Acute and Sub-chronic Toxicities of Aqueous and Ethanol Fruit Extracts of Solanum incanum Linn. in Male Swiss Albino Mice
Abstract
Solanum incanum Linn. (Solanaecae) is used for its medicinal and nutritional values in parts of tropical Africa, including Northeast of Nigeria. The present study evaluated acute and sub-chronic oral toxicities of S. incanum fruit extracts in Swiss albino mice. The fruits were extracted by cold maceration method using distilled water and ethanol as solvents. The acute toxicity studies of the aqueous and ethanol extracts were carried out using Lorke's method. Three groups (Groups 1-3) of 3 mice each received 10, 100 and 1000 mg/kg of aqueous fruit extract orally, respectively, in first phase while the second phase consists of three groups of one mouse each which received 1600, 2900 and 5000 mg/kg orally, respectively. A 28-day sub-chronic toxicity study was performed in accordance with Organization for Economic Cooperation and Development's guidelines using 125, 250 and 500 mg/kg of the ethanol fruit extract and 95, 190 and 380 mg/kg of the queous fruit extract. Mice in group 1 received distilled water orally for 28 days (control for both extracts). Mice in groups 2-4 received aqueous extract at 95, 190 and 380 mg/kg orally once daily for 28 days. Mice in groups 5-7 received ethanol extract at 125, 250 and 500 mg/kg orally once daily for 28 days. After anesthesia, blood samples were collected from cardiac puncture at the end of 28-day administration. The animals were euthanized, followed by removal of the vital organs which were weighed and analyzed for gross and histopathological changes. Aspartate aminotransferase (AST) and Alanine aminotransferase (ALT) of liver function were estimated spectrophotometrically. Oral and intraperitoneal LD50 of the aqueous extract were 3808 mg/kg and 288.5 mg/kg, respectively. The oral LD50 of the ethanol extract was > 5000 mg/kg while intraperitoneal LD50 was 288.5 mg/kg. The extract did not produce significant changes in body weights, relative organ weight and liver enzymes. Histopathological evaluation showed disruption of hepatocyte parenchyma, loss of sinusoidal spaces and increased Kupfer cells at 380 mg/kg of aqueous extract. While ethanol extract at 500 mg/kg showed contracted renal glomeruli interspersed with normal glomeruli. In conclusion, aqueous and ethanol fruit extracts of S. incanum appear relatively safe when administered orally at low-moderate doses but may be toxic at high doses.
Keywords
Solanum incanum, Acute toxicity, Sub-chronic toxicity, Histopathology, Safety
Introduction
Indeed, most of the available chemical drugs used in treatment of various diseases are derived from vegetables [1-3]. It is estimated that 80% of the world's population living in the developing world rely on herbal medicinal products as primary source of healthcare [2,4]. Ertekin, et al. [5] and Koduru, et al. [6] reported that many medicinal plants used in traditional medicine may have adverse effects. This raises concern about the potential toxic effects which may result from short and long-term consumption of such medicinal plants [7]. Thus, acute and sub-chronic toxicity studies are often conducted on medicinal plants to ascertain their safety level when consumed over short- and long-term periods [8-10]. Acute toxicity testing is used to determine the effect of a single large dose on a particular animal species and is also used to determine dose range for subsequent testing. Sub-chronic toxicity testing is used to investigate the adverse effects resulting from repeated exposure in experimental animals over a portion of their average life span. It provides a valuable information on the cumulative toxicity of a substance on target organs and on physiological and metabolic tolerance of a compound at low-dose long-term exposure. They are useful in establishing doses at which no toxicological effects are evident, a critical factor for risk assessment [11].
Solanum incanum Linn. (Solanaceae), commonly called bitter garden egg, apple of Sodom or bitter apple (English Language), "ikan" or "igba" (Yoruba Language) and "gautandaacii" (Hausa Language), is a herb or soft wooded shrub traditionally used for treatment of sore throat, angina, stomach-pain, colic, headache, painful menstruation and liver diseases in tropical Africa [12]. The root decoction of the plant is used in treating snake bite in Senegal, Kenya, Uganda and Zimbabwe [13]. In Niger, Sudan, Rwanda and Namibia, the fruits are used as an ingredient of arrow poison and in Mozambique as fish poison [13]. In Borno State, Northeast Nigeria, S. incanum Linn. is used to colour the teeth for beautification by "Kanuri" women and as vegetable by "Barba-Bura" ethnic group [14]. The leaves, fruits and roots are used as cognitive enhancer in Southwest Nigeria [15,16]. The extracts of the fruits have also been reported to cause skin cancer in animals, toxic in goats and sheep [17,18] and found to cause toxicity in man after oral ingestion [19]. Despite that the fruits of S. incanum Linn. are readily used by various ethnic groups in Northeast Nigeria [14], there is dearth of scientific data on the toxicity of the fruits grown in the region. Hence, the present study investigated the acute and sub-chronic toxicity of the fruit extracts of S. incanum Linn.
Materials and Methods
Sample collection, identification and authentication
Fresh leaves and fruits of S. incanum Linn. were collected from Mulbiya in Hawul Local Government Area, Borno State, Nigeria between January and March, 2017. Identification and authentication of the plant was done by a plant taxonomist, Professor S. S. Sanusi of the Department of Biological Sciences, Faculty of Science, University of Maiduguri, Nigeria. A voucher specimen (Voucher No. DCPT 014) was deposited in Pharmacology Laboratory, Department of Clinical Pharmacology and Therapeutics, Faculty of Basic Clinical Sciences, College of Medical Sciences, University of Maiduguri, Maiduguri, Nigeria.
Preparation of the extracts
The fruits of S. incanum were washed and cleaned. They were cut into small pieces, air dried at room temperature, pulverized into a coarse powder and extracted with distilled water and ethanol using cold maceration method [20]. Briefly, (500 g) each of the powdered fruits was soaked in 99.7 % ethanol (2 L) and distilled water (2 L) for 3 days at room temperature, with agitation at intervals. Afterwards, the solutions were sieved with a muslin cloth and filtered with a Whatman (No. 1) filter paper. The filtrates were dried in oven at 40 ℃ and the dried masses obtained were weighed and stored in a refrigerator until use.
Experimental animals
A total number of eighty-eight (88) mice were randomly selected for the studies, twenty-four (48) mice for acute test, thirty-five (35) mice for sub-chronic test and five (5) mice were used as control in sub-chronic test in the study. Male Swiss albino mice (15-25 g) were procured from the Department of Clinical Pharmacology and Therapeutics, Faculty of Basic Clinical Sciences, College of Medical Sciences, University of Maiduguri and Department of Veterinary Pharmacology and Toxicology, Faculty of Veterinary Medicine, University of Maiduguri, Nigeria. They were housed in a standard environmental condition and fed with rodent feeds (Grand Cereals Meal LTD, Nigeria) and water ad libitum. They were allowed to acclimatize for 2 weeks prior to the commencement of the studies. Animal care and handling conformed to guidelines of Laboratory Animal Care and the Animal Care and Use Guidelines of Biomedical Research Involving Animals [21]. The ethical approval for the study was obtained from Animal Use and Ethics Committee, Faculty of Veterinary Medicine, University of Maiduguri, Nigeria.
Acute oral and intraperitoneal toxicity studies of the extracts
Lorke's method [22] was used to determine S. incanum oral and intraperitoneal acute toxicities as follows:
Phase 1: Nine (9) healthy albino mice of both sexes weighing 15-25 g were selected randomly and divided into three groups (labelled groups as A, B and C) of three animals each. The groups were treated respectively with the extract at incremental doses of 10 mg/kg, 100 mg/kg and 1000 mg/kg orally. The animals were initially observed for 24 hours for signs of toxicity and mortality and then for 14-day post treatment.
Phase II: Three (3) mice were divided into three groups of one mouse each, they were administered graded doses of S. incanum extract at 1600, 2900 and 5000 mg/kg body weight for oral administration and 225, 370 and 600 mg/kg body weight for intraperitoneal administration respectively based on the result of phase I. The mice were given access to food and water ad libitum. They were initially observed for 24 hours for signs of toxicity and mortality and then for 14-day post treatment. The LD50 was calculated using the formula below:
Where a = least dose that kills the animal.
b = highest dose that does not kill the animal.
The two phases (I and II) were repeated using another set of 12 mice that received the extract via intraperitoneal route. These were done for aqueous and ethanol extracts [23].
Sub-chronic toxicity studies
The sub-chronic toxicity study was conducted using an Organization for Economic Co-operation and Development (OECD) guideline for testing of chemicals [24]. Matured male Swiss albino mice (15-25 g) were randomly assigned into seven (7) groups of five (5) mice each. Mice in group 1 received distilled water orally once daily for 28 days. Mice in groups 2-4 received aqueous extract at 95, 190 and 380 mg/kg orally once daily for 28 days, respectively. Mice in groups 5-7 received ethanol extract at 125, 250 and 500 mg/kg orally once daily for 28 days, respectively. The mice were observed daily for any toxic signs and weighed weekly. At the end of the 28-day treatment, the mice were anaesthetized and blood samples were collected by cardiac puncture into plain bottles. Then, they were euthanized and the brain, liver, kidney, heart and testes were harvested and weighed [25].
Mortality and Clinical Sign
In the course of the studies, all mice were observed daily for mortality and clinical sign of toxicity such as, changes in skin, fur, eyes, mucous membranes, occurrence of secretions and excretions, autonomic activity, changes in gait, posture and response to handling, as well as the presence of tremor, writhing, restlessness, stereotypes or bizarre behavior [24,26,27].
Biochemical assays
The blood samples were allowed to clot for 30 minutes, centrifuged at 5000 rpm for 10 min at room temperature and the serum recovered. Liver function was assessed by determining serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities by spectrophotometric technique using enzymatic colorimetric assay kits (Randox Laboratories Limited, Crumlin, County Antrim, BT294QY, United Kingdom), as described by Reitman and Frankel [28].
Histopathology
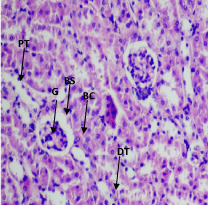
The brain, liver, kidney, heart and testes samples were fixed in 10% buffered form saline in labelled plastic containers. Histological slides stained with haematoxylin and eosin were prepared from sections of all the tissues collected as described by Gupta, et al. [29] Slides were examined under light microscope for probable histological changes and photomicrographs taken with a Leica DM750 Camera Microscope (X 400).
Statistical analysis
Data obtained were expressed as mean ± standard error of mean (SEM) and subjected to statistical analyses (ANOVA) using computer software GraphPad® InStat version 5.01. [30]. Values of p ≤ 0.05 were considered as significant.
Results
Acute oral and intraperitoneal toxicities of the extracts
Oral administration of aqueous fruit extracts of S. incanum at 5000 mg/kg induced restlessness, writhing, tremor and death within 24 hours (Table 1), however, oral administration of the ethanol fruit extracts of S. incanum did not produced toxicity signs at all the dosages. The dose that produced toxic signs of restlessness, writhing, tremor was lower (p < 0.05) for intraperitoneal routes (288.5 mg/kg) than oral routes (3808 mg/kg) of aqueous and > 5000 mg/kg of ethanol extracts. The intraperitoneal administration of aqueous and ethanol extracts, recorded mortality at doses of 370 mg/kg and above, resulting in an LD50 value of 288.5 mg/kg of both extracts (p > 0.05), the oral LD50 of ethanol extract (> 5000 mg/kg) was higher (p < 0.05) than oral LD50 of aqueous extract (3808 mg/kg). The mice manifested restlessness, writhing, tremor and increased breathing at the higher doses before death.
Sub-chronic toxicity testing survival and clinical sign
There were no treatment-related obvious symptom of toxicity in mice following the oral daily administration in sub-chronic study of S. incanum extract for 28 days. No deaths or obvious clinical signs were found in any groups throughout the experimental period. Physical observation of the treated mice throughout the study indicated that none of them showed signs of toxicity in their skin, fur, eyes, mucus membrane, or behavioural changes, diarrhoea, tremors, salivation, sleep and coma.
Effects of the extracts on body weights, relative organ weights and liver functions
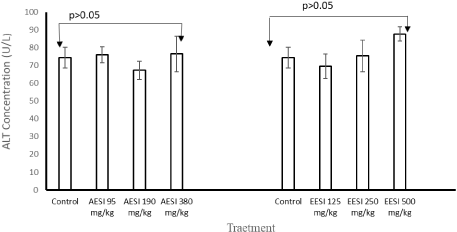
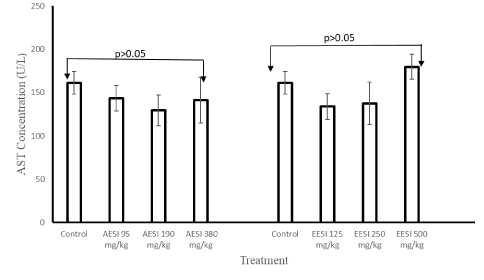
There were no significant changes (p > 0.05) observed in the body weights of the mice in all treatment groups during the 28-day administration of aqueous and ethanol extracts (Tables 2). Similarly, no significant changes (p > 0.05) observed in weight of the organs (liver, spleen, kidney, heart, lung and brain) of the mice treated with both aqueous and ethanol fruit extracts of S. incanum (Supplementary files 1 and Supplementary files 2). There was no significant difference in the concentrations of ALT and AST at all doses of aqueous and ethanol fruits extracts (p > 0.05) [Figures 1 to Figures 2].
Effects of the extracts on histopathology of the vital organs
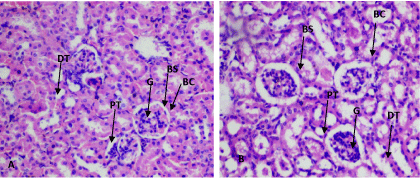
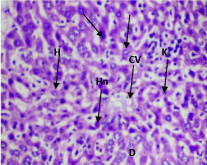
At 190 mg/kg and 380 mg/kg of the aqueous extract, the kidney showed disruption of normal kidney architecture with contracted renal glomeruli, an increase in the Bowman's space and dilatation of the renal tubules (Figure 3). At 380 mg/kg of aqueous extract, the liver showed disruption of hepatocyte parenchyma, loss of sinusoidal spaces and increased Kupfer cells scattered throughout hepatocyte parenchyma. Numerous hepatocytes with pale-staining nuclei were also observed (Figure 4). At 500 mg/kg of the ethanol extract, contracted renal glomeruli interspersed with normal glomeruli was observed (Figure 5). No histopathological changes were observed in other organs (Supplementary files 3).
Discussion
The present study investigated the oral and intraperitoneal acute and oral sub-chronic toxicities of aqueous and ethanol fruit extracts of S. incanum in mice. The oral LD50 of the ethanol fruit extract of S. incanum was > 5000 mg/kg body weight, thus, it could be categorized as practically non-toxic based on Hodge and Sterner scale of toxicity [31-33]. This shows that acute administration of the ethanol extract may not be harmful when consumed orally. This is in agreement with previous studies that demonstrated non-toxic nature of ethanolic fruit extracts grown in Coimbatore, Tamilnadu, India, Addis Ababa and Ethiopia [34,35]. With LD50 of 3808 mg/kg, the aqueous extract could be categorized as slightly toxic using same toxicity scale [32,33]. This could be due to difference in phytochemical constituents of the extracts. Cardenolite, a toxic phytochemical [36,37], is present in the aqueous extract but absent in ethanolic extract [Akanmu et al., in press]. However, both extracts could be classified as moderately toxic [32,33] when administered through intraperitoneal route. The variation in toxic level of oral and intraperitoneal routes could be attributed to the fast absorption and high bioavailability of intraperitoneal route [38]. This could have resulted into high plasma level of the extracts thereby increasing the toxicity. One of the indicators of toxic effect is change in body and organ weights. The adverse effect will be significant if the body weight loss occurred in animals is more than 10% of their initial weight [39]. In the present study, the extracts showed no effects in the body and organ weights over the 28-day period. This is the reflection of the oral acute toxicity that showed that the extracts are relatively non-toxic when administered orally. In addition, the fact that the extracts did not increase weight gain suggests that the energy content of the fruit may be low [40-42] which was done in order to evaluate the effect of extracts on absorption and utilization of the nutrients. Thus, there is need to investigate the proximate contents of the fruits.
The liver is the major organ involved in the metabolism of drugs and other xenobiotics [43]. In this study, AST and ALT were measured because they are one of the most important serum biomarker enzymes usually used in evaluation of toxic effects of medicinal plants or other xenobiotics on liver. Elevated levels of AST and ALT are often used as indicators of compromised liver function [44]. The fact that both extracts did not raise AST and ALT levels is suggestive of safety of the extracts on the liver when given orally at the studied doses. Beside, previous studies have shown that some Solanum spp. possess hepatoprotective properties [45-47].
Histopathological assessment provides a more in-depth assessment of toxicity profile of drugs and other xenobiotics including medicinal plants [48]. The ethanolic extract demonstrated safety tendency when consumed at low, moderate and high doses as indicated by absence of histopathological changes in liver, spleen, kidneys, heart, lungs and brain tissues of experimental mice. In contrast, mice treated with the aqueous extract had renal and hepatic lesions. This could be attributed with harmful effects of some phytochemicals (cardenolites and tannins) found in the aqueous fruits extract [49]. Tannins have been reported to have pro-oxidant effects which causes tissue damage [49]. This is in concordance with the previous study conducted by Thaiyah, et al. [18] in Kenya who reported a necrosis of the Purkinje cells of the cerebellum, tubular necrosis in the kidneys and hepatocyte necrosis in sheep.
Conclusion
The fruit extracts are safer when administered orally compared with IP administration. The results of the sub-chronic effect on the liver function parameters indicated that the fruit extracts is safe when given orally at the studied doses. Oral daily administration of graded doses of the aqueous fruit extract of S. incanum to mice for 28 days adversely affected the morphology of the kidney, liver and heart at the high doses. Therefore, it is recommended that the effects of S. incanum fruit extracts on animal foetus and pregnant animals are needed to complete the safety profile of this herb. Moreover, prolonged oral administration beyond 28 days at high dose may be studied to ascertain the safety of the fruits.
Acknowledgements
The authors are grateful to the entire staff of the Department of Pharmacology, Faculty of Pharmacy, Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria for their support and encouragement at every step of the study.
Ethical Approval
Authors hereby declared that the experimental protocol was approved by the University and Departmental Committee for Research and Ethics. Each animal was used only once. Experimental protocol was followed according to Guidelines of Laboratory Animal Care and with the Animal Care and Use Guidelines of Biomedical Research Involving Animals (2012). All rules were followed as well as specific national laws where applicable.
Conflict of Interests
There is no conflict of interest to report.
References
- Dias DA, Urban S, Roessner U (2012) A historical overview of natural products in drug discovery. Metab 2: 303-336.
- Ekor M (2014) The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety, Front Pharmacol 4: 177-186.
- Tabassum N, Hamdani M (2014) Plants used to treat skin diseases. Pharmacogn Rev 8: 52-60.
- Bandaranayake WM (2006) Quality control, screening, toxicity, and regulation of herbal drugs, In: Modern Phytomedicine. Turning Medicinal Plants into Drugs, (eds) I Ahmad, F Aqil, M Owais, Weinheim: Wiley-VCH GmbH & Co. KGaA 25-57.
- Ertekin V, Selimoglu MA, Altinkaynak S (2005) A combination of unusual presentations of datura stramonium intoxication in a child: Rhabdomyolysis and fulminant hepatitius. J Emerg Med 28: 227-228.
- Koduru S, Grierson DS, Afolayan AJ (2006) Antimicrobial activity of Solanum aculeastrum. Pharm Biol 44: 283-286.
- Ashafa AO, Orekoya LO, Yakubu MT (2012) Toxicity profile of ethanol extract of Azadirachta indica stem bark in male Wistar rats. Asian Pac J Trop Biomed: 811-817.
- Ukwuani AN, Abubakar MG, Hassan SW, et al. (2012) Toxicological studies of hydromethanol leaves extract of Grewia crenata. Int J Pharm Sci Drug Res 4: 245-249.
- Mugisha MK, Ndukui JG, Namutembi A, et al. (2014) Acute and Sub-Acute Toxicity of Ethanolic Leaf Extracts of Rumex abyssinica Jacq. (Polygonaceae) and Mentha spicata L.(Lamiaceae). Pharmacol Ther 5: 309-318.
- Aniagu SO, Nwinyi FC, Akumka DD, et al. (2005) Toxicity studies in rats fed nature cure bitters. Afr J Biotechnol 4: 72-78.
- Meyer O, Svendsen O, Lykkesfeldt J (2011) Animal Models in Pharmacology and Toxicology. In: Handbook of Laboratory Animal Science, (3rd edn), P Svendsen, J Hau, Boca Raton, Fla: CRC Press 150-181.
- Habtamu A, Tamene G, Adane H (2014) Phytochemical investigation on the roots of Solanum incanum, Hadiya zone, Ethiopia. J Med P Stud 2: 83-93.
- Bodart P, Kabengera C, Noirfalise A, et al. (2000) Determination of a solanine a chaconine in potatoes by high performance thin layer chromatography/densiometry. JAOC 83: 1468-1473.
- Bukar A, Danfillo IS, Adeleke OA, et al. (2004) Traditional oral health practices among Kanuri women of Borno State, Nigeria. Trop Dent J 107: 25-31.
- Oladele AT, Cyril-Olutayo CM, Elufiossye TO (2012) Ethnobotanical survey of plants used as memory enhancer and antiaging in Ondo State, Nigeria. Int J Pharm 2: 26-32.
- Olatunji PB, Fasola RT, Onasanwo SA (2016) Ethnobotanical survey of plants used as memory enhancer in three states of Southwestern Nigeria. J Appl Pharm Sci 6: 209-214.
- Mwonjoria JK, Ngeranwa JJ, Kariuki HN, et al. (2014) Ethnomedicinal, phytochemical and pharmacological aspects of Solanum incanum (Lin.). Int J Pharmacol Toxicol 2: 17-20.
- Thaiyah AG, Nyaga PN, Maribei JM, et al. (2011) Acute, sub-chronic and chronic toxicity of Solanum incanum L in sheep in Kenya. Kenya Vet Associ 35: 1-8.
- Verdcourt B, Trump EC (1969) Common poisonous plants of East Africa. Collins, London, 1-254.
- Bandar H, Hijazi A, Rammal H, et al. (2013) Techniques for the extraction of bioactive compounds from Lebanese Urtica dioica. Am Jour of Phytomed & Clinical Therapeut 1: 507-513.
- http://iclas.org/wp-content/uploads/2013/03/CIOMS-ICLAS-Principles-Final.pdf
- Lorke D (1983) A new approach to practical acute toxicity testing. Archi Toxicol 53: 275-287.
- Oyewole OI, Oyedara OO, Olabiyi BF, et al. (2013) Phytochemical, antimicrobial and toxicity studies of Phyllanthus amarus whole plant extract. Int J Bioassays 2: 519-523.
- OECD: Organisation for Economic Co-operation and Development (2008) (OECD) Guideline for Testing of Chemicals (TG 407). Repeated Dose 28-Day Oral Toxicity Study in Rodents. OECD/OEDC. 2008.
- Yemitan OK, Adeyemi OO (2004) Toxicity studies of the aqueous root extract of Lecanoidiscus cupanioides. Nigerian J Heal Biomed Sci 3: 20-23.
- Jaijoy K, Soonthornchareonnon N, Lertprasertsuke N, et al. (2010) Acute and chronic oral toxicity of standardized water extract from the fruit of Phyllanthus emblica Linn. Int J Appl Res Natl Prod 3: 45-58.
- Kengkoom K, Chaimongkolnukul K, Cherdyu S, et al. (2012) Acute and sub-chronic oral toxicity studies of the extracts from herbs in Phikud Navakot. Afr. J. Biotechnol 11: 10903-10911.
- Reitman S, Frankel S (1957) A colorimetric method for determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Path 28: 56-63.
- Gupta E, Bhalla P, Khurana N, et al. (2009) Histopathology for the diagnosis of infectious diseases. Indian J. Med. Microbio 27: 100-106.
- www.graphpad.com
- Ibrahim MB, Sowemimo AA, Sofidiya MO, et al. (2016) Sub-acute and chronic toxicity profiles of Markhamia tomentosa ethanol leaf extract in rats. J Ethnopharmacol 193: 68-75.
- Ahmed M (2015) Acute Toxicity (Lethal Dose 50 Calculation) of Herbal Drug Somina in Rats and Mice. Pharmacol Pharm 6: 185-189.
- https://www.ccohs.ca/oshanswers/chemicals/ld50.html
- Indhumathi T, Mohandass S, Shibi A (2014) Acute toxicity study of ethanol extract of Solanum incanum. L. fruit. Asian J Pharmaceut Clin Res 7: 98-100.
- Feyera T, Assefa S, Mekonnen E, et al. (2017) Phytochemical screening and toxicity profiles of crude extracts of Cissus quadrangularis L. and Solunum incanum L. in mice. Afr J Pharm Pharmacol 11: 411-418.
- Malcolm SB (1991) Cardenolide-Mediated Interactions between Plants and Herbivores. In: Herbivores (2nd edn), GA Rosenthal, MR Berenbaum, Academic Press: San Diego 251-296.
- Blaustein MP (1985) The cellular basis of cardiotonic steroid action. Trends Pharmacol Sci 6: 289-292.
- Madingou NOK, Traore A, Souza A, et al. (2016) Preliminary studies of acute and sub-chronic toxicity of the aqueous extract of Guibourtia tessmannii (Harms) J. Leonard stem barks (Caesalpiniaceae) in mice and rats. Asian Pac J Trop Biomed 6: 506-510.
- Raza M, Al-Shabanah OA, El-Hadiyah TM, et al. (2002) Effect of prolonged vigabatrin treatment on haematological and biochemical parameters in plasma, liver and kidney of Swiss albino mice. Scient Pharmaceut 70: 135-145.
- Odetola AA, Iranloye YO, Akinloye O (2004) Hypolipidaemic potentials of Solanum melongena and Solanum gilo on hypercholesterolaemic Rabbits, Pak J Nutr 3: 180-187.
- Showemimo FA, Olarewaju JD (2004) Agro-nutritional determinants of some garden varieties (Solanum gilo L). J Food Tech 2: 172-175.
- Edijala JK, Asagba SO, Eriyamremu GE, Atomatofa U. Comparative effect of garden egg fruit, oat and apple on serum lipid profile in rats fed a high cholesterol diet. Pak J Nutr. 2005; 4:245-249.
- Almazroo OA, Miah MK, Venkataramanan R (2017) Drug metabolism in the liver. Clin Liver Dis 21: 1-20.
- Kausar MW, Moeed K, Asif N, et al. (2010) Correlation of bilirubin with liver enzymes in patients of falciparum malaria. Int J Path 8: 63-67.
- Vijayan P, Prashanth HC, Vijayaraj P, et al. (2003) Hepatoprotective effect of the total alkaloid fraction of Solanum pseudocapsicum leaves. Pharmaceu Biol 41: 443-448.
- Meenu Krishnan VG, Murugan K (2016) Acute and sub-chronic toxicological evaluation of the purified protease inhibitor from the fruits of Solanum aculeatissimum Jacq. on Wistar rats. Cog Biol 2: 1191588.
- Sambo HS, Pam CS, Dahiru D (2012) Effect of aqueous extract of Solanum incanum fruit on some serum biochemical parameters. Agric Bus Tech J 10: 82-86.
- Zhang Q, Mao Z, Zhang Q, et al. (2018) Acute and sub-chronic toxicological studies of the iridoid glycosides extract of lamiophlomis rotate (Benth.) kudo in rats. Regul Toxicol Pharmacol 92: 315-323.
- Kumar R, Singh M (1984) Tannins: Their adverse role in ruminant Nutrition. J Agric Food Chem. 32: 447-453.
Corresponding Author
Akanmu AO, Faculty of Basic Clinical Sciences, Department of Clinical Pharmacology and Therapeutics, College of Medical Sciences, University of Maiduguri, PMB 1069, Maiduguri, Nigeria, Tel: +234-8037276800.
Copyright
© 2021 Akanmu AO. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.