Tear Electrolytes Concentration in Hospital-derived African Subjects
Abstract
A framework for research on the interactions between macro-elements of the lachrymal functional unit and the ocular surface in health and disease requires data on their physiologic values. This study measured the concentration of sodium, potassium and chloride in tear and serum of apparently healthy hospital subjects.
The measurement was from 25 female and 25 male subjects with a mean age of 52.34 ± 13.17 year. The mean concentrations of sodium, potassium and chloride in tear and serum in mMol/L were 131.06 ± 6.39, 21.40 ± 1.57 and 122.86 ± 7.12 respectively and 135.56 ± 3.85, 4.14 ± 0.70 and 101.58 ± 3.25 respectively. Potassium and chloride were found to have a statistically significant higher in concentration in tear than in serum (p < 0.001 and p = 0.001 respectively). The converse was true for sodium concentration but this was not statistically significant (p = 0.250). Age and sex had no statistically significant effect on tear electrolytes but there appeared to be a relatively good fit between age and tear potassium concentration of inverse nature (R2 = 0.57). The strongest correlation in the two body fluids was found for sodium concentration (Adjusted R2 = 0.019, F = 0.107, p = 0.745).
High potassium concentration in tear is likely protective against extraneous proximal and distal ocular tissue injury and other yet-to-be identified role(s) for electrolytes in ocular health will await further research. There is however need to standardize instruments for electrolyte analysis and a method that minimizes reflex tearing to make measurements readily reproducible.
Keywords
Tear, Serum, Electrolytes, Potassium
Introduction
Tear as an extracellular fluid is of critical importance in the maintenance of ocular surface integrity and achieving optimal vision. The tear film over the ocular surface exists in a 3-layer sub-structure known as the precornea tear film. The outermost lipid layer retards tear evaporation, prevents tear overflow and minimizes friction during eye blinking. An aqueousmid-layer accounts for about 90% of tear film volume and retains the capacity for further volumetric expansion in response to any form of ocular irritation. Beyond its lavage function, the aqueous component is an immunologically-competent medium, antagonizing pathogenic microbes and neutralizing toxins with its array of immuno-modulators [1,2]. The innermost mucin layer ensures an even spread of the aqueous component over the ocular surface. Refraction of light over the ocular surface is most efficient with a uniformly-spread precornea tear film. At least 70% of light refraction within the optical system of the eye is achieved at the air-precornea tear film interphase. In the absence this, the eye will be significantly under-powered with an attendant high hypermetropia [3].
Tear secretion is controlled by the lacrimal functional unit (LFU) which is an integrated system comprising the lacrimal glands, ocular surface (cornea, conjunctiva and meibomian glands) and lids, and the sensory and motor nerves that connect them [4]. Tear fluid contains water, electrolytes, proteins, carbohydrates, lipids and a complex combination of the afore-mentioned macromolecules. The multi-lobular lachrymal gland is the major source of tear and is populated byacinar, ductal, and myoepithelial cells. The acinar cells are the predominant cell type and are responsible for primary tear production. The ductal cells modify the primary tear by absorbing or secreting water and electrolytes as it flows though the lachrymal duct to the outflow orifices. The final electrolyte concentration in tear is therefore determined by ductal cells [5-7].
'The major electrolytes found in tear are sodium, potassium, calcium and magnesium and the corresponding anions chloride and bicarbonate. Tear is not necessarily an ultrafiltrate of blood because of the extensive modification by the lachrymal cells. Studies have shown that tear have higher potassium and chloride concentration in comparison to serum while its protein and sugar content, like other extracellular fluids, falls short of the concentration found in blood [8-11]. As essential components of tear fluid, electrolytes regulate tear pH, stabilize the tear film and contribute to the maintenance of cornea epithelial thickness. The bicarbonate ion (HCO3-) buffers tear to keep the pH within physiologic limits needed for optimal ocular comfort [12].
Tear osmolality is the number of solutes in one liter or kilogram of tear. Electrolytes in tear essentially determine tear osmolality because they are found in much higher concentrations than other dissolved macromolecules such as proteins and sugars. Tear film stability is related to tear osmolality [13]. Abnormalities in tear osmolality therefore results in tear film instability and precedes the development of dry eye disease (DES), one of the commonest ocular conditions affecting up to 5-50% of the global population [14].
Potassium ion has been shown to play an important role in maintaining cornea epithelial thickness [15,16]. Cornea epithelial cells are the primary cells that absorb Ultraviolet light in the wavelength range of 290-315 nM (UVB) [17]. UVB radiation is a component of solar radiation and has been assigned a role in the etiopathogenesis of a wide range of ocular conditions spanning from the anterior to the posterior segments. These disease conditions include pterigium, pingueculum, climatic droplet keratopathy, ocular surface squamous neoplasia (OSSN), cataract and glaucoma through both trabecular meshwork injury and via direct retinal ganglion cell toxicity [18-22].
Tear as a source of biomarker in ocular and systemic conditions is currently increasingly being researched given that it can be accessed in a non-invasive manner. Hagan et al did an excellent review of current pursuits in the field of proteomics as a means of unraveling the presence of biomarker proteins in tear in various disease states [23]. Brain-derived neurotrophic factor (BDNF) have been shown to be low in tear of glaucoma patients [24]. Hypertonicity of tear as indicated by its osmolality is the most consistent objective observation seen in patients with DED [25,26]. Recent studies have suggested that measurement of certain tear electrolytes could be relevant in the assessment of diabetic patients [27,28]. The major cations in tear are sodium and potassium while chloride is the most abundant anion. In this study, these ions will be measured as an initial step in an effort to generate baseline data for their concentration in this important fluid in an African population where apparently none currently exist. For purposes of comparison and because tear is partially ultra-filtrated from blood, the serum electrolytes of the subjects will also be measured.
Study Design
This was an observational cross-sectional study of consecutive and eligible adult subjects aged 30 years and above who presented to the eye clinic of Enugu State University teaching hospital, Parklane, Enugu from December 1st 2015 to February 27th 2016. Enugu is a cosmopolitan city located on latitude 6° 27' 35.8704'' N and longitude 7° 32' 56.2164'' E south-eastern Nigeria with about 722,664 inhabitants [29].
Subjects
A total of 50 patients who fulfilled our inclusion criteria were recruited for the study. The subjects were recruited on the basis of presenting with symptoms of refractive error and/or presbyopia only.
Subjects aged less than 30 years, presence of inflammatory or ocular surface disease, use of potassium supplement, metabolic or chronic systemic disease and pregnancy were exclusion criteria [30,31]. Absence of diabetes mellitus was further excluded with a fasting or random blood sugar using Accu-ChekR Active (Roche) glucometer. Subjects with fasting blood sugar of 120 mg% and above or random blood sugar of 180 mg% or above were excluded [32]. Subjects with glaucoma were equally excluded because they are subject of a related study by the same authors.
Demographic and medical histories of participants were filled using a standardized questionnaire (Appendix 1). Comprehensive ocular examination was carried out on all subjects after documenting their visual acuity and examination with a slit lamp biomicroscope (Haag Streit BM900). Intraocular pressure (IOP was) measured with Haag Streit Goldmann R-type applanation tonometer. The posterior pole with emphasis on the optic nerve head was examined with a +78D super field aspheric lens by Volk R (U.S.A) to exclude glaucoma.
Sample Collection and Analyses
All samples were taken between 10 am and 12 pm to minimize the effect of diurnal variation in electrolyte composition [33]. In order to ensure uniformity, all the participants were subjected to the same instruments, in the same room by the same examiner during the clinical data acquisition all through the study. Four milliliters of venous blood sample was collected from the antecubital vein with a sterile 5 ml hypodermic needle and syringe and transferred to a sterile plane glass tube. The blood was allowed to stand for 30 minutes to clot and was centrifuged with Techmel TM centrifuge (USA) at a speed of 5000 revolutions/minute to yield a supernatant serum. 200 ul of tear sample was collected from the inferior lateral tear meniscus of the ocular surface with a sterile 50 ul plane capillary tube over four collections and emptied into a sterile plane glass test tube [34]. Basal tear collection is difficult in practice, even from anesthetized cornea. However, the difference in basal and reflex tearing is appears to be mostly in its protein content [35]. Tilting the head facilitates tear collection from the lateral canthal area. Both serum and tear samples were analyzed as native samples using ST- 100 B electrolyte analyzer by Sensa Core R, Hyderabad India, an automated electrolyte analyzer which operates on the principle of direct ion selective electrode (ISE). All results from the electrolyte analyzer were included and no measurement was removed as an outlier during analysis.
Data Management
The measured variables were presented in tables, chart and prose. Data analysis was done with SPSS version 16 (SPSS, Inc, Chicago, USA). Bivariate analysis was conducted using cross- tabulations and 2-tailed t-test was used to evaluate associations between means of tested variables. A linear regression was done to investigate the trend within categorical variables. A p-value < 0.05 was considered statistically significant. Diagnostic accuracy test was performed to determine sensitivity and specificity of the test.
The tenets of the Helsinki Declaration regarding the conduct of research involving human Subjects were observed36. Informed verbal consent was duly obtained from all the participants [36] (Appendix 2).
Ethical clearance was obtained from Health Research Ethics Committee of Enugu State University Teaching Hospital, Park lane, Enugu (Appendix 3).
Results
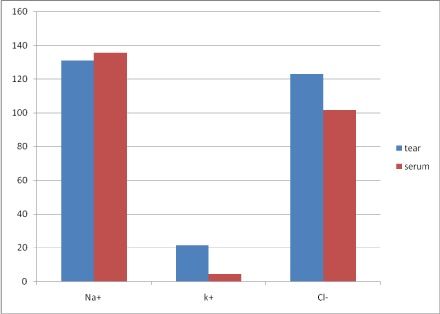
Measurements from 25 females and 25 males were taken. The mean age of the subjects was 52.34 ± 13.17 year. The mean concentrations of sodium, potassium and chloride in tear and serum in mMol/L were 131.06 ± 6.39, 21.40 ± 1.57 and 122.86 ±7.12 respectively and 135.56 ± 3.85, 4.14 ± 0.70 and 101.58 ± 3.25 respectively. These values are depicted in Table 1. A comparison in difference of mean between electrolytes in the two body fluids showed that potassium and chloride were significantly of higher concentration in tear than serum (p < 0.001 and p = 0.001 respectively) while a higher concentration of sodium in serum was observed but this was not statistically significant (p = 0.250)- Table 2. These are depicted in the bar chart shown in Figure 1.
Age as a predictor of electrolyte concentration was not statistically significant (p > 0.05) but there is a relatively good fit between age and tear potassium concentration of inverse nature (R2 = 0.57) - Table 3. Sodium and chloride concentrations in both serum ([Na+] = 136.42, [Cl-] = 101.81) and tear (([Na+] = 132.38, [Cl-] = 124.08) were higher in female subjects compared to males in both fluids (([Na+] = 134.63, [Cl-] = 101.33 and [Na+] = 129.63, [Cl-] = 121.54 respectively) - Table 4. However, males had higher concentrations of potassium in serum ([K+] = 4.21 versus [K+] = 4.07]) and tear ([K+] = 21.80 versus [K+] = 21.04). None of these observed differences was statistically significant- Table 4. In view of the relationship between tear production and circulating blood, a linear correlation using serum ions concentration to predict tear equivalents was done and the strongest correlation was found for sodium (Adjusted R2 = 0.019, F = 0.107, p = 0.745) which is shown in Table 5. Table 6 is a comparison between this study and studies done in other regions in Nigeria.
Discussion
The concentration of major cations and anion namely sodium [Na+], potassium [k+] and chloride [Cl-] found in tear and serum of 50 of subjects aged 30 years and above who had no evidence of ocular surface nor inflammatory eye disease were measured. The average age of the subjects was 55.2 years. The subjects were equally matched for sex with 25 males and 25 females (Table 1). The mean concentrations of sodium potassium and chloride in tear (mmMol/L) were 131.06, 21.40 and 122.86 respectively. This is comparable to values obtained by other authors including Rismondo, et al. who reported a mean potassium ion concentration of 17.0 mmMol/L in tear of laboratory rats [7,8,37]. Potassium ion concentration was also found to rise to about fifty percent (50%) above baseline when laboratory rats were subjected to intense pilocarpine-induced stimulation of its lachrymal gland in one study [37]. This increase did not affect sodium and chloride concentrations. Thaysen and Thorn using the same agent in human subjects, failed to demonstrate any significant increase in potassium concentration in tear [7]. A global paucity of data analyzing tear electrolyte behavior under different conditions have not allowed for a consensus position in potassium concentration changes during basal and reflex tearing. There is a need for further research to resolve this lingering research question. Overall, no statistically-significant sex or age-related changes were observed but a modest reduction in potassium tear concentration with increasing age was seen (p = 0.082). Apparently, no equivalent study has been reported in literature to allow for comparison but indirect evidence using tear osmolarity suggests that increasing age as well as female sex are significant risk factors for increased electrolytes concentration leading to hypertonicity and subsequent development of DED [29-34]. The absence of this trend in the present study might be attributed to the stringent exclusion criteria.
Serum concentration of sodium, potassium and chloride (mMol/L) were 135.56, 4.14 and 101.58 respectively. There is little agreement with other studies done within the country where all appeared uniquely different except for the data from Maanan, et al. which appeared similar to the findings in this study (Table 6). These wide variations underscore the need for global standardization of method and instruments employed in the measurement of serum electrolytes. The multiplicity of the testing algorithms also calls into question the validity of reference values in our various centers.
Sodium ion concentration in tear closely resembled its concentration in serum though in a lower concentration, 131.06 and 135.56 respectively (p = 0.42). It does seem that sodium in tear is ultra-filtrated from blood. This is further buttressed by the fact that a binary regression of serum electrolytes as a predictor of tear electrolytes showed that sodium and tear electrolytes had the best fit (adjusted R2 = 0.019) and a commensurate high p-value (p = 0.75).
The findings for potassium and chloride ion concentrations in tear and serum is remarkably different. Tear have about 5 times higher concentration of potassium than serum, 21.40 and 4.14 mmol/L respectively (p < 0.001). chloride is also significantly higher in tear when compared to serum, 122.86 and 101.58 respectively (p = 0.001). These concentration disparities have been documented in other studies, even with different measuring algorithms [15,16]. It has been suggested that there is an active secretion of K+ and Cl+ into tear fluid by apical acinar cells of the lachrymal gland [5,6]. Studies have shown that high tear [K+] is essential in maintaining a proper cornea epithelial thickness which in turn prevents both proximal and distal UVB-induced cell injury [15-17]. The significance of lower [Na+] in tear compared to serum is unclear but it might be a compensatory mechanism to offset the high [K+]. Further studies are needed to clarify this relationship.
Strenghts and Limitations of the Study
The native samples were used to avoid any confounding influence of a diluting fluid. All the clinical assessments were done by the same person and all the laboratory tests were also carried out by the same laboratory scientists without sample storage and using the same instrument. However, only single tests were carried out for each patient considering the time needed to collect adequate tear sample. The presence of co-existing morbidity cannot be entirely ruled out. Reflex tearing cannot be totally avoided and its effect on measured variables is unknown.
Conclusion and Recommendations
Data on the concentration of the major electrolytes in an important extracelluar fluid such as tear is important considering its known and putative roles in ocular health and disease. Such knowledge can form the basis for further studies on the possible role of electrolytes in the etiopathogenesis of several ocular and systemic conditions and may offer alternative therapeutic options. It is however important that instruments and methods for such measurements are standardized to achieve reproducible outcomes. Such approach should minimizing reflex tearing. The physiologic effects of reflex tearing on tear electrolyte concentration deserves further research considering conflicting extant opinions. Data derived from a population-based study using treatment-naïve eyes could provide a better assessment of the tested parameters.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise in the course of this study. No grant was received for the research.
Acknowledgement
We wish to thank the entire academic staff of Department of Medical Biochemistry for their invaluable advice at one point or the other. We also want to thank Mr. Francis Onyedikachi Eneh for assisting in the statistical analysis.
References
- Zierhut M, Dana MR, Stern ME, et al. (2002) Immunology of the lacrimal gland and ocular tear film. Trends Immunol 23: 333-335.
- Huang LC, Jean D, Proske RJ, et al. (2007) Ocular surface expression and in vitro activity of antimicrobial peptides. Curr Eye Res 32: 595-609.
- Albarran C, Pons AM, Lorente A, et al. (1997) Influence of the tear film on optical quality of the eye. Contact Lens Anterior Eye 20: 129-135.
- Stern ME, Beuerman RW, Fox RI, et al. (1998) The pathology of dry eye: The interaction between the ocular surface and lacrimal glands. Cornea 17: 584-589.
- Mircheff AK (1989) Lacrimal fluid and electrolyte secretion: A review. Curr Eye Res 8: 607-617.
- Alexander JH, VanLennep EW, Young JA (1972) Water and electrolyte secretion by the exorbital lacrimal gland of the rat studied by micropuncture and catheterization techniques. Pflügers Archiv 337: 299-309.
- Yoshimura H, Hosokawa K (1963) Studies on the mechanism of salt and water secretion from the lacrimal gland. Jpn J Physiol 15: 303-318.
- Rismondo V, Osgood TB, Leering P, et al. (1989) Electrolyte composition of lacrimal gland fluid and tears of normal and vitamin A-deficient rabbits. CLAO J 15: 222-228.
- Best LJ, Hendrix DV, Ward DA (2015) Tear film osmolality and electrolyte composition in healthy horses. Am J Vet Res 76: 1066-1069.
- Apweiler R, Haab BB, Simpson RJ, et al. (2005) Overview of the HUPO Plasma Proteome Project: Results from the pilot phase with 35 collaborating laboratories and multiple analytical groups, generating a core dataset of 3020 proteins and a publicly-available database. Proteomics 5: 3226-3245.
- Aass C, Norheim I, Eriksen EF, et al. (2015) Single unit filter-aided method for fast proteomic analysis of tear fluid. Anal Biochem 480: 1-5.
- Carney LG, Mauger TF, Hill RM (1989) Buffering in human tears: pH responses to acid and base challenge. Invest Ophthalmol Vis Sci 30: 747-754.
- Lemp MA, Bron AJ, Baudouin C, et al. (2011) Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol 151: 792 -798.
- Stapleton F, Alves M, Bunya VY, et al. (2017) TFOS DEWS II Epidemiology Report. Ocul Surf 15: 334-365.
- Bachman WG, Wilson G (1985) Essential ions for the maintenance of the corneal epithelial surface. Invest Ophthalmol Vis Sci 26: 1484-1488.
- Green K, MacKeen DL, Slagle T, et al. (1992) Tear potassium contributes to maintenance of corneal thickness. Ophthalmic Res 24: 99-102.
- Kolozsva'ri L, No'gra'di A, Hopp B, et al. (2002) UV Absorbance of the Human Cornea in the 240- to 400-nm Range. Invest Ophthalmol Vis Sci 43: 2165-2168.
- Moran DJ, Hollows FC (1984) Pterygium and ultraviolet radiation: A positive correlation. Br J Ophthalmol 68: 343-346.
- Taylor HR, West SK, Rosenthal FS, et al. (1989) Corneal changes associated with chronic ultraviolet radiation. Arch Ophthalmol 107: 1481-1484.
- Bergbauer K, Kuck J, Su K, et al. (1991) Use of an UV-blocking contact lens in evaluation of UV-induced damage to the guinea pig lens. International Contact Lens Clinic 18: 182-187.
- Ringvold A (1996) The significance of ascorbate in the aqueous humour protection against UV-A and UV-B. Exp Eye Res 62: 261-264.
- Noell WK, Walker VS, Kang BS, et al. (1966) Retinal damage by light in rats. Invest Ophthalmol 5: 450-473.
- Hagan S, Martin E, Enríquez-de-Salamanca A (2016) Tear fluid biomarkers in ocular and systemic disease: potential use for predictive, preventive and personalised medicine. EPMA J 7: 15.
- Ghaffariyeh A, Honarpisheh N, Shakiba Y, et al. (2009) Brain-derived neurotrophic factor in patients with normal tension glaucoma. Optometry 80: 635-638.
- Farris RL (1994) Tear osmolarity-a new gold standard? Adv Exp Med Biol 350: 495-503.
- Bron AJ, Tomlinson A, Foulks G, et al. (2014) Rethinking dry eye disease: A perspective on clinical implications. Ocul Surf 12: S1-S31.
- Wang S, Hou X, Liu Y, et al. (2013) Serum electrolyte levels in relation to macrovascular complications in Chinese patients with diabetes mellitus. Cardiovasc Diabetol 12: 146.
- Okukpon J, Okukpon O (2019) Tear electrolyte assessment of diabetic patients in Southern Nigeria. Afr Health Sci 19: 2839-2845.
- National Population Commission (NPC) Nigeria national census: Population distribution by sex, state, lgas and senatorial district: 2006 Census Priority Tables.
- Khan RN, Saba F, Kausar SF, et al. (2019) Pattern of electrolyte imbalance in Type 2 diabetes patients: Experience from a tertiary care hospital. Pak J Med Sci 35: 797-801.
- Atherton JC, Dark JM, Garland HO, et al. (1982) Changes in water and electrolyte balance, plasma volume and composition during pregnancy in the rat. J Physiol 330: 81-93.
- (2009) International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 32: 1327- 1334.
- Böning D, Schweigart U, Kunze M (1974) Diurnal variations of protein and electrolyte concentrations and of acid-base status in plasma and red cells of normal man. Europ J Appl Physiol Occup Physiol 32: 239-250.
- Choy CK, Cho P, Chung WY, et al. (2001) Water-soluble antioxidants in human tears: Effect of the collection method. Invest Ophthalmol Vis Sci 42: 3130-3134.
- Jordan A, Baum J (1980) Basic tear flow: Does it exist? Ophthalmology 87: 920-930.
- (2000) World Health Organisation Declaration of Helsinki. Ethical principle for medical research involving human subjects. JAMA 284: 3043-3045.
- Thaysen JH, Thorn NA, Schwartz IL (1954) Excretion of sodium, potassium, chloride and carbon dioxide in human parotid saliva. Amr J Physiol 178: 155-159.
Corresponding Author
Ezekiel N Ekweremadu, Department of Ophthalmology, College of Medicine, Enugu State University of Science and Technology, Enugu, Nigeria.
Copyright
© 2021 Ekweremadu EN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.