Aberrant Intrinsic Neural Networks Strength in Individuals with "Smartphone Addiction": An MRI Data Fusion Study
Abstract
Objectives: Excessive smartphone use, sometimes also referred to as "smartphone addiction", has increasingly attracted neuroscientific interest due to its similarities with other behavioral addictions, particularly internet gaming disorder. Little is known so far about the neural mechanisms underlying smartphone addiction. Here, we explored interrelationships between brain structure and function to specify neurobiological correlates of smartphone addiction on a neural system level.
Methods: Gray matter volume and intrinsic neural activity was investigated in individuals with smartphone addiction (n = 20) compared to controls (n = 24), using multimodal magnetic resonance imaging and multivariate data fusion techniques, i.e., parallel Independent Component Analysis.
Results: The joint analysis of both data modalities explored shared information between gray matter volume and intrinsic neural activity which were not revealed by previous modality-specific approaches. Two amplitude of low frequency fluctuations-based independent neural systems significantly differed between individuals with smartphone addiction and controls. A medial/dorsolateral prefrontal system exhibited lower functional network strength lower in smartphone addiction vs. controls, whereas the opposite pattern was detected in a parietal cortical/cerebellar system. Neural network strength was significantly related to duration of smartphone use and sleep difficulties.
Conclusions: We show modality-specific associations of the brain's resting-state activity with distinct and shared smartphone addiction symptom dimensions. In particular, the data suggest contributions of aberrant prefrontal and parietal neural network strength as a possible signature of deficient executive control in smartphone addiction.
Keywords
Functional magnetic resonance imaging, Parallel independent component analysis, Addiction, Smartphone, Multimodal data analysis
Introduction
In the past years, negative physical and psychosocial effects associated with excessive smartphone use have been emphasized by a growing number of studies [1-4]. Recent research highlighted behavioral similarities between excessive smartphone use and other addictive disorders, such as failure to resist use, withdrawal, continuation of use despite being aware of negative consequences, or deception of others regarding the amount of time spent on use [5]. Moreover, excessive smartphone use has been repeatedly linked to impulsiveness and depression [6]. These behaviors also show close similarity to the criteria for Internet Gaming Disorder (IGD) in the DSM-5 [7]. Consequently, criteria for "smartphone addiction" (SPA) were introduced [8] to define a condition characterized by excessive smartphone use and its problematic consequences for work-related achievements and interpersonal relationships, as well as for physical and mental health [1-4].
Although the term "smartphone addiction" has been anchored in several validated psychometric instruments, e.g. the Smartphone Addiction Scale (SAS) [9], or the Smartphone Addiction Inventory (SPAI), it has been criticized for conceptual and taxonomic reasons. In this regard, and because of it possibly being a mobile branch of gaming disorder, alternative terms (e.g. "smartphone use disorder") were suggested [10]. Addictive use of specific internet applications has been recently addressed in the context of the Interaction of Person-Affect-Cognition-Execution (I-PACE) model [11,12], which combines psychological and neuroscientific theories of addictions. According to this model, addictive behavior is a consequence of the interplay of personal characteristics and moderating and mediating variables [12]. This refers to regional brain volume or neural activity, considering the anterior cingulate cortex (ACC), dorsolateral prefrontal cortex (DLPFC), and precuneus as regions of interest in addictive behaviors, such as IGD [13]. Similarly, individuals with SPA show structural and functional changes at the neural structural and functional level 14, such as reduced gray matter volume (GMV), intrinsic neural activity (INA) in ACC [14], altered resting-state connectivity [15], and changes in neural activity in DLPFC, ACC and inferior parietal cortices during affective processing [16]. Recently, differences in cue-reactivity (CR)-related brain activity in individuals with compared to those without SPA, particularly in frontal regions, ACC, precentral gyrus, and anterior insula were shown [17].
Of note, most research in technology-associated behavioral addictions focused on either brain structure or function. To our knowledge, no study so far has provided combined information on both modalities in the same sample. While modality-specific descriptive approaches are clearly useful, such attempts may disregard important sources of joint information between modalities. Such information is essential for directly detecting similarities among patterns of brain structure and function within- and between specific populations.
Data fusion approaches based on multivariate statistical techniques take advantage of a joint examination of various information sources of the same sample at the same time and are able to detect cross-information of data, i.e., co-altered patterns of brain changes that may be partially missed in conventional separate analyses [18,19]. Here, we used parallel Independent Component Analysis (p-ICA), a multivariate method to capture joint information from two data sources [20-22], to multimodally expand the previous descriptive findings on GMV and INA in SPA14 by exploring joint information between these modalities, which cannot be fully detected by conventional mass-univariate statistical approaches [19]. GMV and INA, in terms of the amplitude of low frequency fluctuations (ALFF), were included in a p-ICA model. ALFF captures the relative magnitude of blood oxygen level dependent (BOLD) signal changes of intrinsic neural activity in distinct brain regions. This approach has been shown to be suitable to identify brain regions/networks with aberrant local functioning in patients with substance-use disorders and IGD [23,24].
We expected to find independent components showing differences between individuals with SPA and controls, i.e., individuals showing non-addictive smartphone use, which depict hidden factors, which were not revealed by the former approach of separately analyzing GMV and ALFF data [14]. Furthermore, we explored associations between such hidden factors and distinct SPAI-I dimensions, as suggested by a recent confirmatory factor analysis, i.e., "Time spent", "Compulsivity", "Daily life interference", "Craving", and "Sleep interference" [25].
Materials and Methods
Participants
We used a subsample of participants referred to by 14. Individuals were recruited using flyers and posters distributed at Heidelberg University campus and city center and via ads on social media platforms (i.e. personal Facebook-account of C.M.). In total n = 132 persons expressed their interest in the study. After applying inclusion criteria (i.e., sufficient German language skills, right-handedness, age 18-30 years, no general contraindications for MRI or self-reported neurological or mental illness, no IGD (score on the short-form Internet Gaming Disorder Scale (IGDS-sf) < 6) data from 44 participants (matched for age and gender) were used for final analyses. We defined two user groups based on the short version of the SAS (SAS-SV), where cut-off values of > 31 for males and > 33 for females were used to define a group of excessive smartphone users (SPA; n = 20, 14 female) [26], Participants below the cut-offs were assigned to a group of controls (n-SPA; n = 24, 17 female).
SAS-SV and IGDS-sf were used during a pre-screening process to assign interested persons to one of the two defined groups (either SPA or n-SPA; SAS-SV) and to exclude persons showing IGD (IGDS-sf) to prevent potentially biasing effects of IGD on collected data. Before MRI, participants completed the SPAI, the Beck Depression Inventory (BDI)-II [27] and the Barratt Impulsiveness Scale (BIS) version 11 [28]. The SPAI is a comprehensive scale that measures a wide range of addictive behaviors related to smartphone use [8]. Recently, a confirmatory factor analysis of the SPAI, SPAI-I, suggested a five-factor model of the SPAI, which showed better fit in a European population than the original SPAI [25]. For the purpose of this study, SPAI scores were recalculated according to the SPAI-I. The following factors were considered: (1) Time spent, consisting of four items capturing the difficulty of stopping and devoting more time and resources to use the smartphone. (2) Compulsivity, consisting of four items involving the degree of discomfort and emotional distress when being deprived of using the smartphone and not stopping use in spite of negative consequences. (3) Daily life interference, consisting of eight items describing the interference of smartphone use with other daily activities and interpersonal problems due to smartphone use. (4) Craving, consisting of five items capturing the degree of being unable to resist the urge to continue the behavior, and (5) Sleep interference, including three items focusing on the relationship between smartphone use and sleep duration/disturbance [25]. For completeness, SPAI scores were also calculated following the four-factor solution offered by Lin and colleagues [8], i.e., 1) Compulsive behavior, 2) Functional impairment, 3) Withdrawal, and 4) Tolerance [8]. The BDI and BIS questionnaires were used to assess for depressive symptoms and impulsive personality traits, respectively, as depression and impulsiveness have been previously linked to excessive smartphone use and behavioral addictions [6,29].
The study was approved by the Ethics Committee of the Medical Faculty at Heidelberg University and carried out in compliance with the Declaration of Helsinki. All participants gave written informed consent prior to inclusion in the study. All participants received monetary compensation (30€) for their participation.
MRI Data Acquisition
A 3-T Magnetom TIM Trio MR Scanner (Siemens, Erlangen) equipped with a 32-channel head coil was used to collect whole-brain structural and functional scans in a darkened room. To minimize head motion, the head of the participants was fixated in the head coil using foam cushions. The scanner protocol included four functional measurements including (in this particular order) a resting-state scan, three experimental paradigms, and a structural scan. Modality-specific (structural MRI and resting-state fMRI (rs-fMRI)) findings resulting from univariate statistics are reported in Horvath, et al. [14]. Results of the CR-task employed in this study are reported in Schmitgen, et al. [14].
During acquisition of structural data, 192 T1-weighted images were recorded, which were acquired with an MP-RAGE pulse sequence in a transverse (axial) orientation with the following parameters: Repetition time = 1900 ms, echo time = 2.52 ms, field of view = 350 × 263 × 350 mm, flip angle = 9°, voxel size = 1 × 1 × 1 mm, 192 slices, slice thickness = 1 mm.
For rs-fMRI, participants were instructed to keep their eyes closed, not to fall asleep, and to not think about anything in particular. During rs-fMRI, 200 whole brain echo planar imaging (EPI) volumes were recorded in an axial orientation with the following imaging parameters: Repetition time = 2000 ms, echo time = 30 ms, field of view = 192 mm, flip angle = 90°, voxel size = 3 × 3 × 3 mm, 33 slices, distance factor between slices = 1 mm.
Data preprocessing
Individual data was preprocessed as described in Horvath, et al. [14]. In particular, GMV of T1-weighted sMRI images was calculated using the Computational Anatomy Toolbox, CAT12 (http://dbm.neuro.uni-jena.de/cat/; last access: 05/28/2020) together with SPM12 (http://www.fil.ion.ucl.ac.uk/spm/; last access: 05/28/2020). Preprocessing included segmentation of images into gray matter, white matter, and cerebrospinal fluid, normalization using DARTEL, and smoothing of GMV segments using an 8-mm full-width half-maximum (FWHM) isotropic Gaussian kernel.
Rs-fMRI images were processed using the Data Processing Assistant for rs-fMRI (DPABI/DPARSF) [30]. Preprocessing included slice timing, head motion correction, spatial normalization (Montreal Neurological Institute (MNI) -space; Voxel size 3 × 3 × 3 mm), smoothing with a 4-mm FWHM isotropic Gaussian kernel, regressing out nuisance covariates including mean signals from white matter and cerebrospinal fluid as well as the Friston 24-parameter model [31]. Afterwards, ALFF-maps were calculated based on the preprocessed data.
Parallel ICA
P-ICA [21,32], on GMV and ALFF data was applied using the Fusion ICA Toolbox (FIT; version 2.0d; https://trendscenter.org/software/fit/; last access: 05/28/2020) implemented in MATLAB 9.4.0 (R2018a). The number of components for each modality was estimated using the minimum description length (MDL) and the Akaike Information Criterion (AIC), as described in Calhoun, et al. [33]. Four components were identified for GMV and five components were identified for ALFF. ICASSO [34] was used to assess results after running the approach 20 times to ensure consistency of the components.
For component visualization, the source matrix was reshaped back to a 3D-image, scaled to unit standard deviations (z), and a threshold of z > 3 was applied. Maps from the two components described in Results section were overlaid onto a MNI normalized anatomical template. Anatomical denominations and stereotaxic coordinates were derived from clusters above a threshold of z > 3 by linking the ICA output images (i.e., the chosen components of interest) to the Talairach Daemon data base (http://www.talairach.org/daemon.html; last access: 05/28/2020).
Statistical Analysis
Component pre-selection was performed by two sample t-tests on the loading coefficients of each component, as implemented in FIT at p < 0.1. Three components fulfilled this criterion (one GMV and two ALFF) and loading coefficients of these components were extracted to be included in three ANCOVA models to test for group differences (adjusted for age and gender). The False discovery rate (FDR) was applied to adjust p values for multiple comparisons of the three ANCOVA models and components of interest were defined as models showing a FDR-corrected p-value of < 0.05. Hereby, two components of interest (both ALFF-based) were identified.
The component loadings of the two identified components of interest were used to test for associations with SPAI-I factors and SPAI-I total score by partial correlations (adjusted for age and gender). FDR-correction was applied, and results were defined significant, if the FDR-corrected p-values were < 0.05. ANCOVA models and partial correlations were computed in R (version 3.6.1; https://cran.r-project.org/; last visited 05/28/2020).
Results
Demographic and psychometric data
For demographic and psychometric details of the two groups (Table 1). The groups differed significantly in BDI, BIS-attentional score, BIS-motor score, SAS, SPAI scores, and SPAI-I scores (Table 1).
Parallel ICA
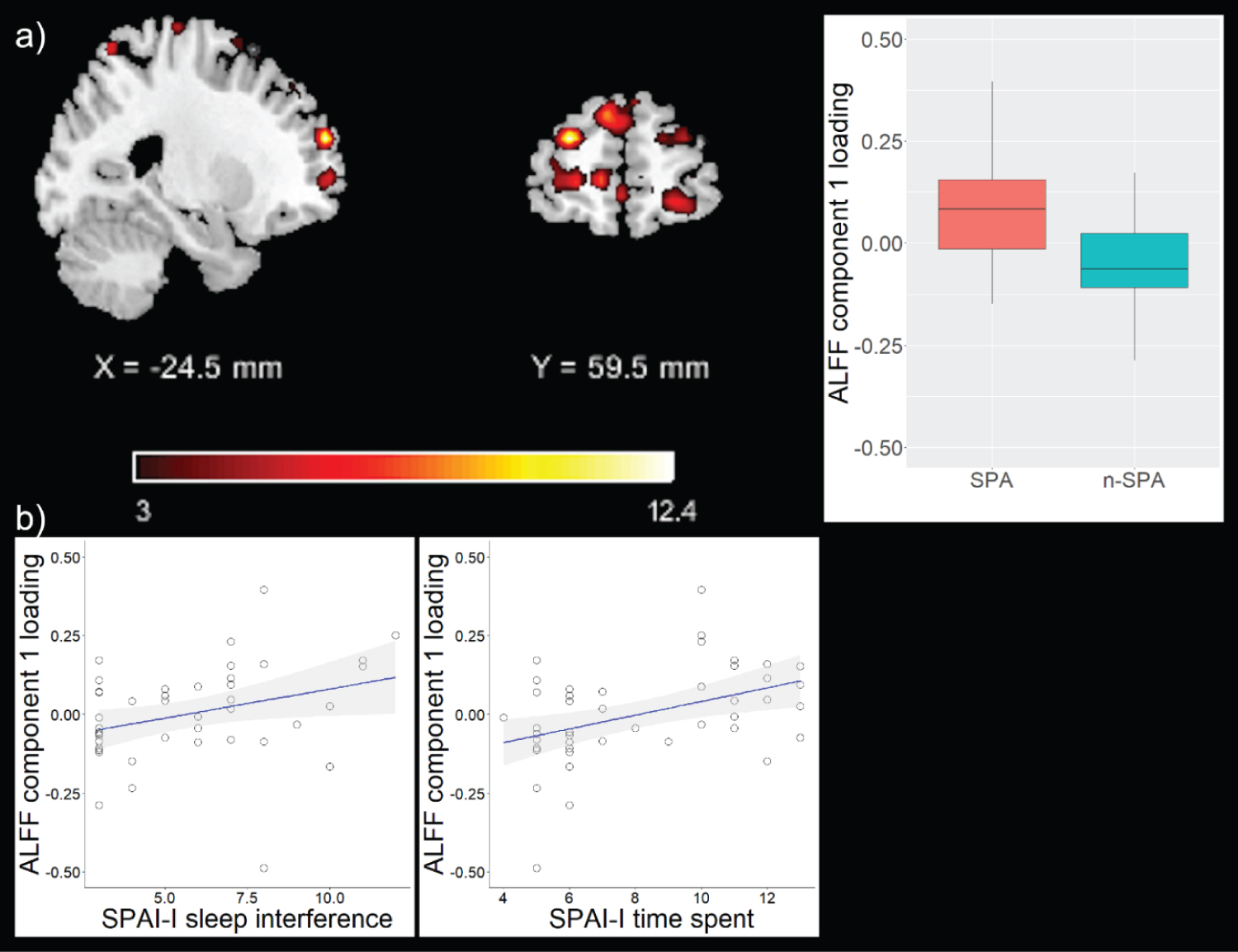
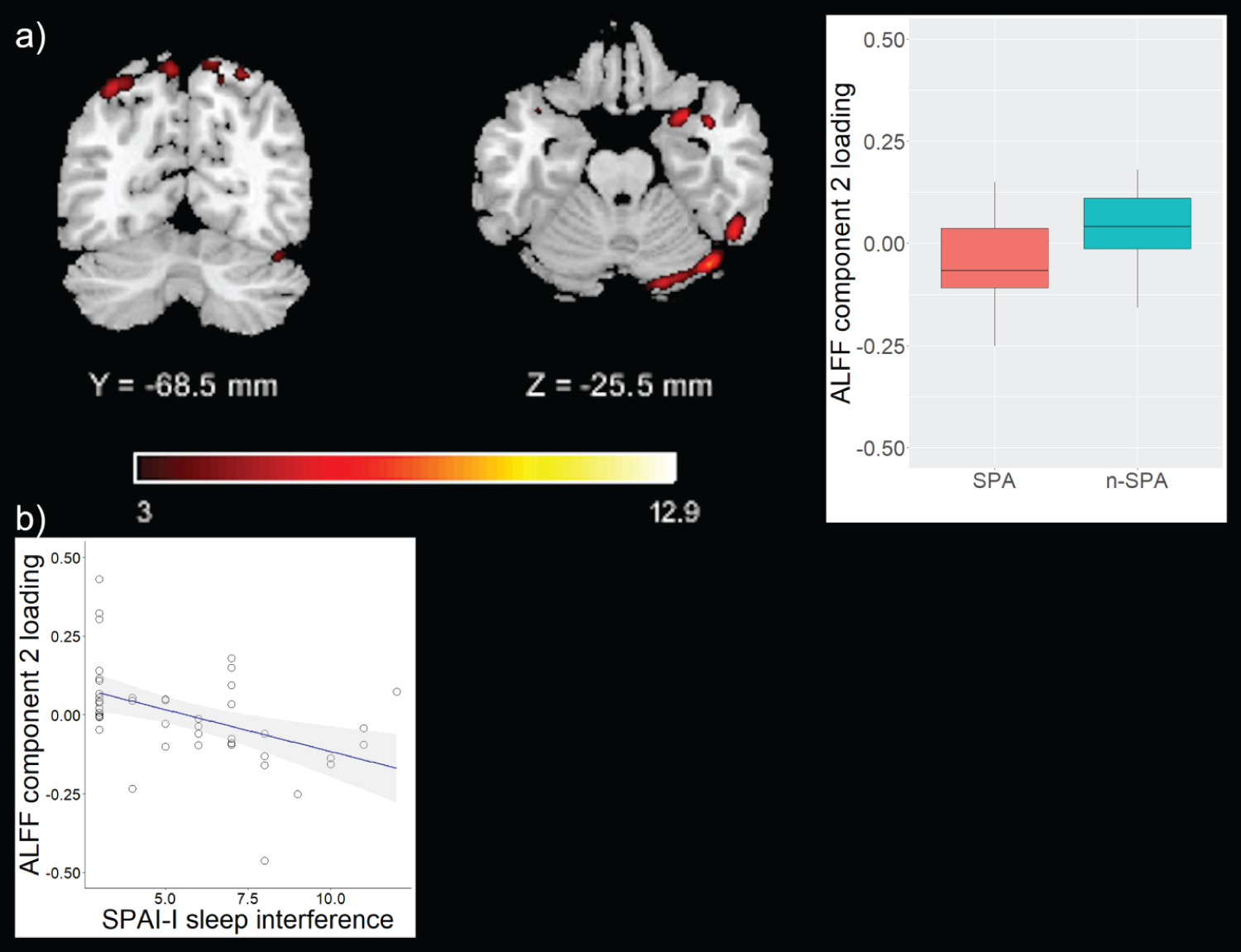
Component pre-selection identified three components (two ALFF, one GMV), whose component loadings were subsequently entered in ANCOVA models to test for group differences. The first ALFF-based component (ALFF 1) predominantly comprised medial and dorsolateral prefrontal regions, but also included temporal and parietal regions. The second ALFF-based component (ALFF 2) predominantly comprised parietal and cerebellar regions, but also included frontal, temporal, and occipital regions (Table 2, Figure 1 and Figure 2). ANCOVA models revealed significant differences of component loadings between groups for ALFF 1 and ALFF 2, but not GMV (ALFF 1: F = 12.85, df = 1, pFDR = 0.003, median SPA = 0.83, mediann-SPA = -0.06; ALFF 2: F = 5.41, df = 1, pFDR = 0.038, median SPA = -0.07, mediann-SPA = 0.04; GMV: F = 3.21, df = 1, pFDR = 0.081, median SPA = 0.03, mediann-SPA = -0.04). ALFF 1 and ALFF 2 were therefore identified as components of interest. For completeness, regions comprised by the GMV component are depicted in Table S1.
Associations between functional networks and SPAI-I
Loading parameters of both components correlated with SPAI-I total score (ALFF 1: pFDR = 0.04, correlation coefficient = 0.38; ALFF 2: pFDR = 0.03, correlation coefficient = -0.38; Table 3. Particularly, loading parameters of ALFF 1 correlated with SPAI-I time spent (pFDR = 0.03, correlation coefficient = 0.45) and SPAI-I sleep interference (pFDR = 0.04, correlation coefficient = 0.38) and loading parameters of ALFF 2 correlated with SPAI-I sleep interference (pFDR = 0.02, correlation coefficient = -0.46; Table 3. For completeness, correlations between component loadings of the GMV component and SPAI factors are depicted in Table S2.
Complementary analyses additionally adjusted for BDI total score showed no significant associations between loading parameters (ALFF 1, ALFF 2, and GMV) with SPAI-I or SPAI factors or respective total score.
SPAI-I time spent and SPAI-I sleep interference were correlated (p < 0.001, correlation coefficient = 0.60), also with an additional adjustment for BDI total score (p < 0.001, correlation coefficient = 0.51).
Discussion
We used a multivariate data-driven approach to study associations of two modalities, i.e., functional, and structural imaging findings in the context of addictive smartphone use. Two key findings emerged: First, a predominantly medial/dorsolateral prefrontal and a mainly parietal/cerebellar functional network differed significantly between SPA and n-SPA. Second, there were specific and shared correlations between these functional systems and dimensional measures of addictive smartphone use.
Our analyses identified two rs-fMRI components, which differ between SPA and n-SPA. The first component showed increased activation in predominantly medial and dorsolateral prefrontal regions in those with SPA vs. n-SPA. In IGD, a diagnosis showing substantial conceptual overlap to SPA [35], common neurobiological mechanisms, particularly with respect to prefrontal cortex among other regions, are suggested [36].
The second component showed decreased activation in predominantly parietal and cerebellar regions in those with SPA vs. n-SPA. Cerebellar function has been associated with habit-formation in addictive disorders [37]. Therefore, it is conceivable, that the found group-difference of incross-information in this component may reflect a link to habit formation in excessive smartphone use. Nevertheless, tailored studies are needed to test this hypothesis.
Taken together, the two components partially depict a fronto-parietal network, which was associated with cognitive control in internet addiction [38] and our findings fit well to the suggested dysbalance between limbic/reward-oriented networks and systems subserving prefrontal control, as suggested by the I-PACE model [11,12].
On a neural systems level, regions depicted by the two functional networks are part of the so-called "default mode network" (DMN), which has been associated to self-awareness in addictive disorders and the salience network, which critically mediates the interplay between executive systems and the DMN [39]. Moreover, the detected patterns share, if at all, only little spatial similarities reported by Horvath, et al. [14]. This indicates that the multivariate fusion approach used here indeed revealed information, which was not detected via separate analyses of GMV and INA.
Remarkably, the functional networks also showed significant correlations with distinct SPAI-I dimensions, i.e. "time spent" (particularly the medial/dorsolateral prefrontal system) and "sleep interference" (both networks). Noteworthy, these SPAI-I dimensions were associated to each other in our sample. The two identified components share the same imaging modality, i.e., rs-fMRI, yet they reflect different physiological properties and cognitive representations on a subnetwork-level. Also, in comparison to joint ICA [40], p-ICA pays more attention to individual linked components and their connections, while joint ICA pays more attention to inter-effects between different data-acquisition modalities as a whole [41]. Moreover, non-fusion ICA based methods on ALFF alone very likely would have not been able to identify the found components without the additional information from GMV. In this line of thought, the found correlations depict component specific and shared associations between temporal features of intrinsic neural activity and measures of addictive smartphone use. Most of the regions found in the prefrontal networks are part of the inhibitory networks depicted by Stevens and coworkers [42], therefore the found associations might represent a lack of inhibitory control in SPA. This notion is also supported by the significant differences between SPA and n-SPA with respect to BIS attention and motor scores. The SPAI-I factor sleep interference includes items of the SPAI that capture the relationship between smartphone use and shorter sleep duration as well as sleep disturbance [25]. Regions found in both networks are part of the DMN, which represents brain activation during a relaxed, resting condition without external stimuli [43]. Altered functional activation in these regions might represent, among others, rumination processes - or even excessive preoccupation with the smartphone -, which in turn disrupt healthy sleeping behavior leading to depressed mood [44]. Taken together, these findings point towards a multifaceted, brain-activation linked interplay of non-capability of not using the smartphone and sleep interfering processes in SPA. The temporal association and causality of these findings remain unclear.
We acknowledge potential limitations of this study, such as the relatively small sample size and missing formal clinical evaluation of potentially confounding comorbid mental disorders. Mental disorders were not reported by the participants, but it is impossible to fully rule out the presence of other mental health conditions that may have an impact on GMV or ALFF. Moreover, the identified associations between loading parameters and measures of SPA did not remain significant, if additionally controlled for depressiveness. Therefore, our interpretations of these findings should be handled with appropriate scientific caution. Also, the cross-sectional design does not allow inference on temporal development and stability of these findings, as much as the correlations do not imply causality. Carefully designed longitudinal studies with sample sizes being more representative of the population showing problematic smartphone use are needed to robustly answer such important questions.
In conclusion, the present study provides further evidence for common neural mechanisms of behavioral addiction in individuals with SPA, which was not accessible via separate mass-univariate analyses of GMV and INA. This study needs replication and extensions using larger and more representative sample sizes, including longitudinal assessments supplemented by ecological momentary assessment. Yet, additionally, this study provides important new findings, suggesting deviant recruitment of resting-state networks and modality-specific associations of resting activation with distinct and shared symptom dimensions of SPA.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. We thank our study participants for their time and interest in this study. Moreover, our special thanks go to Bretta Beer for proofreading the manuscript and valuable comments on improving the text.
Compliance with Ethical Standards
Conflicts of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Research involving human participants
The study was approved by the Ethics Committee of the Medical Faculty at Heidelberg University and carried out in compliance with the Declaration of Helsinki. All participants gave written informed consent prior to inclusion in the study.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (Ethics Committee of the Medical Faculty at Heidelberg University) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Author Contributions
Mike M. Schmitgen, Juliane Horvath, Christina Mundinger, Nadine D. Wolf, and Robert Christian Wolf were responsible for the study concept and design. Mike M. Schmitgen, Juliane Horvath, and Christina Mundinger contributed to the acquisition of human data. Mike M. Schmitgen and Robert Christian Wolf performed the data analysis. Juliane Horvath, Christina Mundinger, and Fabio Sambataro assisted with data analysis and interpretation of findings. Mike M. Sschmitgen and Robert Christian Wolf drafted the manuscript. Nadine D. Wolf, Fabio Sambataro, Dusan Hirjak, Katharina M. Kubera, and Julian Koenig provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved the final version for publication.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Demirci K, Akgonul M, Akpinar A (2015) Relationship of smartphone use severity with sleep quality, depression, and anxiety in university students. J Behav Addict 4: 85-92.
- Duke E, Montag C (2017) Smartphone addiction, daily interruptions and self-reported productivity. Addict Behav Rep 6: 90-95.
- Grant JE, Lust K, Chamberlain SR (2019) Problematic smartphone use associated with greater alcohol consumption, mental health issues, poorer academic performance, and impulsivity. J Behav Addict 8: 335-342.
- Toh SH, Coenen P, Howie EK, et al. (2017) The associations of mobile touch screen device use with musculoskeletal symptoms and exposures: A systematic review. Plos One 12: e0181220.
- Lin YH, Chiang CL, Lin PH, et al. (2016) Proposed diagnostic criteria for smartphone addiction. PLoS One 11: e0163010.
- Kim SG, Park J, Kim HT, et al. (2019) The relationship between smartphone addiction and symptoms of depression, anxiety, and attention-deficit/hyperactivity in South Korean adolescents. Ann Gen Psychiatry 18: 1.
- Petry NM, Rehbein F, Ko CH, et al. (2015) Internet gaming disorder in the DSM-5. Curr Psychiatry Rep 17: 72.
- Lin YH, Chang LR, Lee YH, et al. (2014) Development and validation of the smartphone addiction inventory (SPAI). PLoS One 9: e98312.
- Kwon M, Lee JY, Won WY, et al. (2013) Development and validation of a smartphone addiction scale (SAS). PLoS One 8: e56936.
- Montag C, Wegmann E, Sariyska R, et al. (2019) How to overcome taxonomical problems in the study of Internet use disorders and what to do with "smartphone addiction"? J Behav Addict 9: 908-914.
- Brand M, Wegmann E, Stark R, et al. (2019) The Interaction of person-affect-cognition-execution (I-PACE) model for addictive behaviors: Update, generalization to addictive behaviors beyond internet-use disorders, and specification of the process character of addictive behaviors. Neurosci Biobehav Rev 104: 1-10.
- Brand M, Young KS, Laier C, et al. (2016) Integrating psychological and neurobiological considerations regarding the development and maintenance of specific Internet-use disorders: An interaction of person-affect-cognition-execution (I-PACE) model. Neurosci Biobehav Rev 71: 252-266.
- Yao YW, Liu L, Ma SS, et al. (2017) Functional and structural neural alterations in Internet gaming disorder: A systematic review and meta-analysis. Neurosci Biobehav Rev 83: 313-324.
- Horvath J, Mundinger C, Schmitgen MM, et al. (2020) Structural and functional correlates of smartphone addiction. Addict Behav 105: 106334.
- Kim DJ, Kim JY, Pyeon A (2016) Altered functional connectivity related smartphone overuse in adolescent. Int J Neuropsychopharmacol 19: 9.
- Chun JW, Choi J, Kim JY, et al. (2017) Altered brain activity and the effect of personality traits in excessive smartphone use during facial emotion processing. Sci Rep 7: 12156.
- Schmitgen MM, Horvath J, Mundinger C, et al. (2020) Neural correlates of cue reactivity in individuals with smartphone addiction. Addict Behav 108: 106422.
- Kubera KM, Rashidi M, Schmitgen MM, et al. (2019) Structure/function interrelationships in patients with schizophrenia who have persistent auditory verbal hallucinations: A multimodal MRI study using parallel ICA. Prog Neuropsychopharmacol Biol Psychiatry 93: 114-121.
- Sui J, Adali T, Yu Q, et al. (2012) A review of multivariate methods for multimodal fusion of brain imaging data. J Neurosci Methods 204: 68-81.
- Liu J, Demirci O, Calhoun VD (2008) A parallel independent component analysis approach to investigate genomic influence on brain function. IEEE Signal Process Lett 15: 413-416.
- Liu J, Pearlson G, Windemuth A, et al. (2009) Combining fMRI and SNP data to investigate connections between brain function and genetics using parallel ICA. Hum Brain Mapp 30: 241-255.
- Pearlson GD, Liu J, Calhoun VD (2015) An introductory review of parallel independent component analysis (p-ICA) and a guide to applying p-ICA to genetic data and imaging phenotypes to identify disease-associated biological pathways and systems in common complex disorders. Front Genet 6: 276.
- Liu R, Liu BX, Ma M, et al. (2018) Aberrant prefrontal-parietal-cerebellar circuits in alcohol dependence. Neuropsychiatr Dis Treat 14: 3143-3150.
- Yuan K, Jin C, Cheng P, et al. (2013) Amplitude of low frequency fluctuation abnormalities in adolescents with online gaming addiction. PLoS One 8: e78708.
- Pavia L, Cavani P, Di Blasi M, et al. (2016) Smartphone Addiction Inventory (SPAI): Psychometric properties and confirmatory factor analysis. Comput Hum Behav 63: 170-178.
- Kwon M, Kim DJ, Cho H, et al. (2013) The smartphone addiction scale: Development and validation of a short version for adolescents. PLoS One 8: e83558.
- Beck AT, Ward CH, Mendelson M, et al. (1961) An inventory for measuring depression. Arch Gen Psychiatry 4: 561-571.
- Patton JH, Stanford MS, Barratt ES (1995) Factor structure of the Barratt impulsiveness scale. J Clin Psychol 51: 768-774.
- Grant JE, Potenza MN, Weinstein A, et al. (2010) Introduction to behavioural addictions. Am J Drug Alcohol Abuse 36: 233-241.
- Chao-Gan Y, Yu-Feng Z (2010) DPARSF: A MATLAB Toolbox for "Pipeline" data analysis of resting-state fMRI. Front Syst Neurosci 4: 13.
- Friston KJ, Williams S, Howard R, et al. (1996) Movement-related effects in fMRI time-series. Magn Reson Med 35: 346-355.
- Liu J, Kiehl KA, Pearlson G, et al. (2009) Genetic determinants of target and novelty-related event-related potentials in the auditory oddball response. Neuroimage 46: 809-816.
- Calhoun VD, Adali T, Pearlson GD, et al. (2001) A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 14: 140-151.
- Himberg J, Hyvarinen A, Esposito F (2004) Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage 22: 1214-1222.
- Paik SH, Cho H, Chun JW, et al. (2017) Gaming device usage patterns predict internet gaming disorder: Comparison across different gaming device usage patterns. Int J Environ Res Public Health 14: 1512.
- Kuss DJ, Pontes HM, Griffiths MD (2018) Neurobiological correlates in internet gaming disorder: A systematic literature review. Front Psychiatry 9: 166.
- Noori HR, Cosa Linan A, Spanagel R (2016) Largely overlapping neuronal substrates of reactivity to drug, gambling, food and sexual cues: A comprehensive meta-analysis. Eur Neuropsychopharmacol 26: 1419-1430.
- Wang L, Shen H, Lei Y, et al. (2017) Altered default mode, fronto-parietal and salience networks in adolescents with Internet addiction. Addict Behav 70: 1-6.
- Volkow ND, Wang GJ, Fowler JS, et al. (2012) Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol 52: 321-336.
- Calhoun VD, Adali T, Kiehl KA, et al. (2006) A method for multitask fMRI data fusion applied to schizophrenia. Hum Brain Mapp 27: 598-610.
- Calhoun VD, Adali T, Pearlson GD, et al. (2006) Neuronal chronometry of target detection: fusion of hemodynamic and event-related potential data. Neuroimage 30: 544-553.
- Stevens MC, Kiehl KA, Pearlson GD, et al. (2007) Functional neural networks underlying response inhibition in adolescents and adults. Behav Brain Res 181: 12-22.
- Raichle ME, MacLeod AM, Snyder AZ, et al. (2001) A default mode of brain function. Proc Natl Acad Sci USA 98: 676-682.
- Slavish DC, Graham-Engeland JE (2015) Rumination mediates the relationships between depressed mood and both sleep quality and self-reported health in young adults. J Behav Med 38: 204-213.
Corresponding Author
Robert Christian Wolf, MD, Center for Psychosocial Medicine, Department of General Psychiatry, Heidelberg University, Voßstraße 4, 69115 Heidelberg, Germany, Tel: +49-6221-564405, Fax: +49-6221-564481.
Copyright
© 2022 Schmitgen MM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.