrow progressing into pro B cell, pre B cell, immature B cells and mature B cells. Each mature B cell leaves the bone marrow with a particular antigen-specific B cell receptor on its surface that differentiates into antibody producing plasma cells once encounters its compatible antigen [24]. The depletion of B cells from the circulation is temporary and is highly variable among individual, usually lasting for about six months [8].
The protocol followed in this case of 1000 mg of rituximab in two infusions, usually recommended for rheumatoid arthritis [25], showed efficacy. Risk of infusion-related reaction, common in 30% in the first infusion [25], was prevented with methylprednisolone and diphenhydramine hydrochloride. According to the literature, the dosage and periodicity of the rituximab for Sjögren's syndrome are still to be determined, and the association with other immunosuppressant drugs are still questioned [23,26].
Conclusion
Sjögren's syndrome is a multisystemic and progressive disease with a wide clinical spectrum that varies from benign manifestations to severe life-threatening complications. This vast range of phenotypes highlights the need of improving the classification criteria and outcome measures.
The importance of the biomarkers is very well established for providing directions for diagnosis and prognosis [9,10]. The complement C3 and C4, among the analyzed parameters, are the most significant prognosis biomarkers as it portraits the disease activity. Along with immunoglobulin levels, the complement components compose the biological domain in the ESSDAI, which assists the determination of the degree of risk of the systemic manifestations [17].
The heterogeneity of the SS manifestations is due to its particular physiopathology (T cell infiltration and B cell hyperactivity). B cell target therapy with rituximab has been overall proven effective in many studies, but many aspects remain undefined [10]. Further studies need to be done to determine correct dosage and periodicity of the treatment as well as the interaction with other immunosuppressant drugs that is yet unclear.
New management perspectives arise as the attention towards Sjögren's syndrome increases in the specialized research groups. Molecular biology and microarray technology are very promising as new biomarkers [9] and treatment approach to other immunological mechanisms such as anti-cytokine (BAFF, interleukin-6 and interferon) and inhibition of T-cell stimulation have been recommended for future clinical trials [7].
References
- Patel R, Shahane A (2014) The epidemiology of Sjögren's syndrome. Clin Epidemiol 6: 247-255.
- Stefanski AL, Tomiak C, Pleyer U, et al. (2017) The diagnosis and treatment of Sjögren's syndrome. Dtsch Arztebl Int 114: 354-361.
- Ghafoor M (2012) Sjögren's before Sjögren: Did Henrik Sjögren (1899-1986) really discover Sjögren's Disease? J Maxillofac Oral Surg 11: 373-374.
- Ceballos AD, Restrepo JE, Pulgarín SR (2015) Síndrome de sjögren más que um ojo seco. Archivos de Medicina 15: 343-351.
- Medina-Paz L, Che-Morales JL, Barrera-Pérez HAM, et al. (2016) Síndrome de Sjögren primário y bronquiolitis. Una asociación no usual. Reumatol Clin 997: 10.1016.
- Moutsopoulos HM (1994) Sjogren's syndrome: Autoimmune epithelitis. Clin Immunol Immunopathol 72: 162-165.
- Vivino FB, Carsons SE, Foulks G, et al. (2016) New treatment guidelines for Sjögren's Disease. Rheum Dis Clin North Am 42: 531-551.
- Smith MR (2003) Rituximab (monoclonal anti-CD20 antibody): Mechanism of action and resistance. Oncogene 22: 7359-7368.
- Goules VA, Tzioufas AG (2017) Primary Sjögren's syndrome: Clinical phenotypes, outcome and the development of biomarkers. Immunol Res 65: 331-344.
- Brito-Zéron P, Ramos-Casals M, EULAR-SS task force group (2014) Advances in the understanding and treatment of systemic complications in Sjögren's syndrome. Curr Opin Rheumatol 26: 520-527.
- Vitali C, Bombardieri S, Jonsson R, et al. (2002) Classification criteria for Sjögren's syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 61: 554-558.
- Shiboski SC, Shiboski CH, Criswell L, et al. (2012) American College of Rheumatology classification criteria for Sjögren's syndrome: A data-driven, expert consensus approach in the Sjögren's International Collaborative Clinical Alliance Cohort. Arthritis Care Res (Hoboken) 64: 475-487.
- Tensing EK, Nordström DC, Solovieva S, et al. (2003) Salivary gland scintigraphy in Sjögren's syndrome and patients with sicca symptoms but without Sjögren's syndrome: The psychological profiles and predictors for salivary gland dysfunction. Ann Rheum Dis 62: 964-968.
- Luk WH, Yeung JTH, Fung EPY, et al. (2017) Salivary gland scintigraphy in patients with Sjogren's syndrome: A local experience with dual-tracer. Asia Ocean J Nucl Med Biol 5: 56-65.
- Beckman KA, Luchs J, Milner MS, et al. (2017) The potential role for early biomarker testing as part of a modern, multidisciplinary approach to Sjogren's syndrome diagnoses. Adv Ther 34: 799-812.
- Castro C, Gourley M (2010) Diagnostic testing and interpretation of tests for autoimmunity. J Allergy Clin Immunol 125: S238-S247.
- Seror R, Bowman SJ, Brito-Zerón P, et al. (2015) EULAR Sjögren's syndrome disease activity index (ESSDAI): A user guide. RMD Open 1: e000022.
- Litao MK, Kamat D (2014) Erythrocyte sedimentation rate and C-reactive protein: How best to use them in clinical practice. Pedriatr Ann 43: 417-420.
- Garcia-Garcia C, Jaen-Aguila F, Hidalgo-Tenorio C, et al. (2009) Pure red cell aplasia as first manifestation of primary Sjögren syndrome. Rev Clin Esp 4: 203-204.
- Brito-Zéron P, Retamozo S, Gandia M, et al. (2012) Monoclonal gammopathy related to Sjögren syndrome: A key marker of disease prognosis and outcomes. J Autoimmun 39: 43-48.
- Zadura AF, Theander E, Blom AM, et al. (2009) Complement inhibitor C4b-binding protein in primary Sjogren's syndrome and its association with other disease markers. Scand J Immunol 69: 374-380.
- Ramos-Casals M, Brito-Zéron P, Yagüe J, et al. (2005) Hypocomplementaemia as an immunological marker of morbidity and mortality in patients with primary Sjögren's syndrome. Rheumatology (Oxford) 44: 89-94.
- Randall KL (2016) Rituximab in autoimmune diseases. Aust Prescr 39: 131-134.
- Goodnow CC, Cyster JG, Hartley SB, et al. (1995) Self-tolerance checkpoints in B lymphocyte development. Adv Immunol 59: 279-368.
- Buch MH, Smolen JS, Betteridge N, et al. (2011) Updated concensus statement on the use of rituximab in patients with rheumathoid arthritis. Ann Rheum Dis 70: 909-920.
- Devauchelle-Pensec V, Pennec Y, Morvan J, et al. (2007) Improvement of Sjögren's syndrome after two infusions of rituximab (anti-CD20). Arthritis Rheum 57: 310-317.
Corresponding Author
Rodrigo Binkowski de Andrade, Curso de Biomedicina, Centro Universitário da Serra Gaúcha - FSG, Rua os Dezoite do Forte, 26366, CEP 95020-472, Caxias do Sul - RS, Brazil, Tel: 5554-992-449-976.
Copyright
© 2019 Lorandi J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.