Insights on the Mango Anthracnose and its Management
Abstract
Anthracnose, caused by the fungus Colletotrichum gloeosporioides Linnaeus, is the most common pre-and postharvest disease of mango, causing economic losses of 30-60 percent in the production of fruit in tropical, subtropical countries. C. gloeosporioides is reported to infect a wide range of hosts and has become an increasingly significant pathogen affecting a variety of economically important crops throughout the world. Mango anthracnose management is a popular issue among farmers and agriculturists. The reduction in mango production and fruit quality decline has intensified the demand for a long-term strategy to combat the disease's spread. Though the disease's pandemic nature has been researched for a long time, a lot of work is still unexplored. It needs to be done before any environment-friendly and consistent control strategy comes into existence. This review highlights various aspects of the epidemiology and management of the disease through resistant cultivars, biological controls, hot water treatment with waxing, oxalic acid treatment, Use of essential oils and botanicals.
Keywords
Colletotrichum gloeosporioides, Tear strain, Host-range, Acervuli, Botanicals
Introduction
Mango dates back to 4000 BC at the foot of the Himalayas in South Asia (eastern India, Burma, Andaman Islands), bordering the Bay of Bengal [1]. Mangifera indica L. has been cultivated for thousands of years in South and Southeast Asia and is Asia's most significant fruit crop [2]. King of Fruit; Mango (Mangifera indica L.) is the eighth most produced fruit globally, with 43 million tonnes produced in Bangladesh, India, Nepal, and other tropical countries [3]. Mango plants have fleshy stone fruits rich in polyphenols, vitamins, and phytochemicals of indisputable nutritional value due to their antioxidants, anti-inflammatory, anti-cancer, multifaceted biochemical effects, and beneficial to one's health. Its demand is growing day by day in many developing countries since it is essential sources of diet and nutrient [3,4] (Table 1).
However, All the stages of Mango development are affected by several diseases that might be from plants in the nursery to fruits when stored or transported [5,6]. Mangoes are susceptible to many fungal diseases such as anthracnose, root rot, stem rot, penicillium rot, black rot, mucor rot, pestalotia rot, Macrophoma rot, and powdery mildew to heavy loss [7]. Glomerella cingulata (Stoneman) Spauld and H. Schrenk (anamorphic: Colletotrichum gloeosporioides (Penz.) is one of the most economically critical pathogenic genera of mango anthracnose affecting mango fruits during sorting, packaging, transport, storage, and sale [8]. C. gloeosporioides causes immense economic losses of 30-60 percent in fruit production, which can reach 100 percent in rainy or highly humid circumstances [9]. About a quarter to a third of total mango production losses has been reported due to anthracnose and stem-end rot, spread with raindrops [3]. Mango anthracnose causes premature fruit drop [10], severe spots on young leaves and blackening of the tips; flowers fall, lowered fruit set, and circular, dark, depressed lesions on ripe fruits [11,12].
Among biotic stresses, anthracnose is caused by Colletotrichum gloeosporioides (Penz). Penz. and Sacc. in Penz. (Teleomorph Glomerella cingulata (Stonem) Spauld and Schreule) is the most severe and devastating malady on a wide range of fruit crops worldwide at pre-and postharvest stages, causing severe yield and economic losses to the farmers and traders of Mango [13]. [14] reported that, on average, 17.7% of mangoes are spoiled due to fungal diseases in transit, storage, and marketing. [15] reported losses in mango fruits are as high as 75% because of this disease. Mango plants are commonly naturally infected by anthracnose fungus even before fruiting, but the organism remains quiescent, producing symptoms only on mature fruits on the tree or after harvest [16].
Host range of C. gloeosporioides
The ubiquitous fungus Colletotrichum gloeosporioides or its Glomerella teleomorph have been recorded from many plants, especially in humid areas. It is most frequent in the warm, moist environments encountered in humid and sub-humid tropical zones where it infests stems, leaves, flowers, and fruits. It is the most predominant and significant Colletotrichum pathogen of tropical fruit crops [17]. It is the most common among the preharvest, and postharvest diseases cause marked both pre-and postharvest losses and reduce the postharvest quality attributes in these of a wide variety of tropical and subtropical fruits and vegetables. It is the most important, prevalent, and serious problem in most locations where this crop is cultivated [18] (Table 2).
Symptoms
Colletotrichum gloeosporioides cause different symptoms depending on the type of host and the infected tissue.
Symptoms on leaves
According to a study conducted to measure the disease incidence and severity of mango trees on leaves, the mean incidence of the disease on leaves, panicles, and immature fruits was 76 percent, 71 percent, and 68 percent [19]. Mango anthracnose appears as irregular necrotic black spots on the top and bottom of mango leaves (Mo, et al.). In seedlings of fruit trees, especially mango and rambutan, Symptoms of Colletotrichum gloeosporioides infection can cause up to 40% of reproductive material, previously considered a physiological disease [17]. Typical symptoms are oval or irregular dark brown to dark brown spots of varying sizes, scattered over the entire leaf surface. The fungus proliferates and forms elongated brown necrotic patches with 20-25 mm diameter in humid conditions. Infected leaves often show "shot holes" cause infected young leaves are more vulnerable than older ones [20]. Anthracnose attacks young leaves, causing tough spots and blackening the tips [11,12,21] (Figure 1).
Symptoms on flowers
Infections on the panicles (flower clusters) start as small black or dark-brown spots. These can enlarge, coalesce and kill the flowers. Rainfall during the blossoming and planting of fruits in the region, anthracnose can damage and infest flowers and shed young fruits, leading to severe losses of up to 35% of the harvested fruit [22]. Affected flowers fall off and lower down the fruit set. The demarcating symptom of the disease is circular, dark, depressed lesions with anthracnose on ripe fruits [11,12] (Figure 2).
Symptoms of fruits
Dark, depressed, circular lesions appear during ripening, quickly expanding and covering almost the entire fruit in very severe cases [23]. [24] showed that lesions of different sizes could coalesce to cover large fruits, which is characteristic of the "tear strain" pattern from the basal to the distal part of the fruit. These blisters are usually limited to the peel, but the fungus can invade the pulp and produce large numbers of orange to pink blisters and conidia with a more severe infection. Two fundamental symptoms for mango anthracnose are depressed dark lesions (above, left) or the "tear stain" impact (over right and below, left), straight necrotic districts loaning an alligator-skin effect, often related with the splitting of the epidermis (underneath) found on common mango and other mango varieties [25]. Mango anthracnose causes premature fruit drop and immediate deterioration in ripe fruit quality [10] (Figure 3).
Symptoms on stem and branches
When stems and twigs develop, severe, elongated, darkened lesions and die back apically called twig dieback. The pathogen's abundant sporulation covers the infection's most degraded sites [25].
Epidemiology/Favorable Conditions for Anthracnose
The severity and spread of any disease are majorly decided by Environmental factors. The favorable host, pathogen, and weather conditions cause disease establishment [26]. Thus, a thorough knowledge regarding the epidemiology of the disease should be studied before proposing the management strategies of the disease [26].
Also, Postharvest losses are caused by Anthracnose in tropical fruits and vegetables, which cause a severe obstacle to the expansion of the export trade in fruits such as mangoes. Better understanding the quiescent nature of anthracnose after harvest and the epidemiology is crucial to improving this situation [27].
The optimum temperature for anthracnose infection is around 25 °C [20]. Injuries caused by the anthracnose pathogen are affected by moisture, rain, fog, or excessive dew during flowering; prolonged wet weather during flowering results in severe blooms blight. Relative humidity of 95% or more within 12 hours is crucial for infection and developing C. gloeosporioides in mango fruit. The condition progresses faster in damaged tissues and ripe fruits [20]. The disease is particularly severe on young leaves and, if wet weather prevails during flowering, it causes a severe blossom blight which can destroy the inflorescences and prevent fruit set. Infection can also occur after the fruit set, but the disease usually remains latent until the fruit begins to ripen [28].
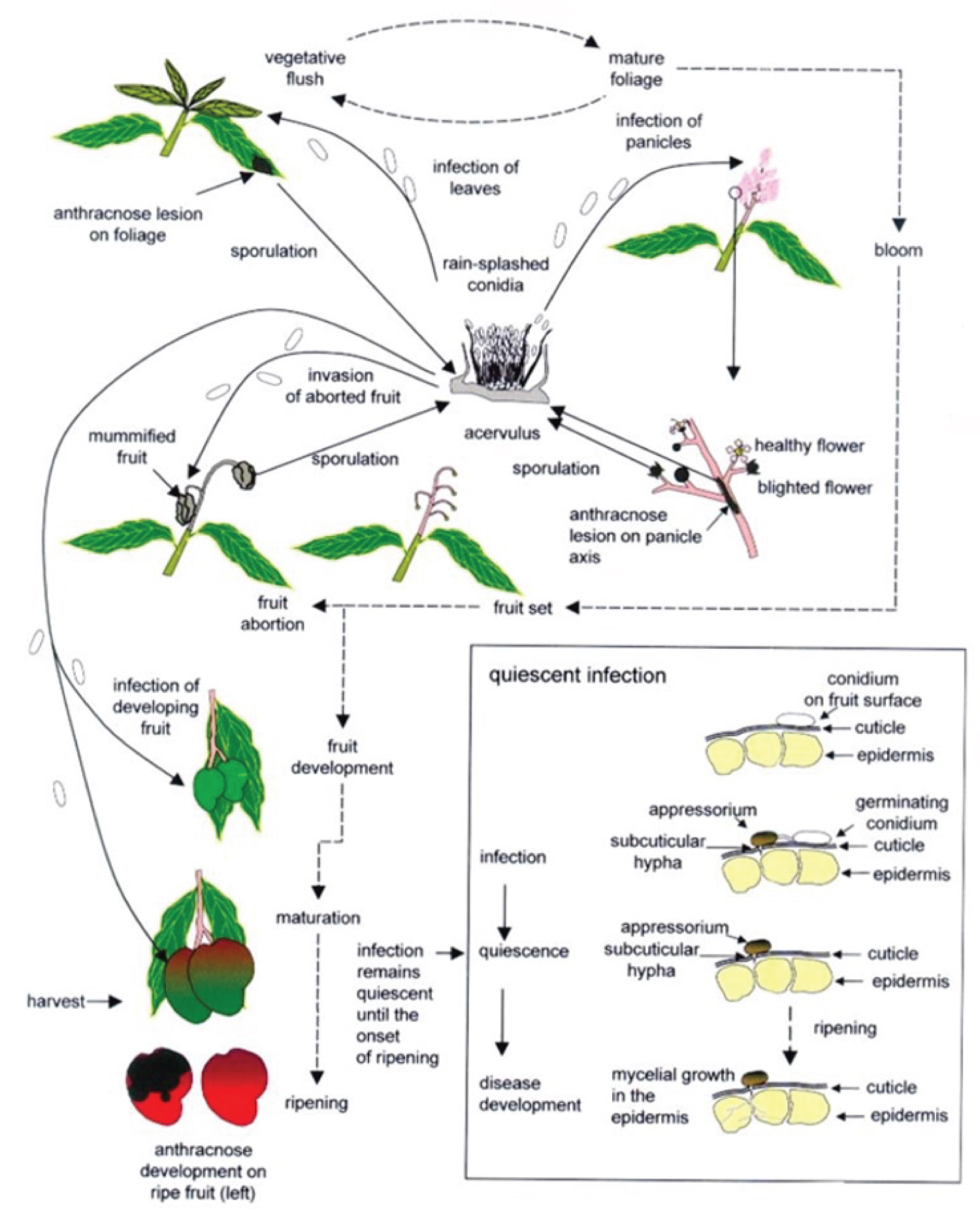
Disease Cycle
1. Dissemination: The splashing rain or irrigation water passively disperses conidia.
2. Inoculation: Spores land on infectious sites such as (panicles, leaves, branch terminals).
3. Symptoms and disease development: Black, sunken, rapidly expanding lesions develop in fruiting bodies (Acervuli).
4. Infection and pathogen development: After germination, the spores enter the cuticle and epidermis of immature fruits and young tissues, ramifying through the tissues. After the spores penetrate the cuticle on mature fruits, infection develops and remains dormant until the beginning of fruit ripening.
5. Symptoms and disease development: On the affected organs, dark, sunken, quickly growing lesions form.
6. Pathogen reproduction: Acervuli on symptomatic tissue generate sticky masses of conidia, especially during wet or humid conditions. As the fungus multiplies during the season, various diseases might develop [3].
7. Pathogen survival: The disease persists between seasons on infected and defoliated branch terminals and mature leaves [29] (Figure 4).
Anthracnose is caused by the water-borne conidia of Colletotrichum gloeosporioides var. Minor [28]. Conidia thrive on the mango canopy, considered the primary source of inoculum producing lesions on twigs, leaves, panicles, and mummified fruits [28]. Conidia are formed from damaged leaves, fallen branches, mummified inflorescences, and bracts [28,30]. Conidia were caught from these sources in gardens at a time when anthracnose flourished during both growth and flowering. Young leaves Lesion captured most conidia. Conidia that develop from lesions of diseased leaves over a wide temperature range (10-30 °C) in humid conditions (95-97% RH) in the laboratory grow or bloom vigorously, and severe disease outbreaks have been recorded [28,30]. After dew, there were no conidia. Ascospore of Glomerella cingulata var. minors were not trapped from the garden during active illness. These spores do not appear to be involved in the infection cycle [28,30]. Conidia spread to other leaves and inflorescences by drizzle. Therefore, they are considered a secondary source of inoculum [28]. This disease is deemed to be polycyclic [31].
The optimum temperature for germination and infestation of conidia is approximately 25-30 °C with moisture and pH 5.8-6.5 [32]. This condition is crucial at the beginning of infection and is generally critical for the successful development of C. gloeosporioides [33].
The developing fruits could be infected, resulting in pre-harvest losses of mango. Developing fruit is infected in the field, but the infection remains dormant until ripening begins. In this case, Anthracnose at postharvest is monocyclic as symptoms appear at a postharvest stage with no fruit-to-fruit infection [34]. The fungus produces vitreous, unicellular, oval to elongated, slightly curved, or dumbbell-shaped conidia with obtuse ends. They are usually borne on distinct, well-developed hyaline conidiophores, typically 12.5-14.8 μm to 4.1-4.7 μm in size [35].
Disease Management Approaches
Mango anthracnose management has been a prevalent issue among agriculturists and farmers. The reduction in mango production and the decline in fruit quality have intensified the need for a long-term strategy to combat the disease's spread. No single management approach has been identified to control the disease effectively. In general, treating the disease using a combination of techniques such as chemical control, biological control, physical control, and inherent resistance has been recommended [26]. Preventive methods, field fungicide sprays, and postharvest treatments are the most effective ways to combat anthracnose [36]. Disease control can be best influenced by an integrated approach that includes pre and postharvest treatments and biological and environmental control factors [27]. Several pre-harvest and postharvest management approaches have been used to control this anthracnose disease of mango fruits, including chemical treatments [3].
Cultural
Since mango anthracnose development depends on wetness or high relative humidity, ideally, orchards should be established in locations with a well-defined dry season so that fruit may develop in conditions unsuitable to disease development [24]. 90% of anthracnose development occurs during the rainy season; therefore, mango cultivation should be timely managed, so the fruit development occurs at the least rainy time. Field sanitation should be regularly practiced. Timely Pruning of the dead, diseased parts, fallen fruit, and tree trash should be done [25]. Leaf inoculum is one of the major sources of disease development, so sanitation is a must. Also, proper spacing between the trees should be maintained, and wide spacing should be provided to prevent the disease epidemic [24].
Intercropping: Epidemics can be avoided by interplanting mango trees with plants that aren't hosts for mango anthracnose [25].
Resistant cultivars are an ideal, simplest and cheapest method for controlling plant disease [3]. Out of several mango cultivars, only the Keitt variety has shown resistance to anthracnose, whereas Himsagar and Ostin offer moderate resistance and other varieties are moderate to highly susceptible to anthracnose [8].
Resistant varieties
Resistant varieties popular in Nepal: A substantial economic loss is caused all around the world due to mango anthracnose. Anthracnose causes 30-60% yield losses on mango across different world countries [31,37]. Developing resistance to the pathogen in the host is seen to be the most significant and long-term strategy for reducing disease-related losses, thereby removing the chemical and mechanical costs of disease control [26]. The goal of using resistant cultivars is to activate the host's defensive response, which then inhibits or slows the pathogen's growth. This is accomplished by using a single gene pair: A host resistance gene and a pathogen a virulence gene [38,39]. Mangifera laurina is a species of mango that is found to be resistant to anthracnose. This species consists of subglabrous and laxly flower panicles that are well adapted to wet climates, making them more resistant to anthracnose and producing hybrids resistant to this disease [40]. Anthracnose affects all commercial mango cultivars. However, Tommy Atkins and Keitt are less sensitive than cultivars like Irwin, Kent, and Edward [24].
Hot water treatment with waxing
For some fruits, treating with warm water is suitable for avoiding discoloration or burns of the rind, taste changes, or pulp softness due to water temperature. The temperature chosen for the HWT is slightly above the target pathogen's thermal death point, which provides excellent potential for minimizing postharvest losses. At high temperatures, pathogens usually die, and most enzymes and proteins are denatured [23]. However, the effectiveness of HWT depends not only on the temperature and the time for which the product is exposed, but also on the degree of ripeness, size, and weight of the fruit, variety, and growing conditions, and this should be taken into account when determining the temperature and duration of treatment [41]. Postharvest anthracnose control in mangoes can also be done using hot water alone or by treating the mangoes with chemicals [42]. Heat treatment of fruits at 50-60 °C for 5-10 minutes is used to combat many pathogens after harvest [43]. Treating mangoes with hot water at 55 °C for 5 minutes can reduce anthracnose severity [44,45]. [44] reported that mangoes were immersed in prochloraz (active ingredient 250 mg/L), azoxystrobin (active ingredient 250 mg/L), or hot water (3 minutes at 55 °C) while stored at 15 °C for ten days. Hot water has proven to be the most effective postharvest treatment. Fruit treated with T6 (Topsin-M @ 1 g L-1 in field dip for 1 min + HWQT @ 48 °C for 60 min), T2 (HWQT @ 45 °C for 75 min), and T8 (HWQT @ 48 °C for 60 min + Carbendazim @ 0.4 g 10 L-1 at 52 °C for 5 min) had the lowest anthracnose incidence score (0.03) when compared to control [44].
Biological control
Innovative techniques to manage infections and postharvest diseases of tropical fruit utilizing regulated biocontrol agents and natural products are encouraged by government intends to restrict pesticides and residues in fresh food [43]. To identify a potential biocontrol agent, 305 epiphytic bacteria isolated from the carposphere of 17 mango cultivars obtained from eight sites on Reunion Island were screened. A first phase in the screening process was based on the isolates' capacity to form a biofilm, thrive under fruit storage conditions, and inhibit the growth of C. gloeosporioides. The potential of chosen isolates to inhibit C. gloeosporioides in vitro mycelial development and conidia germination was evaluated in a second stage, and species were identified. The most effective bacteria belonged to the Enterobacter, Pantoea, Kosakonia, and Leuconostoc genera, but their safe Use has to be demonstrated for some of them. Efficacy in vivo on damaged mature mango fruit was restricted, most likely due to the pathogen-favouring wounding inoculation method. To maximize the protective value of future biocontrol treatments, preharvest applications should be prioritized [46].
Studies have shown that the potential microbial antagonist Colletotrichum gloeosporioides was isolated from mango flowers, leaves, and fruits and filtered using various analytical methods. A total of 648 microorganisms were isolated, including bacteria, yeast, and filamentous fungi, and the growth inhibition of C. gloeosporioides on malt agar extract was tested. Of these isolates, 45 bacteria and yeast inhibited germination. In postharvest commercial testing, both isolates 204 and 558 were tested using various application methods, including the addition of adhesives, peptones, fruit waxes, or sucrose polyesters. The administration of isolate 204 (identified as Bacillus cereus) did not reduce disease progression, while the administration of isolate 558 (Pseudomonas fluorescens) significantly reduced the incidence of anthracnose [47]. The bacterial antagonists (Brevundimonas diminuta) isolate B-62-13, Stenotrophomonas maltophilia L-16-12, and Enterobacteriaceae isolate L-19-13, as well as the yeast, isolates B-65-23 (unknown) and F-58-22 (Candida membranifaciens) reduced anthracnose severity levels below 5% for much of the 12-day experimental period when tested on naturally infected fruit. Furthermore, the fruit treated with these five isolates had only minor, unnoticeable spots that did not influence market attractiveness and were consequently unmarketable due to anthracnose infections [48].
Oxalic acid treatment
Pre-storage application of 5 mM OA for 10 min at 25 °C then stored at 14 °C ± 1 °C for five weeks was a potential technique for preventing postharvest degradation and extending the shelf life of chilled mango fruit, perhaps owing to a combination of its physiological impact in delaying the ripening process and suppression of infections such C. gloeosporioides [49].
Use of essential oils and botanicals
Farmers and exporters use high doses of fungicides to control anthracnose before and after harvest. Although fungicides have many benefits, they lead to fungicidal resistance to pathogens, contamination, and human health problems [13]. In-vitro analyzes with aqueous extracts of lantana, cosmos, marigold, and tamarind showed no suppression of radial growth of the fungus. Still, ethanolic extracts of tamarind and marigold showed the most significant suppression against C. gloeosporioides. In addition to the aesthetic and economic value of the plant, the efficacy of this plant extract must be tested using various solvents (e.g., methanol) as substrates for other organisms as a means of eco-friendly control before and after harvest [26]. The Use of synthetic chemicals before and after harvest, their indiscriminate Use, and high frequency on a commercial scale have negatively impacted agricultural production. Therefore, essential oils (ECs) with antifungal properties and their bioactive components can be an attractive and effective alternative to inhibiting the growth of these fungal pathogens, including anthracnose pathogens, in various host plants [18].
Comparative study of the efficacy and bactericidal properties of citronella essential oil on control groups of citronella essential oil after harvest at Rajamangala University of Technology, Thailand, showed that 4000 ppm citronella oil caused minimal anthracnose when treated with hot water compared to carbendazim 100 ppm lead when treated with hot water [50].
[51] concluded that postharvest Use of thyme essential oil significantly increases the activity of antioxidant and defense-inducing enzymes, including chitinase, 1,3-β-glucanase, phenylalanine ammonia-lyase, and peroxidase. It also maintains high phenol levels in fruit tissues, increases resistance against invasion, and reduces anthracnose spoilage. Cinnamon oil is another essential oil derived from Cinnamomum zeylanicum, with cinnamaldehyde as the main active ingredient [23] (Table 3).
The current appraisal focuses on applying EO obtained from several plant species as bio-fungicides as an alternative to postharvest disease control to modern synthetic chemical fungicides to target pathogenic fungi for anthracnose control [18].
EO is a complex combination of naturally occurring compounds that are volatile and have a strong odour. EO is a liquid, transparent, pale colour, soluble in lipids, soluble in organic solvents less dense than water, and produced as a secondary metabolite [52].
Thymol has shown high in vitro activity against sporulation and mycelium growth of C. gloeosporioides. Recent developments in avocado and mango have demonstrated the effectiveness of thyme and thymol oils in combating postharvest diseases by stimulating biochemical and metabolic pathways involved in natural defense [52]. Treatment with a thymol solution calibrated to 0.025% provided excellent disease control without significantly degrading fruit quality. To fully combat anthracnose, more research is needed using the synergistic effects of several essential oils, including thymol, and optimization based on mango varieties should be considered [52].
The incidence of mango fruit increased over time, with almost all treated mangoes showing symptoms after 17 days of storage and all control plants showing signs after six days. Essential oils (cinnamon and ginger) with different concentrations delayed the occurrence of anthracnose disease and maintained the freshness of the fruits during the first two weeks of storage, and later on, showed few symptoms. Fruit treated with essential oils kept better and had less spoilage, while untreated fruit showed more spoilage [53].
C. gloeosporioides attacks mango fruits at all stages of development. A quiescent infection and symptoms arise when the fruit ripens after harvest. Studies have shown that the tested concentrations of essential oils did not affect some of the quality parameters of the mango fruit for five days but had a significant effect after storage for the next few days. The incidence and severity of the disease were effectively controlled with 0.45% ginger essential oil and 0.075% cinnamon oil. Maximum disease incidence (100%) and severity (65.56%) were recorded for control treatment within 25 days of storage. The minimum incidence (38.1%) and severity (26.67%) of treatment with ginger (0.45%) and cinnamon (0.075%) were recorded 25 days after treatment. The disease's frequency and severity directly impacted several quality parameters such as weight loss, total Soluble solids, titrated acidity, pH value, hardness, and the ratio of total dissolved solids to titrated acidity during the study period [53].
Chemical control
The combination of SA, CaCl2, and M. pulcherrima yeast has the potential for utilization as a safe and effective postharvest tool to control anthracnose while also maintaining and extending the postharvest life of mangoes [54]. Also, Azoxystrobin is a systemic fungicide useful in reducing anthracnose incidence and yield increase; the optimum rate of azoxystrobin is fixed to be at 2.0 ml/l for the control of anthracnose disease. Also, the Use of organic sulphur (Dithiocarbamate) fungicides like zineb, maneb, and heterocyclic nitrogen compounds-captan gave adequate control against anthracnose. However, these fungicides have shown a phytotoxic effect on flowers. Moreover, C. gloeosporioides developed resistance to benomyl (0.1%) a benzimidazoles systemic fungicides to control anthracnose pre and postharvest development [55]. The disease incidence was reduced greater when azoxystrobin was sprayed at 1, 2, and 4 ml/l. Treating trees with these concentrations provided 100 and more than 60% reduction of panicle and leaf anthracnose compared to untreated trees for which disease incidences were 27.73 and 53.68 PDI, respectively [55]. Nativo control anthracnose up to 92.03%, Cabrio top which showed 89.08% control of anthracnose, Topsin-M Score and Shincar were equally effective, offering 76.51-77.17% control of anthracnose and Under field conditions [55]. It has been recommended Boost 500 SC (acibenzolar-S-methyl), which showed excellent activity in reducing the severity of anthracnose on leaves, panicles, and flowers [56]. Bendazim, Funguran, a copper-based fungicide; Copper compounds are not easily washed from leaves by rain since they are relatively insoluble in water [57]. Dimethyl trisulfide (DMTS), a novel antifungal compound, affects the infection process of C. gloeosporioides by suppressing conidial germination and appressorium formation in plants and damaging cytoplasm to cause cells to become vacuolated [58]. Similarly, anthracnose control includes fungicides, biocides, calcium carbonate, chemical inducers, microorganisms, hyperbaric pressure treatments, antioxidants, radiation, heat shock and, at the experimental stage-genetic manipulation [59]. Foliar application of mixed formulation of carbendazim 12% and mancozeb 63% fungicide (SAAF-75 WP) at the recommended dose (90 + 472.5) and double the recommended dose (180 + 945 g a.i.ha-1) also is safe for consumption of mango fruits [60]. Lipoxygenase-related volatiles plays a role in the accompanying wounding of the cell, leading to the production of fatty acids, hydroperoxides, or chemical degradation into C6 and C9-aldehydes, which can be harmful to invading microorganisms [61]. Fungicides such as Cupravit, Bavistin, Dithane M-45, Thiovit, and Redomil were tested against conidial germination of C. gloeosporioides. Dithane M-45 and Redomil were the most effective when the conidia were soaked for 10 ~ 20 minutes at 500 ~ 1000 ppm concentrations [62].
Spraying fungicide before fruit bagging is commonly practiced for mango anthracnose control in Thailand [63] reported that 47 to 60% of fruits treated with azoxystrobin showed no disease development. Prochloraz has been used as a preventative measure or an eradicant spray to reduce inoculum levels in the field and inhibit latent infection [64]. Our results confirmed that sprays of propineb, azoxystrobin, difenoconazole, carbendazim or prochloraz on immature fruit before fruit bagging significantly reduced subsequent anthracnose incidence of ripe fruit. However, prochloraz was ineffective in reducing disease incidence when applied to artificially inoculated fruit [44].
The study shows the preharvest application of salicylic acid (1000 mg L1) or potassium phosphonate (500 or 1000 mg L1), combined with postharvest dipping of mango fruit in 3 percent sodium bicarbonate in hot water (51.5 C), suppresses C. gloeosporioides and improves mango fruit marketability for about 12 days without affecting fruit quality [65].
Fungicides, either as preharvest or postharvest treatments, form the primary approach to reduce losses from anthracnose. However, their Use is increasingly restricted due to public concerns over toxic residues. Moreover, fungicides are unaffordable for many mango growers in developing countries [48]. Exposure to ethanol or acetic acid vapours-controlled mycelium growth and conidia development during storage for seven days at 28 °C to 32 °C, but ethanol was more effective than vinegar and ethanol alone than vinegar [66].
Based on the investigation, postharvest treatment of 'Alphonso' mango with Biosafe at 4 mL L-1 followed by fruit storage at 13 ± 1 °C is the most effective treatment for reducing postharvest rot caused by C. gloeosporioides [67] (Table 4).
Discussion and Conclusion
Anthracnose disease of mango is a significant disease that constrains mango exportation causing 17.7% of mangoes spoilage in transit, storage, and marketing [63].
For successful control of this disease, integration of pathogen biology and preharvest and postharvest management is needed. Usually, fungicides are the primary means of controlling plant diseases. The increasing concern for health hazards, increased consumer preference for healthy agricultural products, and environmental pollution associated with chemical residues in food are the major driving forces for developing alternative strategies to control postharvest diseases of fruits and vegetables. Researches on the Management of postharvest diseases like anthracnose by employing microbial agents, resistant cultivars, biological controls, hot water treatment with waxing, Oxalic acid treatment, Use of essential oils and botanicals have been demonstrated to be the most suitable strategy to replace the chemicals which are either being banned or recommended for limited Use. Also, modifications to traditionally approved cultural practices suiting a particular agro-climatic region will prove helpful in better management of the disease. Though the disease's epidemic character has long been researched, many aspects of the host-pathogen relationship, its transmission, and effective control techniques remain unknown. There is an urgent need for the development of an effective integrated management strategy that considers the many environmental factors and pathogenic resistance that contribute to the pathogen's successful colonization of host tissues.
References
- Yadav D, Singh SP (2017) Mango: History origin and distribution. J Pharmacogn Phytochem 6: 1257-1262.
- Litz RE (2009) Mango. In: Compendium of transgenic crop plants. American Cancer Society 163-174.
- Uddin MN, Shefat SHT, Afroz M, et al. (2018) Management of anthracnose disease of mango caused by Colletotrichum gloeosporioides: A review. Acta Sci Agric 2: 169-177.
- Lauricella M, Emanuele S, Calvaruso G, et al. (2017) Multifaceted health benefits of Mangifera indica L. (Mango): The inestimable value of orchards recently planted in Sicilian rural areas. Nutrients 9: 525.
- Muid S, Ploetz RC, Cooke AW (2003) In: Diseases of tropical fruit crops. CABI Publishing 145-162.
- Prakash O (2004) Diseases and disorders of mango and their management. In: Naqvi SAMH, Diseases of fruits and vegetables diagnosis and management, Kluwer Academic, Springer Netherlands 1: 511-619.
- Ploetz RC (2002) The major diseases of mango: Strategies and potential for sustainable management. Acta Hortic 645: 137-150.
- Bhagwat RG, Mehta BP, Patil VA, et al. (2015) Screening of cultivars/varieties against mango anthracnose caused by Colletotrichum gloeosporioides. Int J Agric Environ Res 1: 21-23.
- Kamle M, Kumar P (2016) Colletotrichum gloeosporioides: Pathogen of anthracnose disease in mango (Mangifera indica L.). In: Kumar P, Gupta VK, Tiwari AK, Current Trends in Plant Disease Diagnostics and Management Practices. Springer International Publishing 207-219.
- Damm U, Cannon PF, Woudenberg JHC, et al. (2012) The Colletotrichum acutatum species complex. Stud Mycol 73: 37-113.
- Jayasinghe CK, Fernando THPS (2009) First report of Colletotrichum acutatum on Mangifera indica in Sri Lanka. Cey J Sci (Biol Sci) 38: 31-34.
- Phoulivong S, Cai L, Chen H, et al. (2010) Colletotrichum gloeosporioides is not a common pathogen on tropical fruits. Fungal Divers 44: 33-43.
- Lakshmi BKM, Anitha Kumari D, Kumar AK, et al. (2013) Mitigating postharvest losses caused by anthracnose disease in mango by using bio agents, botanicals and ISR chemicals. Acta Hortic 1012: 661-670.
- Sharma IM, Harender R, Kaul JL (1994) Studies on post-harvest diseases of mango and chemical control of stem end rot and anthracnose. Indian Phytopathol 47: 197-200.
- Colón-Garay J, Rivera-Vargas LI, McGovern R, et al. (2002) Hypovirulent isolates of Colletotrichum gloeosporioides induce resistance to anthracnose in detached mango fruits and seedlings. J Agric Univ P R 86: 55-64.
- Tucho A, Lemessa F, Berecha G (2014) Distribution and occurrence of mango anthracnose (colletotrichum gloesporioides penz and sacc) in humid agro-ecology of southwest Ethiopia. Plant Pathol J 13: 268-277.
- Alahakoon PW, Brown AE (2008) Host range of Colletotrichum gloeosporioides on tropical fruit crops in Sri Lanka. Int J Pest Manag 40: 23-26.
- Kumar A, Kudachikar VB (2017) Antifungal properties of essential oils against anthracnose disease: A critical appraisal. J Plant Dis Prot 125: 133-144.
- Abera A, Lemessa F, Adunga G (2019) Prevalence and intensity of mango (Mangifera indica L.) anthracnose caused by Colletotrichum species in south-western of Ethiopia.
- Misra AK (2013) Mango diseases and their management. 188-199.
- CABI (2014) Anthracnose on Mango. Plantwise Knowledge Bank.
- Martínez EP, Hío JC, Osorio JA, et al. (2009) Identification of Colletotrichum species causing anthracnose on Tahiti lime, tree tomato and mango. Agron Colomb 27: 211-218.
- Siddiqui Y, Ali A (2014) Chapter 11-Colletotrichum gloeosporioides (Anthracnose). In: Bautista-Baños S, Postharvest Decay, Academic Press 337-371.
- Arauz LF (2000) Mango Anthracnose: Economic impact and current options for integrated management. Plant Dis 84: 600-611.
- Nelson SC (2008) Mango anthracnose (Colletotrichum gloeosporioides). Plant Dis 48: 1-9.
- Alcasid C, Valencia L, Dimasingkil SF (2016) Evaluation of plant extracts against anthracnose of mango (Mangifera indica L.) caused by Colletotrichum gloeosporioides Penz.
- Jeger MJ, Plumbley RA (1988) Post-harvest losses caused by anthracnose (Colletotrichum gloeosporioides) of tropical fruits and vegetables. In: Houghton DR, Smith RN, Eggins HOW, Springer Netherlands, Biodeterioration 7: 642-646.
- Fitzell RD, Peak CM (1984) The epidemiology of anthracnose disease of mango: Inoculum sources, spore production and dispersal. Ann Appl Biol 104: 53-59.
- Abid MK (02:21:57 UTC) Anthracnose of mango.
- Bally ISE (2006) Mangifera indica (mango). Species Profiles for Pacific Island Agroforestry 1-25.
- Akem CN (2006) Mango anthracnose disease: Present status and future research priorities. Plant Pathol J 5: 266-273.
- Sharma M, Kulshrestha S (2015) Colletotrichum gloeosporioides: An anthracnose causing pathogen of fruits and vegetables. Biosci Biotechnol Res Asia 12: 1233-1246.
- Rathod GM (2011) Effect of physical factors on development of anthracnose of mango fruits. Current Botany 2: 15-16.
- Jenny F, Sultana N, Islam M, et al. (2019) A review on anthracnose of mango caused by Colletotrichum gloeosporioides. Bangladesh J Plant Pathol 35: 65-74.
- Xie L, Zhang J, Wan Y, et al. (2010) Identification of Colletotrichum spp. Isolated from strawberry in Zhejiang Province and Shanghai City, China. J Zhejiang Univ Sci B 11: 61-70.
- Nishijima W (1993) Mango diseases and their control.
- Chowdhury MNA, Rahim MA (2009) Integrated crop management to control anthracnose (Colletotrichum gloeosporioides) of mango. J Agric Rural Dev 7: 115-120.
- Flor HH (1971) Current status of the gene-for-gene concept. Annu Rev Phytopathol 9: 275-296.
- Saxena A, Raghuwanshi R, Gupta VK, et al. (2016) Chilli anthracnose: The epidemiology and management. Front Microbiol 7: 1527.
- Bompard (1993) CAB Direct.
- Jacobi KK, MacRae EA, Hetherington SE (2001) Postharvest heat disinfestation treatments of mango fruit. Sci Hortic 89: 171-193.
- Dodd JC, Prusky D, Jeffries P (1997) Fruit diseases. The mango: Botany, production and uses 257-280.
- Tripathi P, Dubey NK (2004) Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biol Technol 32: 235-245.
- Chiangsin R, Wanichkul K, Guest DI, et al. (2016) Reduction of anthracnose on ripened mango fruits by chemicals, fruit bagging, and postharvest treatments. Australas Plant Pathol 45: 629-635.
- Sangchote S (2012) Integrated control of anthracnose (Colletotrichum gloeosporioides) of mango for export. Acta Hortic 973: 55-58.
- Taibi A, Meile JC, Dieudonné H, et al. (2020) New bacterial agents to limit Colletotrichum gloeosporioides development on Mango. Adv Microbiol 10: 691-712.
- Koomen I, Jeffries P (1993) Effects of antagonistic microorganisms on the post-harvest development of Colletotrichum gloeosporioides on mango. Plant Pathol 42: 230-237.
- Kefialew Y, Ayalew A (2008) Postharvest biological control of anthracnose (Colletotrichum gloeosporioides) on mango (Mangifera indica). Postharvest Biol Technol 50: 8-11.
- Zheng XL, Tian SP, Gidley MJ, et al. (2007) Slowing the deterioration of mango fruit during cold storage by pre-storage application of oxalic acid. J Hortic Sci Biotechnol 82: 707-714.
- Duamkhanmanee R (2008) Natural essential oils from lemon grass (Cymbopogon citratus) to control postharvest anthracnose of mango fruit. Int J Biotechnology 10: 104-108.
- Sellamuthu PS, Mafune M, Sivakumar D, et al. (2013) Thyme oil vapour and modified atmosphere packaging reduce anthracnose incidence and maintain fruit quality in avocado. J Sci Food Agric 93: 3024-3031.
- Chillet M, Minier J, Hoarau M, et al. (2020) Optimisation of the postharvest treatment with thymol to control mango anthracnose. Am J Plant Sci 11: 1235-1246.
- Sefu G, Satheesh N, Berecha G (2015) Effect of essential oils treatment on anthracnose (Colletotrichum gloeosporioides) disease development, quality and shelf life of mango fruits (Mangifera indica L). Am Eurasian J Agric Environ Sci 15: 2160-2169.
- Shao Y, Zeng J, Tang H, et al. (2019) The chemical treatments combined with antagonistic yeast control anthracnose and maintain the quality of postharvest mango fruit. J Integr Agric 18: 1159-1169.
- Sundravadana S, Alice D, Kuttalam S, et al. (2007) Efficacy of azoxystrobin on Colletotrichum gloeosporiodes penz growth and on controlling mango anthracnose. J Agric Biol Sci 2: 10-15.
- Nasir M, Iqbal B, Idrees M, et al. (2017) Efficacy of some organic fungicides against anthracnose and powdery mildew of mango. Pak J Agric Sci 54: 493-496.
- Honger JO, Offei SK, Oduro KA, et al. (2015) Chemical control of mango anthracnose disease in Ghana. Ghana Jnl Agric Sci 49: 15-28.
- Tang L, Mo J, Guo T, et al. (2019) Antifungal effects of dimethyl trisulfide against Colletotrichum gloeosporioides infection on mango. Journal of Phytopathology 167: 445-450.
- Jairo AO, Martínez EP, Hío JC (2012) Screening of microbial culture filtrates, plant extracts and fungicides for control of mango anthracnose. Agron colomb 30: 222-229.
- Devi PA, Paramasivam M, Prakasam V (2015) Degradation pattern and risk assessment of carbendazim and mancozeb in mango fruits. Environ Monit Assess 187: 4142.
- Anusha B, Sathya K, Parthasarathy S, et al. (2016) Effect of hexanal on mycelial growth and spore germination of Colletotrichum gloeosporioides and Lasiodiplodia theobromae of mango. Trop Agric 93: 312-322.
- Imtiaj A, Rahman SA, Alam S, et al. (2005) Effect of fungicides and plant extracts on the conidial germination of Colletotrichum gloeosporioides causing mango anthracnose. Mycobiology 33: 200-205.
- Sharma RR, Singh D, Singh R (2009) Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biol Control 50: 205-221.
- Estrada AB, Jeffries P, Dodd JC (1996) Field evaluation of a predictive model to control anthracnose disease of mango in the Philippines. Plant Pathol 45: 294-301.
- Dessalegn Y, Ayalew A, Woldetsadik K (2013) Integrating plant defense inducing chemical, inorganic salt and hot water treatments for the management of postharvest mango anthracnose. Postharvest Biol Technol 85: 83-88.
- Thompson A, Krusong W, Suwapanich R (2019) Postharvest control of anthracnose in mangoes by fumigation with vinegar and ethanol vapours. Int J Postharvest Technol Innov 6: 179-191.
- Pujari KH, Joshi MS, Shedge MS (2014) Management of postharvest fruit rot of mango caused by Colletotrichum gloeosporiodes. Acta Hortic 120: 215-218.
Corresponding Author
Asmita Paudel, Faculty of Agriculture, Agriculture and Forestry University, Rampur, Chitwan, Nepal
Copyright
© 2022 Paudel A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.