Farmer-Cultivated Landraces and Improved Cassava Genotypes Exhibit Varied Response to Cassava Brown Streak Disease Under Natural Infections in Kenya
Abstract
Cassava brown streak disease (CBSD) affects cassava growth and production with resultant foliar symptoms and storage root necrosis causing significant economic losses to farmers. The disease is caused by either Cassava brown streak virus (CBSV) or Uganda cassava brown streak virus (UCBSV), both vectored by whitefly, Bemisia tabaci. Cultivation of CBSD-free or resistant cassava genotypes offers the most long term sustainable CBSD management. Through a field survey, the present study assessed variations for CBSD infections amongst local cassava landraces (LCLs) and improved genotypes (IPGs) cultivated by farmers in Kenya. Foliar symptoms were scored through CBSD severity (CBSD-S) and CBSD incidence (CBSD-I) and relative CBSV and UCBSV titres measured through quantitative RT-PCR with COX as reference gene. Overall results showed varied mean CBSD-I of 12-52% and CBSD-S of 2.0-3.8 between surveyed regions while CBSD-I of 0-100% and CBSD-S of 1.0-4.6 (asymptomatic - severe) between cassava germplasm. The symptoms varied significantly (P ≤ 0.001) enabling identification of CBSD tolerant germplasms (CTGs). RT-PCR revealed 35% no infection, 49% single infections and 16% dual infections. CBSV infected more germplasm (64%) and exhibited higher viral titres compared to UCBSV at 16%. Correlation between CBSD-S and CBSV titre was positive (r = 0.78) and significant (P ≤ 0.004) implicating CBSV with CBSD symptom expression. Out of 112 assessed germplasm, 56% showed low CBSD-S scores and low viral titre thus categorized as CTGs. About 71% of IPGs were CTGs compared to 47% of LCLs. This implied that 29% IPGs and 53% LCLs sampled in this study were CBSD-infected. This indicated potential breakdown of CBSD resistance overtime especially in IPGs, hence could hamper CBSD management. However, the CBSD-free IPGs and LCLs identified in this study could also be bulked for distribution to cassava farmers as clean planting materials for improved cassava production. They could also be potential genetic resources for introgression of virus-resistance traits into farmer-preferred cultivars.
Keywords
CBSD, Improved genotypes, Local landraces, Severity, Virus titre
Introduction
Cassava (Manihot esculenta Crantz) is a perennial woody shrub in the Euphorbiaceae family native to South America but now grown in tropical and subtropical areas worldwide for its edible starchy tuberous roots [1,2]. Majority of cassava cultivation occurs in Africa, where it is gaining popularity due to its low water requirement, survivability in marginal soils, yield under adverse conditions and flexibility in harvest time [3,4]. Due to its inherent tolerance to drought stress [5], the crop has become an important staple for food-vulnerable populations in sub-Saharan Africa (SSA) [6,7]. Despite this significance, cassava's strategic importance as food security crop in SSA [8] has been rendered vulnerable by major viral diseases such as cassava brown streak disease (CBSD) which persistently reduces overall storage root quality and quantity resulting in up to 100% yield losses [9]. CBSD has caused approximately US$750 million annual losses in Kenya, Tanzania, Uganda and Malawi [10]. CBSD is caused by two species of single-stranded RNA viruses (+ssRNA), Cassava brown streak virus (CBSV) and Uganda cassava brown streak virus (UCBSV), belonging to genus Ipomovirus and family Potyviridae [11-14]. CBSV and UCBSV are collectively referred as cassava brown streak viruses (CBSVs). CBSVs are transmitted semi-persistently by whiteflies, Bemisia tabaci, Gennadius [15] and the disease spread through propagation of infected cassava cuttings or planting materials in the field [14,16]. Although endemic at the coastal region of East Africa [17], CBSD outbreaks have been reported in higher altitudes of East and Central Africa [18,19] due to super-proliferation of whitefly vectors and associated changing environmental and climatic conditions [20,21].
Foliar CBSD symptoms include feathery chlorosis along the veins and chlorotic mottling of old leaves, brown elongate necrotic lesions on young green stems with die-back from the shoot tip and brown, corky, necrotic tuberous storage roots with characteristic constrictions [20,22]. The affected roots are unusable as source of food and valueless in the marketplace [20] thus threatening the food and economic security of East and Central Africa [23]. Thus, the impact of CBSD has important implications for smallholder cassava farmers and rural communities [20]. Restriction (quarantine) of movement of planting materials from regions with reported CBSD incidences, control of whiteflies and breeding or development and deployment of CBSD-tolerant/resistant cassava germplasm are some mitigation measures that have been recommended to reduce CBSD-associated yield losses in farmer fields. To contribute towards management of CBSD, the present diagnostic study assessed variations in CBSD infections in local cassava landraces (LCLs) and improved cassava genotypes (IPGs) sampled from farmer fields in Kenya. The objective was to compare response to CBSD between IPGs and LCLs under natural infection by CBSVs. This could aid in identification and selection of CBSD-free or resistant germplasm for bulking and production of clean planting materials and for CBSD resistance breeding purposes.
Materials and Methods
Field survey
Through a field survey carried out in April 2018, a total of 112 cassava germplasm (Table 1) were assessed for CBSD severity (CBSD-S) and CBSD incidence (CBSD-I) in five major cassava growing areas of Kenya (Figure 1). The multi-stage field survey design and systematic identification of farms for sample collection was adopted from Koima, et al. [24] and Mware, et al. [15]. This involved selection of cassava plants more than three months old at regular intervals of 2 to 5 km along motorable roads traversing each sampling location. In each farm, 30 plants were sampled at regular intervals along the diagonals of an X-shaped transect (IITA, 2009; 27]. Farmers providing the names and age of the cassava variety or genotype on their farms (Table 1). CBSD-S was visually scored on a scale of 1-5 as described by Abaca, et al. [25] where 1 = no apparent symptoms; 2 = slight foliar chlorotic leaf mottle, no stem lesions; 3 = foliar chlorotic mottle and blotches with mild stem lesions, no die-back; 4 = foliar chlorotic mottle and blotches and pronounced stem lesions with no die-back severe and 5 = defoliation with stem lesions and pronounced die-back. CBSD-I was determined as the proportion number of CBSD symptomatic plants relative to total number of number of plants assessed.
Virus diagnostics and quantification
Out of the 30 pre-selected plants per cassava germplasm, five plants per germplasm (biological replicates) were randomly sampled for CBSV and UCBSV diagnostics and quantification. Both fourth and fifth fully expanded leaf from the top of each plant [26] was harvested and pooled into one sample. Total RNA was extracted from three biological replicates per germplasm using CTAB-based protocol as described by Orek [65]. Quantity, quality and integrity of each RNA sample was measured using NanoDrop® ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and 1.5% agarose gel electrophoresis (AGE). All RNA isolates were digested by RNAse-free DNase I to eliminate genomic cassava DNA contamination according manufacturer's recommendations (Thermo Fisher Scientific, Waltham, MA, USA). DNase-treated RNA were then subjected to cDNA synthesis using AccuPower® RocketScript™ Cycle RT First strand cDNA Synthesis kit following manufacturer (Bioneer, Daejeon, South Korea) instructions. Due to differences in RNA quantity, the samples were normalized to a working concentration of 100 ng/µl by addition of an appropriate amount of sterile water [27]. Duplex reverse transcriptase polymerase chain reaction (RT-PCR) was performed on the synthesized complementary DNA (cDNAs) for simultaneous detection of both CBSV and UCBSV in a single reaction. Primers CBSVF2 (5'-GGRCCATACATYAARTGGTT-3'), CBSVR7 (5'-CCCTTTGCAAARCTRAAATARC-3') and CBSVR7 (5'-CCATTRTCTYTCCAMADCTTC-3') were used to amplify approximately 441 and 345 bp of UCBSV and CBSV respectively [28]. RT-PCR reaction and cycling conditions were adopted and modified from Maruthi, et al. [16]. RT-PCR products were then resolved by 1.5% agarose gel electrophoresis (AGE) and visualized using the Gel Doc XR+ imaging and documentation system (Bio-Rad, New York, USA).
Using 7900 Fast RT PCR System (Applied Biosystems®, Foster City, CA), real-time quantitative PCR (RT-qPCR) was performed on the synthesized cDNA samples to determine the virus loads. The RT-qPCR was based on TaqMan chemistry with primers and probe sequences adopted from Adams, et al. [29]. CBSV probe was 5'-FAM-TAMRA-3' labeled, UCBSV probe 5'-VIC-TAMRA-3' labeled and cytochrome oxidase gene (COX) used as an internal control with primers COX-F (5'- CGTCGCATTCCAGATTATCCA-3'), COX-R (5'- CAACTACGGATATATAAGRRCCRRAACTG-3') and probe (5'- [FAM]-AGGGCATTCCATCCAGCGTAAGCA-[TAMRA]-3') [29]. Mitochondrial COX is a widely used reference or endogenous gene to normalize cycle threshold (Ct) values validated by Adams, et al. [29] and has been widely used for CBSV and UCBSV quantification [27,30,31]. The reaction composed of three cDNA samples (biological replicates) for each cassava germplasm and three technical replicates per biological replicate. The 20 µl reaction volume consisted of 10 µl DreamTaq PCR master mix (2X) (Thermo Fisher Scientific, Waltham, MA, USA), 1 µl each forward and reverse primer (7.5 µM), 0.5 µl Taqman probe (5 µM), 2 µl cDNA template and 5.5 µl nuclease-free sterile H2O. For every assay, non-template H2O control (no cDNA) was included to check for reagent contamination. The reactions were incubated at 95℃ for 10 min for initial denaturation, followed by 40 cycles at 95℃ for 15 sec, annealing at 60℃ for 1 min and extension at 60℃ for 1 min (Ct collected).
Data Analysis
Descriptive statistics was applied in analysis of CBSD-S and CBSD-I using SPSS software version 22.0 with group means separated based on standard deviations to determine significant differences amongst germplasm. For RT-PCR, presence (+) or absence (-) of CBSV (345 bp) and UCBSV (441 bp) was based on the detectability of the expected RT-PCR band sizes as visualized underage. For RT-qPCR, Ct values collected at 60℃ for 1 min, were generated on default settings of the 7900 Fast System SDS software and then used to compute CBSV and UCBSV fold change (virus loads or titres) in a cassava germplasm relative to COX reference gene using the comparative 2-∆∆CT method [32]. All cassava germplasm that had Ct values of 40 for UCBSV or CBSV were considered to be free of these viruses [27]. Variations for mean CBSD-S and viral fold change (VFC) or load were used to classify each germplasm as either CBSD tolerant germplasm (CTGs) or CBSD susceptible germplasm (CSGs). CTGs had mean foliar CBSD-S scores of 0.0-2.4 (no apparent symptoms to slight foliar chlorotic leaf mottle) with low VFC (0.0-1.4), while CSGs had foliar CBSD-S scores of 2.5-5.0 (foliar chlorotic mottle to defoliation with stem lesions and pronounced dieback) and high VFC (> 1.5). Pearson correlation coefficient (r) was used to determine relationships between virus load or VFC, CBSD-S and CBSD-I.
Results
CBSD incidence and severity
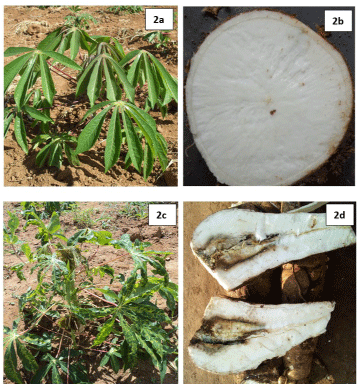
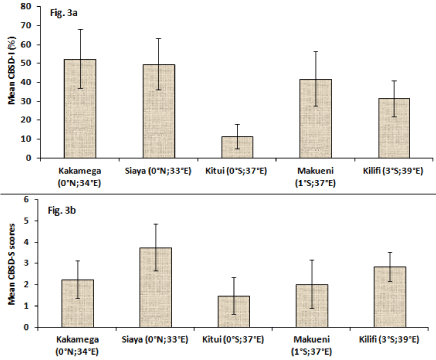
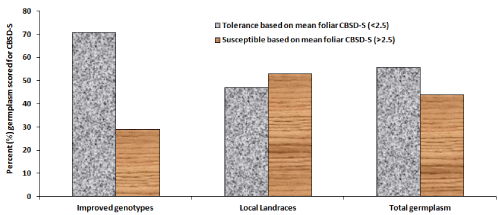
Compared to CBSD-free plants (Figure 2a and Figure 2b), typical CBSD symptoms were observed on infected cassava plants including leaf chlorosis that appeared yellowish and mottled (Figure 2c) and as well yellowish to dark-brown necrotic storage roots (Figure 2d). An overall mean CBSD-I of 36.5% and mean CBSD-S of 2.3 was computed for all germplasm. Mean CBSD-I and CBSD-S varied significantly (P ≤ 0.001) between surveyed regions and cassava germplasm (Table 2), (Figure 3). For example, Kitui area (0°S;37°E) had significantly lower CBSD-I (~12%) and relatively lower CBSD-S (~1.5) compared to the other four sampled regions that showed between 32-52% CBSD-I and CBSD-S of 2.0-3.8 (Figure 3a and Figure 3b). Siaya (0°N; 33°E) generally showed higher incidence and severity. Wider variations for the two foliar parameters were observed amongst germplasm. For instance, mean CBSD-I varied between 0-100% and mean CBSD-S scores ranged from 1.0-4.6 (Table 3). Amongst germplasm with mean CBSD-S scores of 0-2.4 (or < 2.5) majority (71%) were improved genotypes (IPGs) compared to local cassava landraces (LCLs) at 47% (Figure 4). These were visually classified as CBSD tolerant germplasm (CTGs). Alternatively, the 29% IPGs and 53% LCLs that showed mean CBSD-S scores of 2.5-5.0 (or > 2.5) (Figure 4) were categorized as CBSD susceptible germplasm (CSGs). When combined, out of 112 sampled germplasm, 56% visually constituted CTGs compared to 44% that were CSGs (Figure 4).
RT-PCR diagnosis of CBSV and UCBSV
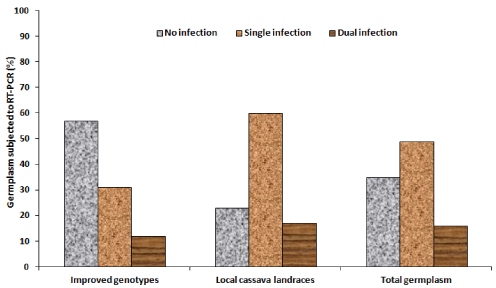
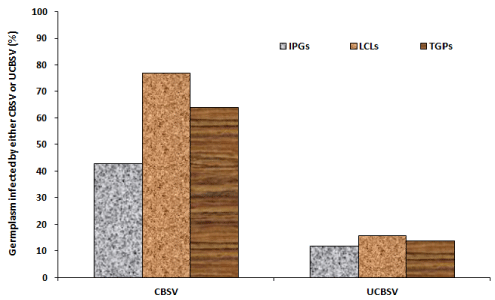
Detection of CBSV and UCBSV through the duplex RT-PCR revealed no infection, single infections (CBSV or UCBSV) and dual infections (CBSV + UCBSV) in different cassava germplasm (Table 4). The different levels of infections were shown by the 441 bp and 345 bp amplicons sizes of UCBSV and CBSV respectively (Figure 5). Generally, out of 112 tested germplasm, 35% were non-infected, 49% had single infection and 16% had dual infection (Figure 6a). For specific germplasm, majority (57%) of IPGs compared to 23% LCLs were non-infected, most (60%) of LCLs had single infections compared to 31% IPGs and dual infections were respectively analyzed in 12 and 17% of IPGs and LCLs (Figure 6a). For specific viruses, generally CBSV and UCBSV were respectively detected in 64 and 14% of combined cassava germplasm (Figure 6b). For different germplasm, CBSV was respectively detected in 43 and 77% of IPGs and LCLs compared to UCBSV that infected in 12% IPGs and 16% LCLs (Figure 6b).
RT-qPCR quantification of CBSV and UCBSV
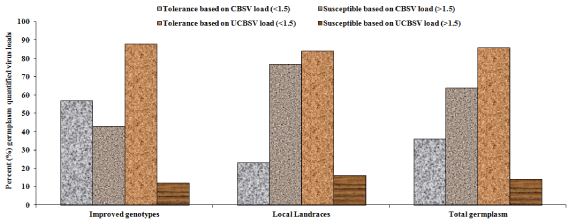
Through amplification plots (data not shown), Ct values for CBSV in most germplasm were detected earlier compared to Ct values of UCBSV. RT-qPCR revealed differences in viral loads or titres between CBSV and UCBSV amongst germplasms. For example, CBSV titres widely varied between 0-39.3 compared to UCBSV loads of 0-4.2 (Table 5). Majority of IPGs (20) with zero (0) viral tires for both viruses were sampled from Kitui (Thika273, Kiboko275, Kiboko300, Thika279, Kiboko276, Kiboko11 among others) compared to three IPGs in Kakamega (MM98/1313-HS, MM08/2206 & MM96/0686) and one in Siaya (Lady Gay) (Table 5). The remaining IPGs in other areas had more than 3.0 viral load especially for CBSV. Among LCLs with with zero (0) viral tires for both viruses included six in Kakamega (Mukulusu, Inzakula, Lugusisti, Isambe, Shamiloli & Magana) and six in Makueni (Kasioni, Kisimba, Kitwa-a, Kaliluni, Muvila & Kalimbini-a) (Table 5). LCLs from other regions had high viral loads especially for CBSV. More LCLs had higher CBSV loads (> 10) compared to IPGs. Based on viral load less than 1.5, more IPGs (57%) were classified as resistant to CBSV compared to LCLs (23%) and relatively equal IPGs (88%) and LCLs (84%) were resistant to UCBSV (Figure 7). For viral load more than 1.5, more LCLs (77%) were susceptible to CBSV compared to IPGs (43%) with relatively similar trends (16 and 12%) respectively observed for UCBSV (Figure 7). In summary, more combined cassava germplasm were resistant to UCBSV (86%) compared to CBSV (36%) or more combined germplasm were susceptible to CBSV (64%) compared to UCBSV (16%) (Figure 7).
Correlations between virus load and CBSD symptom
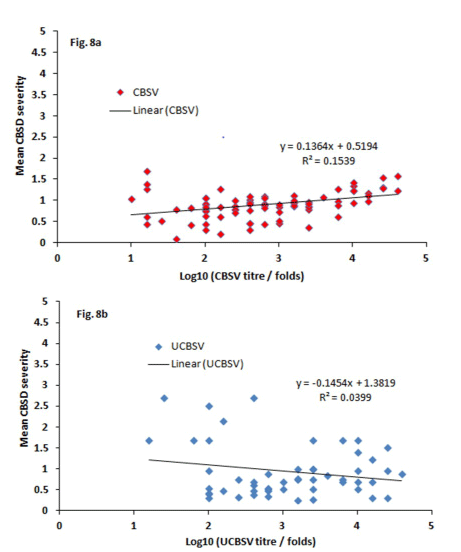
All the 2-tailed correlation coefficients (r) between CBSD symptoms and virus load were strong positive and significant (Table 6), (Figure 8). For example, r = 0.78 and P ≤ 0.004 for CBSD severity with CBSV load (Table 6a) and r = 0.51, P ≤ 0.006 for CBSD incidence and CBSV load (Table 6b). Weak and positive correlations were observed between UCBSV loads, severity and incidence. For example, r = 0.25, P ≤ 0.008 for CBSD severity and UCBSV load (Table 6c) and r = 0.24, P ≤ 0.01 for CBSD incidence and UCBSV load (Table 6d). Correlations between CBSV load and UCBSV load was also positive and significant (r = 0.43, P ≤ 0.03) (Table 6e). Correlations of determination (r2) was also calculated and graphed between log10 of the virus titre fold change and mean CBSD severity (Figure 8). The correlations results showed weak association with r2 = 0.15 for severity with CBSV titre (Figure 8a) and r2 = 0.04 for severity and UCBSV titre (Figure 8b).
Discussion
The present survey identified asymptomatic and symptomatic cassava plants with the latter exhibiting typical CBSD symptoms such as leaf chlorosis that appeared yellowish and mottled and yellowish to dark-brown constricted and necrotic storage roots. Previous studies have characterized similar CBSD symptoms in cassava [9,20,27,33,34]. The necrotic lesions on the storage roots render them inedible and unmarketable resulting in economic losses for farmers. The significant variations for foliar CBSD severity and incidence between surveyed regions and amongst cassava germplasm reported in this study could have three implications. First it can enable identification of CBSD hot spots across the country that can be used establish resistance breeding programs. In contrast, such hot spots could also be recommended for quarantine measures to restrict the spread of CBSD. Secondly, the wider genotypic variations for CBSD can aid in selection of CBSD-free planting materials or parental sources for CBSD resistance breeding. Lastly, regions with low or no CBSD could be used to establish and bulk CBSD-free seeds or cuttings for distribution to farmers in affected areas. Planting CBSD-free cuttings is a mitigation measure that reduces the effects of CBSD on cassava yield [35-37]. The present study identified Siaya (0°N; 33°E) and Kakamega (0°N; 34°E) in western Kenya as CBSD hot spots and Kitui (0°S; 37°E) in eastern as an area with low CBSD infections. Further, the study identified about 56% CBSD resistant and 44% CBSD susceptible cassava germplasms. These regional and cassava germplasm variations for CBSD severity and incidence were corroborated by relatively similar reports of related studies in Kenya [15,24,38,39]. The reasons that have been attributed to these variations include occurrence, distribution, and abundance of the whitefly vector (Bemisia tabaci) [40,41], virus species (CBSV or UCBSV) causing the symptoms [42,43], differential cultivar sensitivity to CBSD (15,38], age or growth stage of the plant [9,44,45], dissemination of infected planting materials [19,40] and varied growing or weather conditions [42,46].
Molecular detection and quantification of CBSVs can identify virus-latent genotypes and explain observed variations in symptom severity. In this study, the presence/absence, and concentrations of CBSV and UCBSV in cassava germplasm were respectively determined by duplex RT-PCR and RT-qPCR. This resulted in identification of CBSVs-free germplasms as well those with single and dual or mixed infections of CBSV and UCBSV. Results showed single infections with high CBSV concentration as predominant and UCBSV exhibiting minor infections with low titres. Similarly, Mukiibi, et al. [30] reported more prevalent single-CBSV infections compared to single-UCBSV infections. Although dual or co-infections with both virus species is common [14,47], only 16% of all germplasm had dual infections in the present study. Genotypic differences for CBSV and UCBSV were also observed where most improved genotypes were CBSVs-free or with low viral loads compared to local landraces that had high concentrations of CBSV. This concurs with previous research which similarly reported higher concentration of CBSV and insignificant or low UCBSV in cassava varieties [27,48].
The genotypic variations in response to both viruses could perhaps be associated with differences in mechanism of host-virus interactions between CBSV and UCBSV [46]. The large differences in virus loads could also be due to competition between the viruses with CBSV outcompeting UCBSV, differences in pathogenicity or differential reaction of genotype to each virus [27]. Compared to UCBSV, CBSV is a more aggressive and virulent CBSD viral pathogen generally accumulating higher titres in most host genotypes in either field or controlled experiments [13,14,27,42,49,50]. Further, CBSV isolates are often more detectable, have higher infection rates and induces more severe symptoms in cassava compared to the "less virulent" UCBSV that generates milder foliar symptoms [42,48]. Although both CBSV and UCBSV cause similar symptoms, the underlying factors behind their varied virulence levels are yet to be established to date [13,42,49,50]. In summary, response CBSV and UCBSV is genotype specific.
In this study, more improved genotypes showed least CBSD severity and low titres of CBSV and UCBSV thus were classified as resistant to CBSD compared to more CBSD susceptible local landraces with high viral loads. Literature reviews indicate that most of these improved genotypes had been bred for CBSD resistance thus low CBSD. Such genotypes included MM96/0686 and MM96/0876 [25] and Thika272, Thika273, Kiboko275, Kiboko274, Thika280, Kiboko300, Kiboko271, Thika279, Thika289, Kiboko295, Kiboko277, Kiboko276, Thika278 and Kiboko281 [34]. Further, majority of the improved genotypes were sampled from farms in Kitui (0°S; 37°E), a region in eastern Kenya that concurrently showed low CBSD incidence and severity. Some of the improved genotypes analyzed in this study also showed susceptibility to CBSD, through high severity and high viral titres, although they had been bred for CBSD resistance. These included TC14, TC4-Katune, 99-0005 and Thika5 [51,52], Kaleso [27,53,54], Katsuhanzala/990132, Tajirika/KME0802 and MH95/0183 [55], MM97/0293, MM96/1871, MM97/0293 and MM96/0686 [56-58]. These results showed potential breakdown of CBSD resistance overtime in improved varieties. Most of the farmer preferred varieties or landraces were classified as susceptible to CBSD. Previous reports also showed farmer-preferred cassava varieties to be highly susceptible to CBSD [51,59,60]. These cultivars are often cultivated by farmers irrespective of availability of improved virus resistant genotypes due to their unique attribute preferences beyond CBSD resistance [59].
Possible inferences could be drawn from the significantly positive correlations observed between virus loads and foliar severity or incidences. For instance, higher CBSD severity scores could be associated with increased accumulation or concentration of CBSVs in a plant. Indeed, in the present study, improved genotypes and landraces that had higher visual CBSD severity also showed higher concentration of viruses especially CBSV. This however contrasted with other findings that did not correlate symptom severity with viral load [27,61]. Ogwok, et al. [50] similarly reported no clear correlation between CBSVs and CBSD symptom types and variability. Although positive, the weak correlations observed between CBSD severity and UCBSV load, implied the lesser or limited effect of UCBSV on CBSD symptoms in the present study. This further confirmed UCBSV as a "lesser" virulent species. Shirima, et al. [62] also reported insignificant or weak correlation between UCBSV titre and symptom severity. The significant and positive correlation between CBSV load and UCBSV load implied potential synergistic effect or interaction between the two viruses. Higher CBSV titres has been recorded in cassava varieties with dual or mixed infections than in singly infected samples suggesting the possible existence of synergism between CBSV and UCBSV in field-infected cassava [63]. CBSD symptoms depend on whether cassava is infected with a single virus, or if there is a concurrent infection of two or more viruses resulting in synergistic interactions [64]. In the current study, 16% of the germplasm that had dual infections also had higher CBSD foliar severity or incidences and viral loads.
Conclusion
In conclusion, the present survey revealed differences in response to CBSD infections between local cassava landraces and improved genotypes with more candidates from the later exhibiting lesser CBSD severity or incidences and viral loads. Most improved genotypes were therefore considered tolerant or resistant to CBSD. However, some of the improved genotypes were also susceptible to CBSD implying potential breakdown of CBSD resistance overtime. Although most local landraces were susceptible to CBSD, a few of them were CBSD-free. Both CBSD-free or resistant germplasms (improved genotypes and landraces) could be bulked for distribution to cassava farmers as clean planting materials for improved production. They could also be potential genetic resources to introgress virus-resistance traits into farmer-preferred varieties.
Acknowledgements
This research did not receive any funding. The author acknowledges Dr. Evans Nyaboga from the department of Biochemistry at University of Nairobi where molecular analysis was carried out. The author thanks Mr. Migwa Yussuf of Kenya Agricultural and Livestock Research Organization-Katumani (KALRO-Katumani), farmers, research assistants and farmhands who that assisted with mapping field sites for data collection.
Competing Interests
The author declares no competing interests.
Author Contributions
Orek Charles: Designed the study, set methodologies and objectives, carried out the survey, sampled germplasms, collected, and analyzed data and developed the manuscript.
References
- Kuete V (2014) 22- Physical, hematological and histopathological signs of toxicity induced by African Medicinal Plants. Toxicological Survey of African Medicinal Plants 635-657.
- Clifton P, Keogh J (2016) Starch. In: Caballero B, Finglas PM, Toldrá F (Edn), Encyclopedia of Food and Health, Academic Press, 146-151.
- Shigaki T (2016) Cassava: Nature and uses. In: Caballero B, Finglas P, Toldra F (Edn), Encyclopedia of Food and Health, Oxford: Elsevier 687-693.
- Okogbenin E, Setter TL, Ferguson M, et al. (2013) Phenotypic approaches to drought in cassava: Review. Front physiol 4: 1-15.
- Orek C, Gruissem W, Ferguson M, et al. (2020) Morpho-physiological and molecular evaluation of drought tolerance in cassava (Manihot esculenta Crantz). Field Crops Research 255.
- Lobell DB, Burke MB, Tebaldi C, et al. (2008) Prioritizing climate change adaptation needs for food security in 2030. Science 319: 607-610.
- Rosenthal DM, Slattery RA, Miller RE, et al. (2012) Cassava about-FACE: Greater than expected yield stimulation of Cassava (Manihot esculenta) by future CO2 levels. Global Change Biology 18: 2661 - 2675.
- Githunguri CM, Njiru EN (2021) Role of cassava and sweetpotato in mitigating drought in semi-arid makueni county in Kenya. African Handbook of Climate Change Adaptation 241-259.
- Valentor AO, Ochwo-Ssemakula M, Kaweesi T, et al. (2018) Plot based heritability estimates and categorization of cassava genotype response to cassava brown streak disease. Crop Prot 108: 39-46.
- Hillocks R, Maruthi MN (2015) Post-harvest impact of cassava brown streak disease in four countries in Eastern Africa. Food Chain 5: 116-122.
- Monger WA, Alicai T, Ndunguru J, et al. (2010) The complete genome sequence of the Tanzanian strain of Cassava brown streak virus and comparison with the Ugandan strain sequence. Arch Virol 155: 429-433.
- Mbanzibwa DR, Tian YP, Tugume AK, et al. (2011) Simultaneous virus-specific detection of the two cassava brown streak associated viruses by RT-PCR reveals wide distribution in East Africa, mixed infections, and infections in Manihot glaziovii. J Virol Methods 171: 394-400.
- Winter S, Koerbler M, Stein B, et al. (2010) Analysis of cassava brown streak viruses reveals the presence of distinct virus species causing cassava brown streak disease in East Africa. J Gen Virol 91: 1365-1372.
- Ndunguru J, Sseruwagi P, Tairo F, et al. (2015) Analyses of twelve new whole genome sequences of cassava brown streak viruses and Ugandan cassava brown streak viruses from East Africa: Diversity, supercomputing and evidence for further speciation. PLoS ONE 10: e0139321.
- Mware BO, Ateka EM, Songa JM, et al (2009) Transmission and distribution of cassava brown streak virus disease in cassava growing areas of Kenya. Journal of Applied Biosciences 16: 864-870.
- Maruthi MN, Bouvaine S, Tufan HA, et al. (2014) Transcriptional response of virus-infected cassava and identification of putative sources of resistance for cassava brown streak disease. PLoS ONE 9: e96642.
- Hillocks RJ, Raya MD, Mtunda K, et al. (2001) Effects of brown streak virus disease on yield and quality of cassava in Tanzania. J Phytopathology 149: 389-394.
- Legg JP, Sseruwagi P, Boniface S, et al. (2014) Spatio-temporal patterns of genetic change amongst populations of cassava Bemisia tabaci whiteflies driving virus pandemics in East and Central Africa. Virus Res 186: 61-75.
- Patil BL, Legg JP, Kanju E, et al (2015) Cassava brown streak disease: A threat to food security in Africa. J Gen Virol 96: 956-968.
- Wagaba H, Beyene G, Aleu J, et al. (2017) Field level RNAi-mediated resistance to cassava brown streak disease across multiple cropping cycles and diverse East African Agro-Ecological Locations. Front Plant Sci 7: 1-18.
- Jeremiah SC, Ndyetabula IL, Mkamilo GS, et al. (2015) The dynamics and environmental influence on interactions between cassava brown streak disease and the whitefly, Bemisia tabaci. Phytopathology 105: 646-655.
- Legg JP, Jeremiah SC, Obiero HM, et al. (2011) Comparing the regional epidemiology of cassava mosaic and cassava brown streak virus pandemics in Africa. Virus Res 159: 161-170.
- Fermont AM, Babirye A, Obiero HM, et al. (2010) False beliefs on the socio-economic drivers of cassava cropping. Agron Sustain Dev 30: 433-444.
- Koima, IN, Orek CO, Nguluu SN (2018) Distribution of cassava mosaic and cassava brown streak diseases in agro-ecological zones of lower Eastern Kenya. IJISRT18JA192 3: 391-399.
- Abaca A, Kawuki R, Tukamuhabwa P, et al. (2012) Evaluation of local and elite cassava genotypes for resistance to cassava brown streak disease in Uganda. Journal of Agronomy 11: 65-72.
- Rwegasira GM, Momanyi G, Rey MEC et al. (2011) Widespread occurrence and diversity of Cassava brown streak virus (Potyviridae: Ipomovirus) in Tanzania. Phytopathology 101: 1159-1167.
- Kaweesi T, Kawuki R, Kyaligonza V, et al. (2014) Field evaluation of selected cassava genotypes for cassava brown streak disease based on symptom expression and virus load. Virology Journal 11: 2-14.
- Abarshi MM, Mohammed IU, Jeremiah SC, et al. (2012) Multiplex RT-PCR assays for the simultaneous detection of both RNA and DNA viruses infecting cassava and the common occurrence of mixed infections by two cassava brown streak viruses in East Africa. J Virol Methods 179: 176-184.
- Adams IP, Abidrabo P, Miano DW, et al. (2013) High throughput real-time RT-PCR assays for specific detection of cassava brown streak disease causal viruses, and their application to testing of planting material. Plant Pathology 62: 233-242.
- Mukiibi DR, Alicai T, Kawuki R, et al. (2019) Resistance of advanced cassava breeding clones to infection by major viruses in Uganda. Crop Protection 115: 104-112.
- Owomugisha G, Nuwamanya E, Quinn JA, et al. (2020) Early detection of plant diseases using spectral data. APPIS 2020: 1-6.
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-??CT Method. Methods 25: 402-408.
- Nyirakanani C, Pierre BJ, Yves K, et al (2021) Farmer and field survey in cassava-growing districts of Rwanda reveals key factors associated with cassava brown streak disease incidence and cassava productivity. Front Sustain Food Syst 5: 1-14.
- Koima IN, Orek CO (2018) Response to cassava brown streak disease infections in local and improved cassava genotypes under field and greenhouse assays in lower Eastern Kenya. Int J of Path Res 1: 1-14.
- McQuaid CF, van den Bosch F, Szyniszewska A, et al. (2017) Spatial dynamics and control of a crop pathogen with mixed-mode transmission. PLoS Comput Biol 13: e1005654.
- Legg JP, Ndalahwa M, Yabeja J, et al. (2017) Community phytosanitation to manage cassava brown streak disease. Virus Res 241: 236-253.
- Mohammed IU, Ghosh S, Maruthi MN (2017) Generating virus-free cassava plants by in vitro propagation with chemical and heat treatment. Afr j Biotechnol 16: 1551-1560.
- Masinde EA, Ogendo JO, Maruthi MN, et al. (2016) Occurrence and estimated losses caused by cassava viruses in Migori County, Kenya. AJAR 11: 2064-2074.
- Osogo AK, Muoma J, Nyamwamu P, et al. (2014) Occurrence and distribution of cassava brown streak viruses in Western Kenya. Journal of Agri-Food and Applied Sciences 2: 184-190.
- Maruthi MN, Jeremiah SC, Mohammed IU, et al. (2017) The role of the whitefly, Bemisia tabaci (Gennadius), and farmer practices in the spread of cassava brown streak ipomoviruses. Journal of Phytopathology 165: 707-717.
- Njoroge MK, Mutisya DL, Miano DW et al. (2017) Whitefly species efficiency in transmitting cassava mosaic and brown streak virus diseases. Cogent Biology 3: 1-8.
- Alicai T, Ndunguru J, Sseruwagi P, et al. (2016) Cassava brown streak virus has a rapidly evolving genome: Implications for virus speciation, variability, diagnosis and host resistance. Sci Rep 6: 36164.
- Ogwok E, Patil BL, Alicai T, et al. (2010) Transmission studies with cassava brown streak Uganda virus (Potyviridae: Ipomovirus) and its interaction with abiotic and biotic factors in Nicotiana benthamiana. J Virol Methods 169: 296-304.
- Ndyetabula IL, Merumba SM, Jeremiah SC, et al. (2016) Analysis of interactions between cassava brown streak disease symptom types facilitates the determination of varietal responses and yield losses. Plant Dis 100: 1388-1396.
- Rwegasira GM, Rey CME (2012) Response of selected cassava varieties to the incidence and severity of cassava brown streak disease in Tanzania. Journal of Agricultural Science 4: 237-245.
- Ogwok E, Muhammed IU, Alicai T, et al. (2016) Comparative analysis of virus derived small RNAs within cassava (Manihot esculenta Crantz) infected with cassava brown streak viruses. Virus Res 215: 1-11.
- Amuge T, Berger DK, Katari MS, et al. (2017) A time series transcriptome analysis of cassava (Manihot esculenta Crantz) varieties challenged with Ugandan cassava brown streak virus. Scientific Reports 7.
- Mohammed IU, Abarshi MM, Muli B, et al. (2012) The symptom and genetic diversity of cassava brown streak viruses infecting cassava in East Africa. Adv Virol.
- Patil BL, Ogwok E, Wagaba H, et al. (2011) RNAi-mediated resistance to diverse isolates belonging to two virus species involved in Cassava brown streak disease. Mol Plant Pathol 12: 31-41.
- Ogwok E, Alicai T, Rey MEC, et al. (2015) Distribution and accumulation of cassava brown streak viruses within infected cassava (Manihot esculenta) plants. Plant Pathology 64: 1235-1246.
- Koros JC, Runo SM, Yusuf M, et al. (2018) Screening selected cassava cultivars for resistance against cassava viruses and cassava green mites under advanced yield trials in Kenya. IOSR-JBB 4: 37-52.
- Kalro (2016) Annual Report 2015-2016.
- Kanju E, Mkamilo G, Mgoo V, et al. (2010) Statistical evidence linking the zigzag stems habit with tolerance to cassava brown streak disease. Roots 12: 4-6.
- Kavil S, Otti G, Bouvaine S, et al. (2021) PAL1 gene of the phenylpropanoid pathway increases resistance to the Cassava brown streak virus in cassava. Virol j 18: 184.
- Kivuva B, Munga TL, Woyengo V, et al. (2019) Inventory of climate smart agriculture cassava technologies, innovations and management practices. Kalro: Under Kenya Climate Smart Agriculture Project (Kcsap).
- Musungayi EM, Ngugi K, Muthomi JW, et al. (2018) Evaluation of resistance of cassava half-sib progenies to Cassava mosaic disease and their agronomic performances in western Kenya. Journal of Agricultural Science 10: 78-91.
- Ntawuruhunga P, Dixon AGO, Kanju E, et al. (2013) Successful innovations and lessons learnt in cassava improvement and deployment by IITA in the Eastern African Region. AJRTC 10: 41-54.
- Obiero HM, Whyte JAB, Legg JP, et al. (2007) Successful restoration of cassava production in Western Kenya. Proceedings of the 13th ISTRC Symposium 682-685.
- Kasule F, Wasswa P, Mukasa SB, et al. (2020) Farmer preference of cassava cultivars in Eastern Uganda: A Choice beyond Disease Resistance. Agri Sci 2: 169-177.
- Elegba W, McCallum EJ, Wilhelm G, et al. (2021) Efficient genetic transformation and regeneration of a farmer-preferred cassava cultivar from Ghana. Front Plant Sci 12: 1-12.
- Tomlinson KR, Bailey AM, Alicai T, et al. (2018) Cassava brown streak disease: Historical timeline, current knowledge and future prospects. Mol plant pathol 19: 1282-1294.
- Shirima RR, Daniel G, Kanju ME et al. (2017) Absolute quantification of cassava brown streak virus mRNA by real-time qPCR. J Virol Methods 245: 5-13.
- Otti G, Bouvaine S, Kimata B, et al. (2016) High throughput multiplex real time PCR assay for the simultaneous quantification of DNA and RNA viruses infecting cassava plants. J Appl Microbiol 120: 1346-1356.
- Ogwok E, Odipio J, Halsey M, et al. (2012) Transgenic RNA interference (RNAi)-derived field resistance to cassava brown streak disease. Mol Plant Pathol 13: 1019-1031.
- Orek, CO (2018) An optimised cetyltrimethylammonium bromide (CTAB)-based protocol for extracting RNA from young and old cassava leaves. J Adv Biol Biotechnol 19: 1 -7.
Corresponding Author
Orek Charles, Department of Agricultural Sciences, South Eastern Kenya University, P.O Box 170-90200, Kitui, Kenya.
Copyright
© 2022 Charles O. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.