Translational NIRS: Parallel Alteration of Brain Metabolism Following Alcohol Intake in Rodents and Man
Abstract
Near-infrared spectroscopy (NIRS) provides a non-invasive, non-ionizing means to monitor total haemoglobin concentration and oxygen saturation in the living tissue allowing monitoring oxygen sufficiency. Main applications of NIRS are in the study of the transport of the oxygen to the muscles, the cellular metabolism and the cerebral haemodynamics. Fewer NIRS studies have been performed at the Central Nervous System (CNS), mainly to monitor oxygen sufficiency, brain functions and diseases.
Recently, the NIRS methodology has been set to monitor the brain of rodents and then confirmed to be apt to analyzing non-invasively and in real time the influence of systemic pharmacological treatments upon brain metabolism. Furthermore, it has been proven able to determining penetration of drugs into the brain.
Alcohol is the second most widely abused psychoactive drug after caffeine. Thus, in this work, a novel translational medicine approach has been undertaken i.e. the influence of alcohol on brain levels of NIRS parameters is monitored either in rodents as well as in humans.
The present NIRS data show that administration of alcohol to rodents as well as to man is resulting in modified levels of the NIRS parameters and in particular significant decrease of oxygenated hemoglobin (HbO2).
Altogether they indicate that NIRS could be applied to study neurobiological processes and psychiatric diseases in preclinical and also in a translational strategy from preclinical to clinical investigations.
Keywords
Near-infrared spectroscopy (NIRS), Alcohol, Brain, Rodents, Humans
Introduction
Near-infrared spectroscopy (NIRS) is a relatively new methodology that provides a non-invasive, non-ionizing means to monitor total haemoglobin concentration and oxygen saturation in the living tissue. NIRS has been introduced since about four decades, when in 1977, F Jobsis presented his key work that established the technique allowing monitoring oxygen sufficiency [1]. Since then, it has been greatly improved [2,3].
The technique founds itself on the use of harmless radiations, which have wavelength in the spectral range of the near infrared (650-1000 nm). It depends on the relatively good transparency of biological tissue in the near-infrared range, which allows for transmission of photons through the tissue, so that they can be detected at the exit from the tissue. In particular, oxygenated hemoglobin (HbO2) and deoxy-hemoglobin (Hb) are the dominant absorbing elements between 700 nm and 1,000 nm, and the transmission of light is relatively unaffected by water in the same region. Thus, the near-infrared region of the spectrum is the most favorable to the optical measurement of these parameters that is done by radiating near-infrared light from light source(s) into the biological tissue and by measuring changes in the amount of light reaching the detector(s). Thus, changes in absorption in the area of the body analyzed can be detected. In such way, NIRS provides a non-invasive, non-ionizing means to monitor total haemoglobin concentration (HbO2 + Hb) that is considered as total blood volume (HbT or V) [4], as well as oxygen saturation in the living tissue. All together, these measurements are indicative of the state of vascular activity and the state of the metabolism in the tissue analyzed. Thus, NIRS has been proposed as another non-invasive method to study tissue haemodynamics. Furthermore, NIRS is relatively simple and inexpensive (even if that is not always considered as a "plus" by some) compared to more "dignified" techniques such as functional magnetic resonance imaging (fMRI) and positron emission tomography (PET).
Generally, main applications of NIRS are in the study of the transport of the oxygen to the muscles, the tissue oxygenation index, the cellular metabolism and the cerebral haemodynamics.
In clinical studies NIRS is currently employed to monitor fetal hypoxemia [5,6] and in newborn infants to detect birth asphyxia and/or apnoea and hypoxia [7-10]. In addition, changes in oxygenation during occlusion of the carotid artery, or during cardiopulmonary bypass, circulatory alterations and/or arrest are also analyzed using NIRS [11-13].
Besides fewer NIRS studies have been performed at the Central Nervous System (CNS) level, mainly to monitor oxygen sufficiency and brain functions and/or brain mapping [14-16] as well as brain diseases [17-20] while only a limited amount of studies have used NIRS to analyze "vascular CNS" functions in animals. In order to implement such type of analysis, we have developed a Near Infrared Continuous Wave Spectroscopy instrument (NIR-CWS), based on the low extinction coefficient of tissue in the near infrared region [21,22] that allows in vivo, real time non-invasive NIRS measurements in the rat brain [23-25].
In a recent work, the NIRS methodology is confirmed to be apt to analyzing non-invasively and in real time the influence of systemic pharmacological treatments upon brain metabolism. Moreover, in vivo PK/PD-NIRS parallel experiments performed with various classes of chemicals supported the feasibility of a comparable evolution of in vivo PK/PD values with NIRS values. Indeed, the observed parallel evolution of NIRS data and blood levels of the compounds in time indicated that there might be a relationship between brain penetration and brain metabolism and that this can be monitored in real time by NIRS [26]. Thus, non-invasive NIRS allows determining penetration of drugs in brain and therefore could be used to study neurobiological processes and psychiatric diseases in preclinical but also in a translational strategy from preclinical to clinical investigations [2,3].
The aim of this study was indeed to verify this possibility via direct comparison of data gathered from NIRS analysis of rodents and from humans during alcohol intake.
Material and Methods
NIRS apparatus
The schematic configuration of the NIRS system consists of four main blocks: the optical head, the emitter unit, the receiver unit, and the control unit, which includes the personal computer (see Scheme 1).
The optical head is placed and fixed onto the surface of the tissue under test in order to monitor non-invasively the oxygenation. These boards are connected to a laptop PC. The front-end electronics has been designed to maximize the signal to noise ratio, to reject continuous and alternate ambient light, and to reduce the effects of artifacts induced by probe movements. It consists of two channels including an instrument amplifier, a low-pass filter, and a time-variant gated integrator.
For each illumination light pulse generated by the laser diodes, i.e. a single wavelength and single spatial injection point, the receiver unit produces two voltage signals proportional to the excitation and back-diffused light intensity respectively. The system bandwidth is selectable within the range 2.3-27 Hz, i.e. a maximum of 20 channels (five chromatic and four spatial channels) can be acquired 27 times for each measuring second, whereas the amplification can be set to measure optical density ranging from 3.5 to 8.5.
One of the channels, named the reference channel, processes the reference signal generated by the optical source, whereas the other channel, named the measuring channel, processes the voltage signal generated by the photodetector.
Algorithm for the 'differential path-length factor' (DPF) quantification
DPF is the correction factor needed to take into account the modification of the optical path that photons take due to scattering phenomena. We developed an algorithm for the quantification of the DPF on the basis of the water peak absorption algorithm. This algorithm allows the calculation of absorption and reduced scattering coefficients of the medium analyzed. Briefly, three mathematical steps define this algorithm: diffusion equation, analytical solution for the examined medium and extraction of the optical parameters of the medium [27].
Once the optical parameters were obtained, the DPF value was calculated by applying the following equation:
where D = 1/3μ_ is the diffusion coefficient and ρ is the source-receiver geometric distance. The DPF value at different wavelengths was then taken into account in the modified Lambert-Beer law [28], allowing to obtain changes in the concentrations expressed in μmol/L.
The same NIRS apparatus can be used for both human and rodents analysis as the settings can be selected based on the parameters shown in Table 1 and that have been established in previous applications (see ref. [21-26]).
Experimental
Two groups of four adult male rats (230-250 g) each were supplied by Charles-River (Italy) and were kept in temperature and humidity-controlled rooms (22 ℃, 50%, respectively) with lights on from 07.00 h to 19.00 h with water and food available ad libitum. All procedures concerning experimentation, transportation and care of the animals were carried out in accordance with the Italian law (Legislative Decree no.116, 27 January 1992), which acknowledges the European Directive 86/609/EEC, and were fully compliant with GlaxoSmithKline policy on the care and use of laboratory animal and codes of practice. Furthermore, all efforts were made to minimize the number of animals used and their suffering.
These two groups of animals were prepared for NIRS analysis as described previously: briefly each rat was anesthetized using urethane (1.4 g/kg ip) and placed on a stereotaxic apparatus (D. Kopf, USA). Then the input system (four optic fibers, 200 μm diameter each) and the receiver system were both firmly placed using a stereotaxic micromanipulator against the surface of the rat's head, close to the sagittal line without any surgery, as shown in Scheme 2. The stereotaxic was then fully covered so that to protect the measurements from the light.
Then, in order to evaluate the sensitivity of NIRS measurements to exogenous oxygen capability and therefore quantify the eventual modification of the NIRS parameters each one of the 8 rats was supplied with pure O2 (0.5 bar, 1 min) via a tubing positioned into the animal mouth.
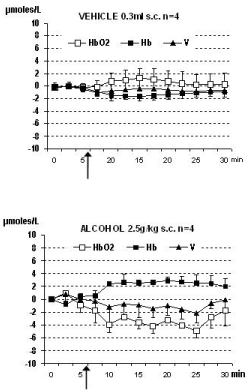
Subsequently, rats were treated with vehicle (water, 1 ml/kg s.c., control group, n = 4) or alcohol (2.5 g/Kg n = 4), respectively.
In parallel, three human male volunteers (aged 26-28) not alcohol drinkers were prepared for NIRS measurements: i.e. they were wearing a cap in which the optic fibers and the receiver were embedded so that external light cannot influence the measurements. The optical components were positioned in order to involve the prefrontal region of the brain accordingly to previous work indicating this area among the most sensitive to alcohol [29]. See Scheme 2.
The three men were offered a single drink of water (control treatment) followed 5 min later with the ingestion of 150 ml alcohol 12%. The alcohol amount corresponds to approximately 0.25 g/Kg for 70 Kg weight. This dosage was selected the day before the NIRS experiment as it was corresponding to the minimal dosage of alcohol that was giving rise to subjective feeling (sensitivity) to each one of the not drinkers men in condition of empty stomach. The NIRS test was taking place the following day at approximately 11 am. i.e. the time at which was performed the NIRS analysis in the anesthetized rodents.
Ethical considerations concerning the three volunteers
Ethical clearance and permission was obtained from the Ethical Review Committee of Public Health and Medical Sciences. Data were collected from the participants after getting informed consents. All the information obtained in due time were kept confidentially.
Top left: Scheme of the positioning of the receiver R and of the laser sources S1-S4: placed at about 1 mm aside from the sagittal line and between bregma and lambda of the rat [30].
Top right: In vivo non-invasive NIRS measurements in man. Scheme of the positioning of the receiver R and of the laser sources S1-S4 deeply embedded in a cap to avoid influence of external light.
Bottom: Theoretical brain areas monitored in the rat brain: computer simulation of photon paths based on photon migration theory The red area indicate the delimited region monitored i.e. by the second source only [31,32].
Statistics
Statistical analysis have been performed using Statistica 6. Row data were subjected to ANOVA, with comparison between "control" (vehicle) and "treatment" values performed using the Bonferroni (Dunn's) test. Results are presented as µmoles/L, mean ± s.e.m., *p < 0.05, **p < 0.001.
Results
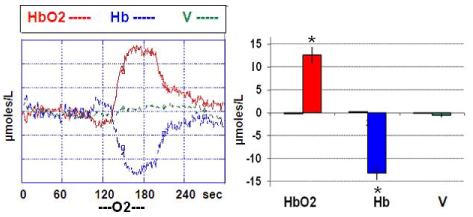
It appeared that pure O2 supply was increasing significantly HbO2 levels from steady state baseline (considered as zero) up to approx. + 13 μmoles/L while significantly decreasing Hb to a similar (negative) extent. This effect was reversible as soon as the influx of O2 is stopped.
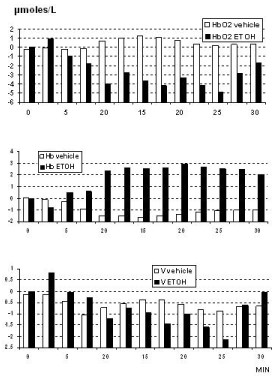
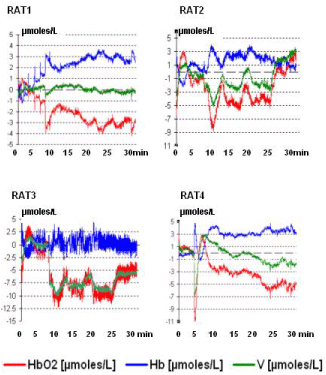
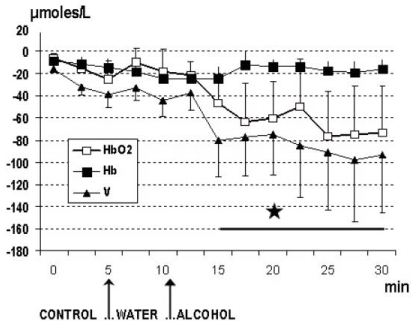
Similar data were obtained in all the animals and they are shown in Figure 1. Furthermore, in the rodents the treatment with a single dose of alcohol was followed by significant change of the NIRS parameters when compared to NIRS data collected from the control group of rats treated with vehicle (see Figure 2). In particular a significant, constant decrease of HbO2 levels from steady state baseline (that is considered = zero μmoles/L value) was detected together with a similar change of total volume (V). In contrast, increase of Hb values was monitored. See Figure 2, Figure 3 and single traces of each one of the alcohol treated rats in Figure 4.
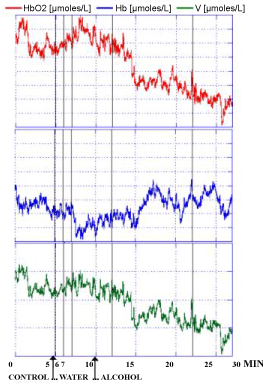
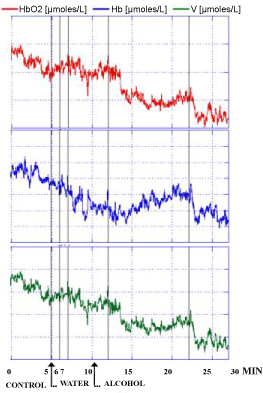
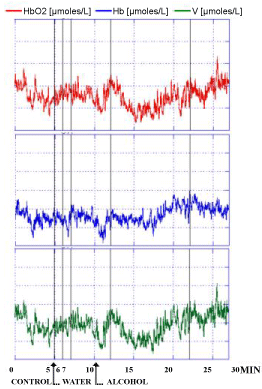
Similar evolution of the NIRS parameters were observed in the 3 human volunteers after the intake of alcohol at a dose at which each one of the 3 people was preliminary feeling subjective sensibility i.e. approximately 0.25 g/kg for 70 Kg weight. Indeed, this dose was shown able to modulate locomotor activity in rodents and in particular in rats that are exhibiting increased spontaneous motor activity already after doses of 0.25 g/kg [33,34]. In particular a significant decrease of HbO2 and V levels were monitored. In contrast the intake of water was not changing significantly NIRS values when compared to the control period of the recordings as shown in Figure 5 and see the single traces for each subject in Figure 6, Figure 7 and Figure 8, respectively. Specifically in these traces the first 5 min of recordings were considered as period of stabilization of the signals, so that the control period was starting at time 5 min.
Discussion
Alcohol is the second most widely abused psychoactive drug after caffeine. In 1990, the American Medical Association formally recognized alcohol abuse as pathology. In the aim to develop new drugs for the treatment of alcohol abuse, recent studies have shown that ethanol interacts with several central neurotransmitter systems, such as:
• The GABAergic system activity via increasing the number of GABA receptors [35,36].
• The dopaminergic system: resulting either in increase [37,38] or decrease [39] and for a review see [40].
• The serotoninergic system that seems to play a role in the control of ethanol intake [39,41].
• The CCK system with the evidence of the development of preference for ethanol in naïve rats when treated with CCK receptor antagonists [42,43].
• The NO system: where ethanol, at pharmacologically significant doses, strongly inhibits striatal NO• production and release apparently through inhibition of NMDA receptor function. Inhibition of NMDA receptor-mediated activation of the NO• pathway could be a primary neurobiological mechanism contributing to the effects of ethanol [44].
In the present work, a novel translational medicine approach has been undertaken i.e. the influence of alcohol on brain levels of HbO2 is monitored either in rodents as well as in humans. In particular, NIRS data monitored in rats receiving alcohol indicate significant decreased levels of HbO2 and blood volume V and increase of Hb levels. This may indicate a possible direct influence of alcohol on brain metabolism via alteration of blood flow and haemoglobin levels. Similarly, in the 3 male volunteers receiving alcohol, a significant decrease of HbO2 and V was also observed, supporting the observations performed in rodents and confirming the influence of alcohol on brain metabolism also in men. This is in accord with PET [45,46] and SPECT [47,48] studies in man that have confirmed and extended earlier findings that the prefrontal regions are particularly susceptible to decreased metabolism in alcoholic patients. The decrease in whole brain metabolism is in accord with former PET work viewing consistent decrease in CBF in alcoholics [45,46].
In addition, a salient finding in alcohol-dependent patients is the atrophy detected in these areas, and this is significantly correlated with the degree of hypometabolism in the medial frontal area of the cerebral cortex and therefore with the severity of the clinical neurological impairment [47]. In particular, in parallel to a decreased frontal lobe glucose utilization, emission computed tomography have reported a reduced cerebral blood flow (CBF). Subsequent neuropsychological studies have shown that there are specific deficits in alcoholism that suggest frontal lobe dysfunction and propose the concept of frontal lobe pathology in alcoholism [48].
Accordingly, the present data are detecting reduced levels of cerebral HbO2 in such region following alcohol intake, hence decreased amount of O2 available for metabolic purposes.
In addition, evidence for the inhibitory action of ethanol on haemoglobin synthesis have been proposed in in vitro studies suggesting that the effect is mediated through inhibition of haemin synthesis at a site influenced by pyridoxine [49]. Indeed, haemin is necessary for maximal protein synthesis in intact reticulocytes, therefore alcohol is a direct toxin to developing red cell precursors via its effect on mitochondrial haem synthesis [50]. Altogether these data underline the possibility of an altered condition of red cells following alcohol intake which can influence negatively blood efficacy and blood flow including CBF.
During the past three decades, a number of researchers have used near-infrared spectroscopy (NIRS) and, more recently, functional NIRS (fNIRS) to produce novel insights about the neural mechanisms underlying cognitive and perceptual functions [51,52]. Moreover, this recently developed technique is non-invasive, easy-to-use, portable, restraint-free, and replicable [53]. Consequently, it is relatively psychologically undemanding, which makes it advantageous for clinical applications and translational approaches [54]. The present results performed with NIRS both in rodents and man support NIRS as a valuable tool for analysis of brain metabolism and its reliable efficacy on direct, rapid translational studies from animals to man.
Acknowledgment
Acknowledgment to Francesco Congestri' and Maurizio Donini for technical support.
References
- Jobsis FF (1977) Non-invasive infrared monitoring of cerebral and myocardial sufficiency and circulatory parameters. Science 198: 1264-1267.
- Obrig H (2014) NIRS in clinical neurology - a 'promising' tool? Neuroimage 85: 535-546.
- Piper SK, Krueger A, Koch P, et al. (2014) A wearable multi-channel fNIRS system for brain imaging in freely moving subjects. Neuroimage 85: 64-71.
- Chia-Wei Sun, Ching-Cheng Chuang (2012) Hemodynamics Study Based on Near-Infrared Optical Assessment. In: A Seda Artis, Hemodynamics - New diagnostic and therapeutic approaches. InTech pub, 47-89.
- Aldrich CJ, Wyatt JS, Spencer JAD, et al. (1994) The effect of maternal oxygen administration on human fetal cerebral oxygenation measured during labour by near infrared spectroscopy. Br J Obstet Gynaecol 101: 509-513.
- Douglas-Escobar M, Weiss (2015) Hypoxic-ischemic encephalopathy: A review for the clinician. JAMA Pediatr 169: 397-403.
- Brazy JE, Lewis DV, Mitnick MH, et al. (1985) Noninvasive monitoring of cerebral oxygenation in preterm infants: Preliminary observations. Pediatrics 75: 217-225.
- Wyatt JS, Cope M, Delpy DT, et al. (1986) Quantification of cerebral oxygenation and haemodynamics in sick newborn infants by near infrared spectrophotometry. Lancet 2: 1063-1066.
- Meek JH, Elwell CE, McCormick DC, et al. (1999) Abnormal cerebral haemodynamics in perinatally asphyxiated neonates related to outcome. Arch Dis Child Fetal Neonatal Ed 81: 110-115.
- Pellicer A, Del Carmen Bravo M (2011) Near-infrared spectroscopy: A methodology-focused review. Semin Fetal Neonatal Med 16: 42-49.
- Quaresima V, Lepanto R, Ferrari M (2003) The use of near infrared spectroscopy in sports medicine. J Sports Med Phys Fitness 43: 1-13.
- Pogue BW, Poplack SP, McBride TO, et al. (2001) Quantitative hemoglobin tomography with diffuse NIRS: Pilot results in the breast. Radiology 218: 261-266.
- Durduran T, Choe R, WB Baker, et al. (2010) Diffuse optics for tissue monitoring and tomography. Rep Prog Phys 73: 076701.
- Villringer A, Chance B (1997) Non-invasive optical spectroscopy and imaging of human brain function. Trends Neurosci 20: 435-442.
- Obrig H, Wolf T, Doge C, et al. (1996) Cerebral oxygenation changes during motor and somatosensory stimulation in humans, as measured by near-infrared spectroscopy. Adv Exp Med Biol 388: 219-224.
- Colier W, Quaresima V, Oeseburg B, et al. (1999) Human motor-cortex oxygenation changes induced by cyclic coupled movements of hand and foot. Exp Brain Res 129: 457-461.
- Hock C, Villringer K, Muller-Spahn F, et al. (1996) Near infrared spectroscopy in the diagnosis of Alzheimer's disease. Ann N Y Acad Sci 777: 22-29.
- Suto T, Fukuda M, Ito M, et al. (2004) Multichannel near-infrared spectroscopy in depression and schizophrenia: Cognitive brain activation study. Biol Psychiatry 55: 501-511.
- Kubota Y, Toichi M, Shimizu M, et al. (2005) Prefrontal activation during verbal fluency tests in schizophrenia, a near--infrared spectroscopy (NIRS) study. Schizophr Res 77: 65-73.
- Ehlis AC, Schneider S, DreslerT, et al. (2014) Application of functional near-infrared spectroscopy in psychiatry. Neuroimage 85: 478-488.
- Rovati L, Bandera A, Donini M, et al. (2003) A novel tissue oxymeter combining the multidistance approach with an accurate spectral analysis. Proc. Instrumentation and Measurement Technology Conference, Colorado, USA.
- Crespi F (2013) Parallel effect of nicotine and mk-801 on brain metabolism: An in vivo non invasive near-infrared spectroscopy analysis in rats. Curr Synthetic Sys Biol 1: 101.
- Crespi F, Bandera A, Donini M, et al. (2005) Non-invasive in vivo infrared laser spectroscopy to analyse endogenous oxy-haemoglobin, deoxy-haemoglobin, and blood volume in the rat CNS. J Neurosci Methods 145: 11-22.
- Crespi F, M Donini, A Bandera, et al. (2006) Near infrared oxymeter biosensor prototype for non-invasive in vivo> analysis of rat brain oxygenation: Effects of drugs of abuse. Journal of Optics A: Pure and Applied Optics 8: 528-534.
- Crespi F (2007) Near-infrared spectroscopy (NIRS): A non invasive in vivo methodology for analysis of brain vascular and metabolic activities in real time in rodents. Curr Vasc Pharmacol 5: 305-321.
- Crespi F, Cottini S, Bandera A, et al. (2016) In vivo real time non invasive monitoring of brain penetration of chemicals with near-infrared spectroscopy: Concomitant PK/PD analysis. J Neurosci Methods 258: 79-86.
- Martelli F (2002) Analytical solutions of the diffusion equation in homogeneous media. Department of Physics, University of Florence, Italy.
- Wahr JA, Tremper KK, Samra S (1996) Near-infrared spectroscopy: Theory and applications. J Cardiothorac Vasc Anesth 10: 406-418.
- Sano M, Wendt PE, Wirsén A, et al. (1993) Acute effects of alcohol on regional cerebral blood flow in man. J Stud Alcohol 54: 369-376.
- Paxinos G, Watson C (1986) The rat in stereotaxic coordinates. (2nd edn), Plenum Press, New York, USA.
- Matcher SJ, Kirkpatrick PJ, Nahid K, et al. (1995) Absolute quantification methods in tissue near-infrared spectroscopy. SPIE 2389.
- Scholkmann F, Kleiser S, Metz AJ, et al. (2013) A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage 85: 6-27.
- Frye GD, Breese GR (1981) An evaluation of the locomotor stimulating action of ethanol in rats and mice. Psychopharmacology (Berl) 75: 372-379.
- Waller MB, Murphy JM, McBride WJ, et al. (1986) Effect of low dose ethanol on spontaneous motor activity in alcohol-preferring and -non preferring lines of rats. Pharmacol Biochem Behav 24: 617-623.
- Ticku MK, Lowrimore P, Lehoullier P (1986) Ethanol enhances GABA induced 36Cl-influx in primary spinal cord cultured neurons. Brain Res Bull 17: 123-126.
- Roberto M, Madamba SG, Moore SD, et al. (2003) Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A 100: 2053-2058.
- Di Chiara G, Imperato A (1986) Alcohol and barbiturates preferentially stimulate dopamine release in the nucleus Accumbens of freely moving rats. Symposia in Neuroscience, Fidia Research Series 3: 89-96.
- Di Chiara G, Bassareo V, Fenu S, et al. (2004) Dopamine and drug addiction: The nucleus accumbens shell connection. Neuropharmacology 47: 227-241.
- Gessa G, Colombo G, Fadda F (1990) Modelli sperimentali di alcolismo. Recenti Progressi in Medicina 81: 162-165.
- Ma H, Zhu G (2014) The dopamine system and alcohol dependence. Shanghai Arch Psychiatry 26: 61-68.
- Johnson BA (2004) Role of the serotonergic system in the neurobiology of alcoholism: implications for treatment. CNS Drugs 18: 1105-1118.
- Crespi F (1998) The role of cholecystokinin (CCK), CCK-A or CCK-B receptor antagonists in the spontaneous preference for drugs of abuse (alcohol or cocaine) in naive rats. Methods Find Exp Clin Pharmacol 20: 679-697.
- Crespi F, England T, Gaviraghi G (1996) Spontaneous preference for ethanol in naive rats is influenced by cholecystokinin A receptor antagonism. Alcohol 14: 327-332.
- Rossetti Z, Crespi F (2004) Inhibition of Nitric Oxide release in vivo by ethanol. Alcohol Clin Exp Res 28: 1746-1751.
- Berglund M (1981) Cerebral blood flow in chronic alcoholics. Alcoholism: Clinical and Experimental Research 5: 295-303.
- Volkow ND, Hitzemann R, Wang GJ, et al. (1992) Decreased brain metabolism in neurologically intact healthy alcoholics. Am J Psychiatry 149: 1016-1022.
- Gilman S, Adams K, Koeppe RA, et al. (1990) Cerebellar and frontal hypometabolism in alcoholic cerebellar degeneration studied with positron emission tomography. Ann Neurol 28: 775-785.
- Hamdy F Moselhy, George Georgiou, Ashraf Kahn (2001) Frontal lobe changes in alcoholism: A review of the literature. Alcohol Alcohol 36: 357-368.
- Ali M, Brain C (1974) Ethanol inhibition of haemoglobin synthesis: In vitro evidence for a haem correctable defect in normal subjects and in alcoholics. British Journal of Haematology 28: 311-316.
- Freedman ML, Cohen HS, Rosman J, et al. (1975) Ethanol inhibition of reticulocyte protein synthesis: The role of haem. Br J Haematol 30: 351-363.
- Ferrari M, Quaresima V (2012) A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage 63: 921-935.
- Hoshi Y, Huang J, Kohri S, et al. (2011) Recognition of human emotions from cerebral blood flow changes in the frontal region: A study with event-related near-infrared spectroscopy. J Neuroimaging 21: e94-e101.
- Kono T, Matsuo K, Tsunashima K, et al. (2007) Multiple-time replicability of near-infrared spectroscopy recording during prefrontal activation task in healthy men. Neurosci Res 57: 504-512.
- Molteni E, Contini D, Caffini M, et al. (2012) Load-dependent brain activation assessed by time-domain functional near-infrared spectroscopy during a working memory task with graded levels of difficulty. J Biomed Opt 17: 056005.
Corresponding Author
Francesco Crespi, Biology Department, GSK, Verona, Italy.
Copyright
© 2018 Crespi F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.