Human Papillomavirus & its Role in Oral Cavity Cancers: Pathogenesis, Transmission and Prognosis
Abstract
Oral cancers are in a rising trends nowadays. In Asia, it is among the most common cancers that carry significantly high morbidity and mortality. A steady rise in incidence in oral cavity cancers especially among young males with no history of tobacco or alcohol use has given rise to a new risk factor, the human papillomavirus (HPV). The HPV-OSCC is a unique, under covered clinical entity. The role of HPV and its oncogenic potential in the development of oral squamous cell carcinoma are evident. HPV-positive status significantly improves survival and prognostically better. This article reviews on the pathogenesis, transmission and prognosis of HPV-related oral squamous cell carcinoma (OSCC).
Keywords
Human papillomavirus, Oral squamous cell carcinoma, Oral cancer
Introduction
The link between human papillomavirus (HPV) in oral squamous cell carcinomas (OSCC) is evident in few studies and need to be taken into consideration while battling these cancers [1-3]. One of the earliest studies to suggest this link was in 1983 in which 40 biopsy samples of oral cancers were studied under the light microscopy with emphasis on histopathological features suggesting HPV lesion. It was found that 16 samples with morphological signs with 8 of them showing HPV-positive nuclei [4].
A meta-analysis highlighted the prevalence of HPV at 24.5%, particularly HPV type 16 and 18, among dysplastic and invasive cancers of oral cavity and oropharynx supports the assumption that HPV infection occurs during early phase of these cancers etiopathogenesis [3]. Another study estimated proportion of HPV infection among oral and oropharyngeal SCC at 35% [5].
International Agency for Research on Cancer (IARC), in its monograph released in 2007, have concluded there is sufficient evidence in humans for the carcinogenicity of HPV 16 in oral cavity and oropharynx but limited evidence in humans for the carcinogenicity of HPV 18 in oral cavity [6].
Overview of Human Papillomavirus (HPV)
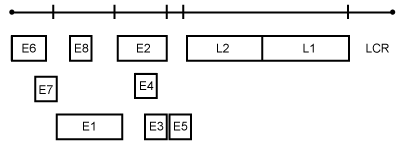
HPV belongs to Papillomaviridae virus family in which HPV 16 belongs to Alfa-papillomavirus genus [7]. They are icosahedral, non-enveloped particles, 55 nm in diameter, double-stranded DNA viruses containing approximately 7900 base pairs [8]. The genome organization includes 8 open reading frames and a non-coding region. The six early open reading frames (E1-E6) encode proteins involved in DNA replication, transcription and cellular transformation; the capsid proteins (L1 & L2) are encoded by late open reading frames (Figure 1) [7,9].
Both E1 and E2 genes encode proteins that bind to DNA and act as transcriptional activators or repressors which regulates virus transcription and genome replication [8,9]. The E4 gene is involved in maturation and release of papillomavirus particles which is expressed relatively late in virus replication. The E6 and E7 genes, which are the main culprit in viral oncogenesis, are responsible in producing oncogenic proteins while the non-coding region or long control region (LCR) contains regulatory sequences that respond to steroid receptor hormones. The summary of these genes (Table 1) [10].
HPV can be classified as low-risk and high risk types based on the established cervical oncogenicity [11]. Low risk HPV types (eg. type 6 and 11) induce benign lesions with minimum risk of progression to malignancy such as in laryngeal papilloma. [9,12-14] On the other hand, high risk HPV have higher oncogenic potential with HPV 16 being the most prevalent followed by types 18, 31 & 33 [9,12]. A particular HPV genotype is considered as a distinct one when the nucleotide sequence of L, E6 and E7 genes differs from any other by at least 10% [15]. Infection by more than one strain of HPV was possible but HPV-positive oral squamous cell carcinoma were associated with oncogenic or high-risk type HPV. While the other types could be dormant [16]. Table 2 lists down the various types of HPV based on risk classification.
Pathogenesis: Function of E6, E7 Genes
These papilloma viruses infect epithelial cells and initial infection involves exposure of infectious particles to basal layer. Expression of viral gene product occurs as infected basal cell migrates to epithelial surface [9]. The E6 gene encodes a protein that binds to tumour suppressor protein p53 which induces its degradation while the E7 gene encodes a protein that binds to retinoblastoma protein (Rb). Since over-expression of p53 and Rb inhibits viral replication, expression of E6 and E7 in epithelial layers causes degradation of these proteins and drives cells into S-phase, conducive for viral genome replication and cell proliferation. In progression towards carcinogenesis, viral genome integrates into host genome and this disrupts E2. As a result, E6 and E7 expression is accelerated and bind to p53 and retinoblastoma tumour suppressors as mentioned earlier.
E6 protein binds to p53 protein via cellular ubiquitin-ligase, recruiting the ubiquitin complex of enzymes, ubiquitinating lysines on p53 and causing its proteolysis. This becomes the prime cause of chromosomal instability via bypassing the normal growth arrest signals at G1/S and G2/M checkpoints in addition of enhanced integration of foreign DNA into host-cell genome [17]. In addition, this oncoprotein also inhibits degradation of SRC-family kinases which stimulates mitotic activity [18] and activates telomerase by transcriptional activation of human telomerase reverse transcriptase (hTert) gene thus disrupting the natural pathway that leads to senescence and cell death [19]. E6 proteins also induce ubiquitin-mediated degradation of several PDZ domain-containing substrates which control cell proliferation, polarity and adhesion thus contributing to malignancy [20].
E7 protein binds with retinoblastoma gene product (pRb) to facilitate progression into S-phase of cell cycle [18] by preventing pRb binding with E2F transcription factors (normally they bind to form complexes that repress transcription) thus promoting cell cycle progression. In addition, 2 other members of pRb family, p107 and p130 which usually down regulates E2F transcription, are also affected by E7 and causes cell cycle progression [19]. E7 also stimulates the S-phase genes cyclin E and cyclin A and evades the inhibitory activities of cyclin-dependent kinase inhibitors (CKIs) [18]. Aneuploidy of cells expressing E7 is induced by centriole amplification which contributes to tumour genesis [18].
However, only a certain fraction of infected individuals present with oral cavity cancers as seen in cervical cancers. As such, consideration needs to be given to additional factors. Two other factors, which have been given a lot of attention are p53 polymorphism and human leukocyte antigen (HLA).
Cervical cancer research has shown p53 polymorphism proline/arginine at codon 72 does play a role in an individual’s susceptibility to HPV 16 and 18 induced degradation [21]. Results have shown that p53Arg is more susceptible than p53Pro to degradation induced by HPV. The study also found individuals with homozygous p53Arg are 7 times more likely to develop HPV-associated cervical cancer than individuals having one or more p53Pro alleles. In a cumulative series, homozygous mutant (Arg/Arg) had an overall OR of 1.2 in comparison with heterozygous mutant (Arg/Pro) but this figure differs according to HPV genotype and country [22]. However, in contrary, no association was found between type of p53 allele and head and neck carcinoma [23]. In fact, the arginine allele appears to protect against head and neck cancers. Zur Hausen suggested that same virus in condylomata acuminate were found in cervical cancer and uncovered more than 95% cervical cancer contains high-risk HPV genomes. It was also found that HPV 16 were commonly identified in HPV-positive tonsillar SCC were similar to those found in cervical cancers [24].
Human leukocyte antigen (HLA), human version of major histocompatibility complex (MHC) found in many species, consists more than 200 genes located on chromosome 6 and can be divided into 3 basic groups: class I, class II and class III p [25]. Class I genes in humans consists of 3 main genes known as HLA-A, HLA-B and HLA-C. The proteins are present on the surface of almost all cells of the body and these proteins display peptides produced by the cell to the immune system. If the immune system detects the peptide as foreign, it triggers the self-destruction of the infected cell. There are 6 main MHC class II genes in humans consisting of HLA-DPA1, HLA-DPB1, HLA-DQA1, HLA-DQB1, HLA-DRA, and HLA-DRB1. They encode proteins that are present almost exclusively on the surface of certain immune system cells and have similar function to MHC class I proteins. Proteins from MHC class III involve in inflammation and other immune system activities. Studies on HLA have shown none of the alleles have consistently shown increased risk of cancer [26].
On the other hand, p16 is a tumour-suppressor gene located on chromosome 9p21 which acts as a cell-cycle checkpoint regulation. It is a reliable surrogate marker for high-risk HPV testing and p16 positivity served as a favourable prognosis in head and neck squamous cell carcinoma by increased radiosensitivity [27].
Transmission
Transmission of HPV in these cancers point to oro-genital contact and an increase in oral sex as mode of HPV transmission to oral mucosa [28]. Increased number of sex partners was noted to be a consistent risk factor as studies have shown strong association between lifetime number of sex partners and genital HPV acquisition both in women and men [11,28,29].
Interestingly, Gillison, et al. in 2012 uncovered that transmission were by casual sexual contact, more common among sexually-experienced individual rather inexperience individual. It was also reported that oral HPV was 8-fold higher among sexually active person and significantly higher with increase sexual partners. The prevalence was 3-fold higher among men might due through oral sex on women [16].
Prognosis
Clinically, HPV-positive tumours present at an earlier tumour (T) stage but more advanced nodal (N) stage and have better treatment outcomes than HPV-negative tumours [1,28]. When treated with radiation, HPV-positive OSCC have significantly higher survival rate than HPV-negative OSCC at all stages [1,30,31]. Significant differences in HPV-positive and HPV-negative head and neck squamous cell carcinoma supports the fact that HPV-positive cancers represent a tumour entity of its own that is biologically and clinically different from HPV-negative cancers [32]. HPV carcinogenesis is less frequently linked to polyploidy and have smaller tumour nuclei than cancers caused by smoking and alcohol.
HPV have also been persistently detected in post treatment HPV-positive patients irrespective of tumour site, time interval, type of treatment and presence of recurrence [33]. This suggests that the patients can still be tested post treatment whether it is post surgery, post radiotherapy or post chemoradiotherapy.
Conclusion
The analysis on HPV-related OSCC teaches us a few pearls on this matter. Incidence of HPV-related OSCC is on a rising trend with high-risk HPV showed significant relation in developing the cancer. Firm knowledge on the virus biology, pathogenesis and transmission will help us on the therapeutic strategies on them and subsequently prognosticate the disease as well.
References
- Chaturvedi AK, Engels EA, Anderson WF, et al. (2008) Incidence trends for human papillomavirus-related and unrelated oral squamous cell carcinomas in the US. Journal of Clinical Oncology 26: 612-619.
- Ryerson AB, Peters ES, Coughlin SS, et al. (2008) Burden of potentially human papillomavirus-associated careers of the oropharynx and oral cavity in US, 1998-2003. Cancer 113: 2901-2909.
- Jayaprakash V, Reid M, Hatton E, et al. (2011) Human papillomavirus types 16 and 18 in epithelial dysplasia of oral cavity and oropharynx: A meta-analysis, 1985-2010. Oral Oncol 47: 1048-1054.
- Syrjänen K, Syrjänen S, Lamberg M, et al. (1983) Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg 12: 418-424.
- Hansson BG, Rosenquist K, Antonsson A, et al. (2005) Strong association between infection with human papillomavirus and oral and oropharyngeal squamous cell carcinoma: A population-based case-control study in southern Sweden. Acta Otolaryngol 125: 1337-1344.
- International Agency for Research on Cancer (2007) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans - Volume 90- Human Papillomaviruses.
- Huertas-Salgado A, Martin-Gamez DC, Monero P, et al. (2011) E6 molecular variants of human papillomavirus (HPV) type 16: An updated and unified criterion for clustering and nomenclature. Virology 410: 201-215.
- Boulet G, Horvath C, Vanden Broeck D, et al. (2007) Human papillomavirus : E6 and E7 oncogenes. The International Journal of Biochemistry & Cell Biology 39: 2006-2011.
- Torrente MC, Ojeda JM (2007) Exploring the relation between human papilloma virus and larynx cancer. Acta Otolaryngol 127: 900-906.
- Kajitani N, Satsuka A, Kawate A, et al. (2012) Productive life cycle of human papillomaviruses that depends upon squamous epithelium differentiation. Front Microbiol 3: 1-12.
- Baseman JG, Koutsky LA (2005) The epidemiology of human papillomavirus infections. J Clin Virol 32: S16-S24.
- You J (2010) Papillomavirus interaction with cellular chromatin. Biochim Biophys Acta 1799: 192-199.
- Almadori G, Cadoni G, Cattani P, et al. (1996) Detection of human papillomavirus DNA in laryngeal squamous cell carcinoma by Polymerase Chain Reaction. Eur J Cancer 32: 783-788.
- Garcia-Milian R, Hernandez H, Panade L, et al. (1998) Detection and typing of human papillomavirus DNA in benign and malignant tumours of laryngeal epithelium. Acta Otolaryngol 118: 754-758.
- de Villiers EM, Fauquet C, Broker TR, et al. (2004) Classification of papillomaviruses. Virology 324: 17-27.
- Gillison ML, Broutian T, Pickard RK, et al. (2012) Prevalence of oral HPV infection in the United States, 2009-2010. JAMA 307: 693-703.
- Thomas M, Pim D, Banks L (1999) The role of the E6-p53 interaction in the molecular pathogenesis of HPV. Oncogene 18: 7690-7700.
- Zur Hausen H (2002) Papillomaviruses and cancer: From basic studies to clinical application. Nat Rev Cancer 2: 342-350.
- Fehrmann F, Laimins LA (2003) Human papillomaviruses: Targeting differentiating epithelial cells for malignant transformation. Oncogene 22: 5201-5207.
- Massimi P, Gammoh N, Thomas M, et al. (2004) HPV E6 specifically targets different cellular pools of its PDZ domain containing tumour suppressor substrates for proteosome-mediated degradation. Oncogene 23: 8033-8039.
- Storey A, Thomas M, Kalita A, et al. (1998) Role of a p53 polymorphism in the development of human papillomavirus associated cancer. Nature 393: 229-234.
- Jee SH, Won SY, Yun JE, et al. (2004) Polymorphism p53 codon-72 and invasive cervical cancer: a meta-analysis. Int J Gynaecol Obstet 85: 301-308.
- Sanches S, Jocelan P, Sobrinho JS, et al. (2004) Analysis of human papillomavirus prevalence and TP53 polymorphism in head and neck squamous cell carcinomas. Cancer Genet Cytogenet 150: 44-49.
- zur Hausen H (2009) Papillomaviruses in the causation of human cancers - a brief historical account. Virology 2: 260-265.
- https://ghr.nlm.nih.gov/.
- Hildesheim A, Wang SS (2002) Host and viral genetics and risk of cervical cancer: a review. Virus Res 89: 229-240.
- Fischer CA, Zlobec I, Green E, et al. (2010) Is the improved prognosis of p16 possitive oropharyngeal squamous cell carcinoma dependent of the treatment modality? Int J Cancer 126: 1256-1262.
- Timbrell R (2011) HPV-Associated oropharyngeal cancers. Radiation Therapist 20: 115-127.
- Anaya-Saavedra G, Ramirez-Amador V, Irigoyen-Camacho ME, et al. (2008) High association of human papillomavirus infection with oral cancer: a case control study. Arch Med Res 39: 189-197.
- Marur S, D’Souza G, Westra WH, et al. (2010) HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol 11: 781-789.
- O’Rorke MA, Ellison MV, Murray LJ, et al. (2012) Human papillomavirus related head and neck cancer survival: A systematic review and meta-analysis. Oral Oncol 48: 1191-1201.
- Kotb WF, Petersen I (2012) Morphology, DNA ploidy and HPV in lung cancer and head and neck cancer. Pathol Res Pract 208: 1-8.
- Morbini P, Dal Bello B, Alberizzi P, et al. (2013) Oral HPV infection and persistence in patients with head and neck cancer. Oral Surg Oral Med Oral Pathol Oral Radiol 116: 474-484.
Corresponding Author
Rohaizam Bin Jaafar, Department of Otorhinolaryngology-Head and Neck Surgery, Hospital Miri, Jalan Cahaya, Malaysia, Tel: +60-85460-600, Fax: +60-85416-514.
Copyright
© 2017 Ramalinggam G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.