Nanoparticles for Drug Delivery and Strategies for Reversing Tumor Drug Resistance
Abstract
Cancer poses a great threat and challenge to human health. However, the most widely used chemotherapy drugs show resistance due to various reasons, such as cellular efflux, poor targeting, obvious side effects. On the other hand, as a new drug delivery vehicle, nanoparticles have attracted extensive attention due to their good targeting and low toxicity. Various nanoparticles for drug delivery, including liposomes, polymer nanoparticles, micelles, drug-polymer conjugates, dendrimers, nanoshells, magnetic nanoparticles, drug nanocrystals, and extracellular vesicles, are reviewed in this paper. The basic property and characteristics of various nanoparticle were summarized and compared, and that strategies of nanoparticle in reversing drug resistance of tumors were analyzed.
Keywords
Nanoparticle, Drug delivery, Tumor drug resistance, Reversal drug resistance
Introduction
Cancer, as a disease with the highest mortality rate for a long time, is a great challenge for human health. Drug treatment plays a vital role in cancer treatment. Since the first compound nitrogen mustard with anti-tumor activity was found in the 1940s, 130-150 anti-cancer drugs have been approved and marketed worldwide. However, different mechanisms and different degrees of drug resistance have become one of the most important reasons for the failure of clinical chemotherapy [1-4]. There are many mechanisms for drug resistance in tumors, such as reduced accumulation of drugs in tumor cells due to cellular efflux, drug inactivation, inhibition of cell apoptosis, increase or change of drug targets, resistance of target proteins due to gene mutation, changes in tumor microenvironment, and enhanced repair of drug-induced injury [5-9]. Clinically, tumor resistance to chemotherapeutic drugs is often associated with multiple mechanisms, which is also one of the reasons leading to multi-drug resistance. Based on these mechanisms, different strategies have been proposed to reverse drug resistance, including inhibition of over-expression of drug efflux transporters, targeted delivery, delivery of miRNA, siRNA and other gene drugs to regulate the tumor cell apoptosis signaling pathway, and combined drug administration [10-15]. The practice of these strategies needs appropriate delivery vehicles. Therefore, nanoparticles with the advantages of small size, high delivery efficiency, and low immunogenicity have entered the attention of researchers.
Nanoparticles refer to artificial or natural solid particles with at least one dimension in the nanometer size (0.1-100 nm) in three-dimensional space, also known as nano dust or nano dust. The ultra-small size of nanoparticles enables them to have extraordinary penetration, permeability and great specific surface area, which are not available in other materials. As a result, they become a new type of materials, which is expected to be applied in various fields. In the field of drug delivery, the characteristics of nanoparticles give them significant advantages in crossing physiological barriers such as the gastrointestinal tract, cell uptake, and targeting, which is expected to enhance the therapeutic effect of anticancer drugs and reverse drug resistance. In this paper, we introduce the basic characteristics and respective advantages of various nanoparticles for the delivery of anticancer drugs, with emphasis on the analysis of strategies for the realization of drug resistance reversal by nanoparticles.
Introduction of Nanoparticles
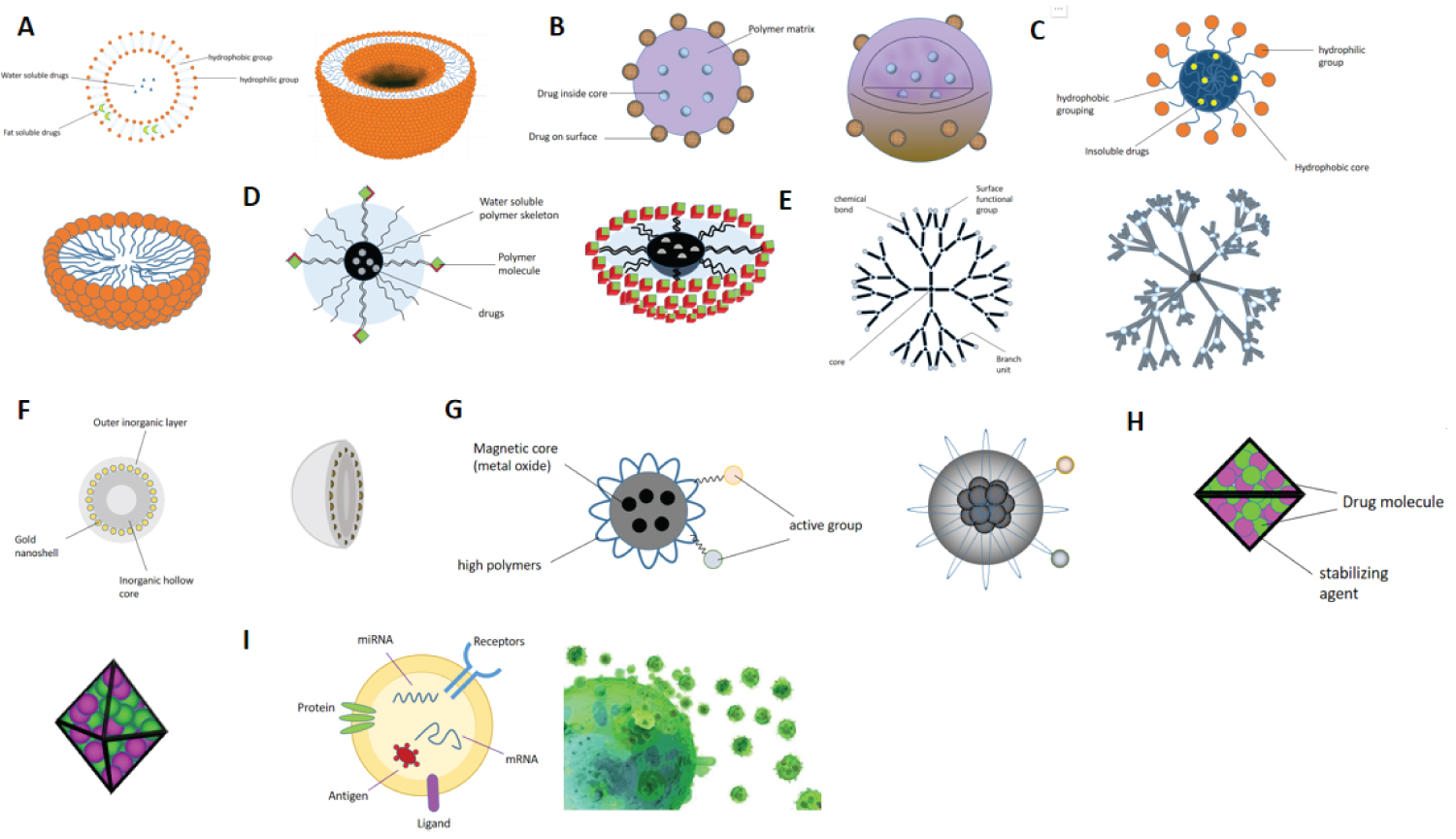
The types of nanoparticles are diverse, and the properties of nanoparticles with different particle sizes, shapes, structures and even materials are also different. At present, the nanoparticles with more studies mainly include liposomes [16-19], polymeric nanoparticles [20], micelles [21,22], drug-polymer conjugates [23], dendrimers [24], nanoshells [25], magnetic nanoparticles [26], drug nanocrystals [27], and nanoparticles (extracellular vesicles) of biological origin [28]. The schematic diagram of the planar and three-dimensional structure is shown in Figure 1. These different nanoparticles have respective advantages in reversing the resistance of anticancer drugs (Table 1).
Liposomes
Liposome is a artificial membrane. The hydrophilic head of that phospholipid molecule in the water is insert into the water, and the hydrophobic tail of the liposome extends to the air to form the spherical liposome with double-layer lipid molecules aft stirring, and the diameter is 25-1000 nm. In terms of drug delivery, liposomes have the following advantages:
Low toxicity, low immunogenicity: The liposome consists of phospholipid and additive. The phospholipid includes natural phospholipid (lecithin) and synthetic phospholipid from egg yolk and soybean. The additive is cholesterol or sterol. These components are similar to biomembrane and have low toxicity and immunogenicity. In addition, liposomes can deliver toxic drugs into cells through membrane fusion, thereby reducing the non-targeted distribution of drugs in the body and reducing their toxicity.
Targeting: The passive targeting of liposomes depends on the endocytosis of cells, while the active targeting is mainly achieved through engineering. For example, folic acid receptor is abundantly expressed in most malignant tumor cells and almost not expressed in normal cells. Using this, we can design and link folic acid molecules on liposomes to achieve targeted effects [29].
Polymer nanoparticles
The system formed by drug dispersion, encapsulation and adsorption in polymer materials is called polymer nanoparticles, The size of polymer nanoparticles is usually 10-10-1000 nm, Polymer materials include polyalkylene glycol, copolymer of lactide and glycolide, poly (L-lactide), polylactic acid, etc, [30,31]. In terms of drug delivery, polymer nanoparticles have the following advantages:
The polymer is a nanoparticle that improves the properties of the drug molecule. Compared with other nano delivery systems, polymer nanoparticles can be functionalized more easily and finely in terms of size adjustment and surface modification, thereby improving the targeting of drugs to target organs and increasing the half-life of drugs in the blood stream [32].
The polymer nanoparticles can enhance the stability of drugs, especially protein drugs, and avoid the loss of effective drug amount caused by hydrolysis and leakage before reaching the action site. This effect is achieved through a variety of polymer-to-drug molecule fixation: The nanocapsules are porous systems in which the drug molecules are adsorbed in the pores without easy leakage; The nanoparticles were a homogeneous system in which the drug was homogeneously dispersed.
Polymer micelles
When a surfactant is dissolved in water and its concentration increases so that the surface of the solution is saturated that it can no longer be adsorbed, the hydrophobic parts of many surfactant molecules (generally 50 to 150) attract and associate with each other to form an association called a micelle or micelle, which has various shapes such as a sphere, a layer, a rod, and usually 10 to 10-100 nm in size. In drug delivery, micelles have the advantage that:
Targeting: Due to their small size, micelles are more likely to carry drug to target sites and be taken up by cells by penetrating through various physiological barrier. Similar to liposomes, micelles can also be actively targeted by surface modification [33-35].
One significant advantage of micelles over other nanoparticles is their ability to improve the stability of poorly soluble drugs. The hydrophobic ends of the surfactant molecules constituting the polymer micelles can be crosslinked to form a hydrophobic inner core through the reduction of the system free energy, and the micelles can increase the existence of insoluble drugs in the hydrophobic inner core and keep the stability from aggregation and settlement.
Drug-polymer conjugates
The drug-polymer conjugate model comprises a biocompatible water-soluble polymer backbone and a delivered drug or prodrug carried within the backbone with a particle size between 50 and 50-1000 nm. Common polymer materials include polyethylene glycol (PEG), N-(2- hydroxypropyl) methacrylamide (HPMA), polyglycolic acid (PGA) and polylactide-co-glycolide (PLGA) [36,37]. These materials have the advantages of biodegradability and good water solubility. In terms of drug delivery, drug-polymer conjugates have the following advantages:
High drug loading: The polymer molecules are combined or coupled with the drugs by covalent bonding, and the framework structure provides enough space for the drug to be contained, so that a larger amount of drugs can be loaded.
Dendrimers
Dendrimers, also known as dendrimers, are linear polymers with a dendrimer on each repeating unit. Dendrimers have precise nanostructures and their synthesis methods include divergence method and convergence method. The precise generations and volume of dendrimers are determined by the synthesis steps. The diameter of the dendritic polymer ranges from 10 nm to 130 nm from G0 generation to G10 generation respectively. There are three kinds of drug loading sites for dendrimers:
(I) Void space (through molecular entrapment); (ii) Branch point (by hydrogen bonding); And (iii) Through an outer surface group (charge-charge interaction) [38].
In terms of drug delivery, dendrimers have the following advantages:
High stability: According to different generations, the dendrimers have different diameters, thus forming a stable rigid structure with thick core and thin end. Intermolecular forces, hydrogen bonds, and other forces can maintain the stability of its internal structure. In addition, the drug can be integrated with the dendritic polymer by covalent connection, so that the original structure of the nanocarrier is not affected, and the stability of the nanocarrier is maintained.
Nanometer shell
Nano-shell is a new type of nanoparticles with adjustable optical properties. It is a hollow metallic sphere, which is only one twentieth the size of red blood cells and has a diameter of 10-500 nm. The nano-shell can be directly formed on the surface of the target drug by methods such as atomic layer deposition (ALD) to complete the preparation of the drug-loaded nano-shell. In terms of drug delivery, the advantages of nanoshells are:
Sustainable drug release: After the drug-loaded nano-shells enter the plasma, due to the friction force generated by blood flow, the pH value of the plasma and the like, the nano-shell layers are gradually dissolved, and the drug is gradually released, so that local high concentration and sub-therapeutic effects are avoided.
Flexible adjustability: In the process of preparing the nano shell, the thickness of the shell layer can be controlled by controlling the reaction, so that the optical properties such as the efficiency of absorbing light of the nano shell are changed. The thickness of the nanoshell when it was most sensitive to light could be determined by continuous adjustment, so as to achieve a more sensitive photoresponse-drug release [39]. In addition, the nanoshell can be directly injected into the tumor and the tumor can be irradiated with visible light, laser, and the like to increase the temperature, thereby eliminating the tumor [40,41].
Magnetic nanoparticles
Magnetic nanoparticles are nano-sized particles, generally composed of a magnetic core composed of metal oxides such as iron, cobalt, nickel and a high-molecular polymer/silicon/hydroxyapatite shell layer wrapped outside the magnetic core, with a size of 10-1000 nm. It is different from other types of nanoparticles due to its special properties such as magnetism. The most common nuclear layer is made of Fe3O4 or γ-Fe3O3 with super paramagnetic or ferromagnetic properties, with magnetic orientation (targeting). Under the action of the external magnetic field, it can achieve directional movement, convenient positioning and separation from the medium. The most common shell layer is composed of high-molecular polymers. The active groups coupled on the shell layer can combine with a variety of biomolecules, such as protein, enzymes, antigens, antibodies, nucleic acids, and so on, to achieve its functionalization. In terms of drug delivery, magnetic nanoparticles have the following advantages:
Has special magnetic guidance: The materials of the magnetic nanoparticles comprise metal compounds, so that the magnetic nanoparticles can be purposefully and targeted enriched in tumor tissues under the action of an external magnetic field or a magnetic field in vivo [42,43].
Magnetic nanoparticles can interact with alternating magnetic fields to convert electromagnetic energy into thermal energy. Based on this feature, after the targeted delivery of magnetic nanoparticles to the tumor site, the application of an alternating magnetic field can increase the temperature of the nanoparticles to kill the tumor cells [44].
Drug nanocrystals
Drug nanocrystals refer to pure solid drug particles present as crystals with an average particle size of not more than 1000 nm. Compared with the nanocarrier drugs listed above, the most unique advantage of nanocrystals is that they are not constrained by the encapsulation efficiency: The drugs are directly nanocrystallized without the need of using carrier materials, so there is no problem of encapsulation efficiency or drug loading [45]. In addition, the advantages of diverse dosage forms, controllable particle size, and easy mass production make nanocrystals occupy an important position in the research and development of nano drug delivery. In terms of drug delivery, the advantages of nanocrystals are:
And that effective drug load is large. Except for a small amount of stabilizer, drug nanocrystals were mainly composed of drugs, and their drug content at the same grade size was greater than that of other kinds of nanoparticles composed of drugs and carriers. In addition, the higher crystal density further increases the actual drug content of the nanocrystalline drug.
Improve the solubility of insoluble drugs: Amorphous particles in nanocrystalline drugs have the special advantage that their saturated solubility is much higher than that of crystalline drugs at the same particle size, which can greatly improve the bioavailability. Smaller particle size and larger specific surface area of nanocrystals are also beneficial to improving the solubility of poorly soluble drugs.
Extracellular vesicles
Extracellular vesicles are collectively referred to the tiny vesicles with membrane structures actively secreted by cells, and almost all cells will secrete EVs [46]. In 1983, the vesicles smaller than 50 μM secreted by reticulocytes during maturation were found to have protein transport function. Since then, the delivery of EVs has attracted increasing attention and become a research focus in the field of drug delivery. According to the difference in size, biological characteristics and formation process, EVs is mainly divided into three categories: Exosomes, microvesicles and apoptotic bodies. In which exosomes with a diameter in the range of 40-200 nm and microvesicles in the range of 200-1000 nm have a function of transferring mRNA, miRNA and protein to receptor cells. Unlike the synthetic nanoparticles described above, extracellular vesicles are of biological origin and offer unique advantages in drug delivery:
Low immunogenicity: EVs are derived from natural biological cells and are biocompatible and biodegradable, with lower toxicity and immunogenicity than other nanoparticles. By contrast, the structure of EVs is similar to that of liposomes which are also lipid bilayer, but the components of EVs are more similar to its parent cells, so it is not easy to be captured by the immune system in the recipient organism, thus ensuring the release of drugs after reaching the target site.
And is easy to be taken up by target cell. EVs has a phospholipid bilayer structure, which is the same as that of biological target cells, so it is easy to achieve endocytosis through the mobility of the membrane. In addition, EVs often has a biological recognition ligand on the surface, which can recognize each other with the receptor to promote cell uptake; The small size of EVs is also one of the reasons for its easy uptake by cells.
Strong targeting: EVs derived from a particular parental cell line have tropism for their cognate cells. The ability of EVs to target tumor cells is significantly enhanced compared to liposomes of comparable size, which is associated with optimized EVs internalization by receptor ligand binding on the receptor cells. EVs itself is rich in miRNA, and many tumors have characteristic miRNA expression down-regulation, so using EVs to deliver miRNA to target tumor sites is an ideal solution.
Mechanism of Nanoparticles Reversing Drug Resistance
Reducing cellular efflux
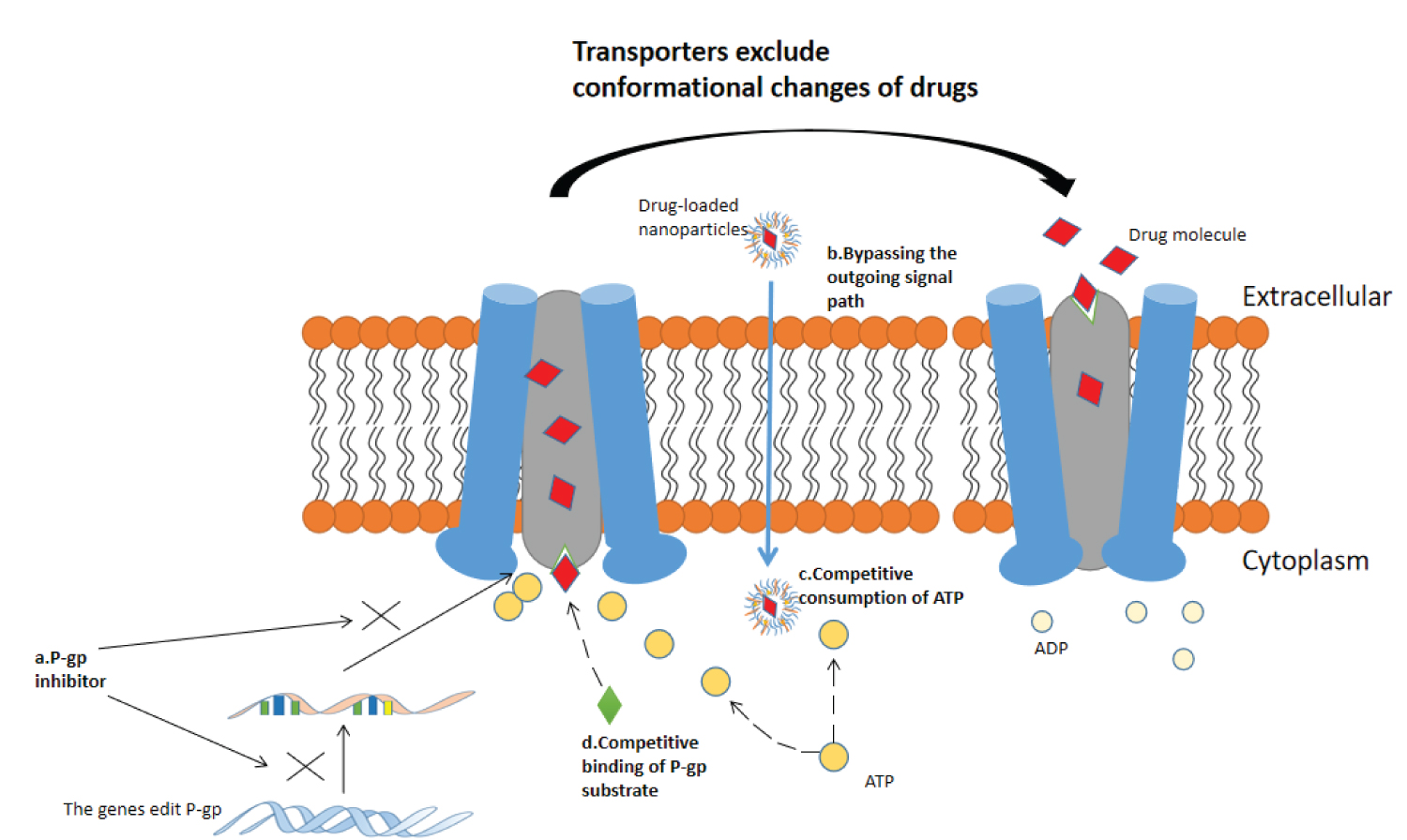
Tumor resistance is largely related to the expression of (membrane transporter) resistance-related proteins. Various drug resistance protein genes represented by ATP-binding cassette (ABC) transporter family expel drug molecules out of cells to reduce drug accumulation by expressing membrane transporters such as P-gp, MRPs, and TfR, thereby directly affecting the efficacy of drug therapy [47-50]. Specifically, many studies have shown that using nanoparticles as drug delivery vehicles can reverse drug efflux [51-53]. Figure 2 shows the conformational changes of P-gp when transporting drugs and various pathways for reversing the action of P-gp.
Nanoparticles can deliver drug efflux transporter inhibitors to directly reverse drug efflux, including but not limited to organic polymers [54]; Chemical [55,56]; SiRNA and other gene drugs [57-59]. Transporter inhibitors can silence ABCB1, ABCC and other genes to down-regulate the expression of P-gp and MRPs and reverse the drug efflux. Nuclear factor κB (NF-κB) is highly correlated with P-gp expression. Chemotherapy drugs such as doxorubicin and paclitaxel can activate NF-κB [60]. The chemotherapeutic sensitizer pyrrolidine dithiocarbamate (PDTC) can inhibit P-gp expression by inhibiting the high activity of NF-κB spontaneously or activated by chemotherapy drugs [61]. Based on this, Xu Cheng, et al, [56] delivered PDTC through polymer nanoparticles, and the results showed that compared with blank nanoparticles and free PDTC group, PTDC-NP could effectively inhibit NF-κB nuclear translocation and down-regulate P-gp expression. RNA interference technology (RNAi) refers to the highly conservative phenomenon of efficient and specific degradation of homologous mRNA induced by double-stranded RNA (dsRNA) in the evolution process [62]. The use of RNAi technology can specifically eliminate or shut down the expression of genes associated with drug efflux transporters, and nanoparticles are an ideal vehicle for implementing this technology in reversing the resistance of anticancer drugs. Bao-an Chen, et al. [57] designed short hairpin RNA (shRNA) directly targeting the target sequences of MDR1 mRNA (nucleotides 3491-3509, 1539-1557 and 3103-3121) to be delivered to K562 cells through magnetic ferroferric oxide nanoparticles. FCM method detected that the shRNA-containing nanoparticles had higher accumulation in K562 cells than the blank nanoparticles.
Nanoparticles can carry drugs around the efflux signaling pathway of tumor cells. Due to their extremely small size and large specific surface area, the drug-loaded nanoparticles can be ingested by cells through a passive diffusion process, thereby avoiding the activation of efflux signaling pathways. In addition, nanoparticles such as liposomes, polymer micelles, and extracellular vesicles can enter cells through endocytosis and then release drugs due to their good fat solubility, which can greatly increase the content of drugs in cells. Sandeep Kumar Vishwakarma, et al. [63] synthesized and transfected sorafenib-gold nanoconjugates (SF-GNP) with an average particle size of only 7nm into a HepG2 drug-resistant cell model, and transcriptional expression analysis showed that after treatment with SF-GNP nanoconjugates. A highly reduced expression of ABCG2 was observed in SF-resistant HepG2 cells from the corresponding colonies (P < 0.0001), suggesting that SF-GNP entry into cells did not activate the efflux signaling pathway in SF-resistant HepG2 cells.
P - The drug efflux process of gp is an active transport process, which needs to consume energy. The energy is also consumed in the process of drug-loaded nanoparticles being swallowed into cells. Therefore, nanoparticles can directly compete with P-gp for ATP to inhibit its transport function. Jing Miao, et al., [64] compared the toxicity of free paclitaxel and paclitaxel-loaded solid lipid nanoparticles on drug-resistant MCF-7 cells. Cell uptake experiments showed that the percentage of cell uptake of SLN-loaded PTX was significantly increased compared with that of free paclitaxel. In addition to ATP competition, nanoparticles can also deliver substrates of certain drug efflux transporters that bind to the transporters and competitively inhibit drug efflux. Therefore, according to the structural characteristics of P-gp binding sites, compounds can be targeted designed to competitively bind to P-gp or to simultaneously deliver more than two drugs, one of which acts as a competitive inhibitor of P-gp to reverse the efflux of the other drug. The main problem with this strategy today is the control of the amount of drug.
P-gp expression in cells is limited. When the amount of drugs entering cells is large, the transport function of P-gp reaches saturation, and the bioavailability of drugs increases sharply in a short time to the level of killing tumor cells. Nanoparticles can deliver larger amounts of drug compared to free administration to achieve this strategy. However, this strategy needs to be further refined in view of the potential and uncontrolled toxicity of drug overdoses.
Combined dosing
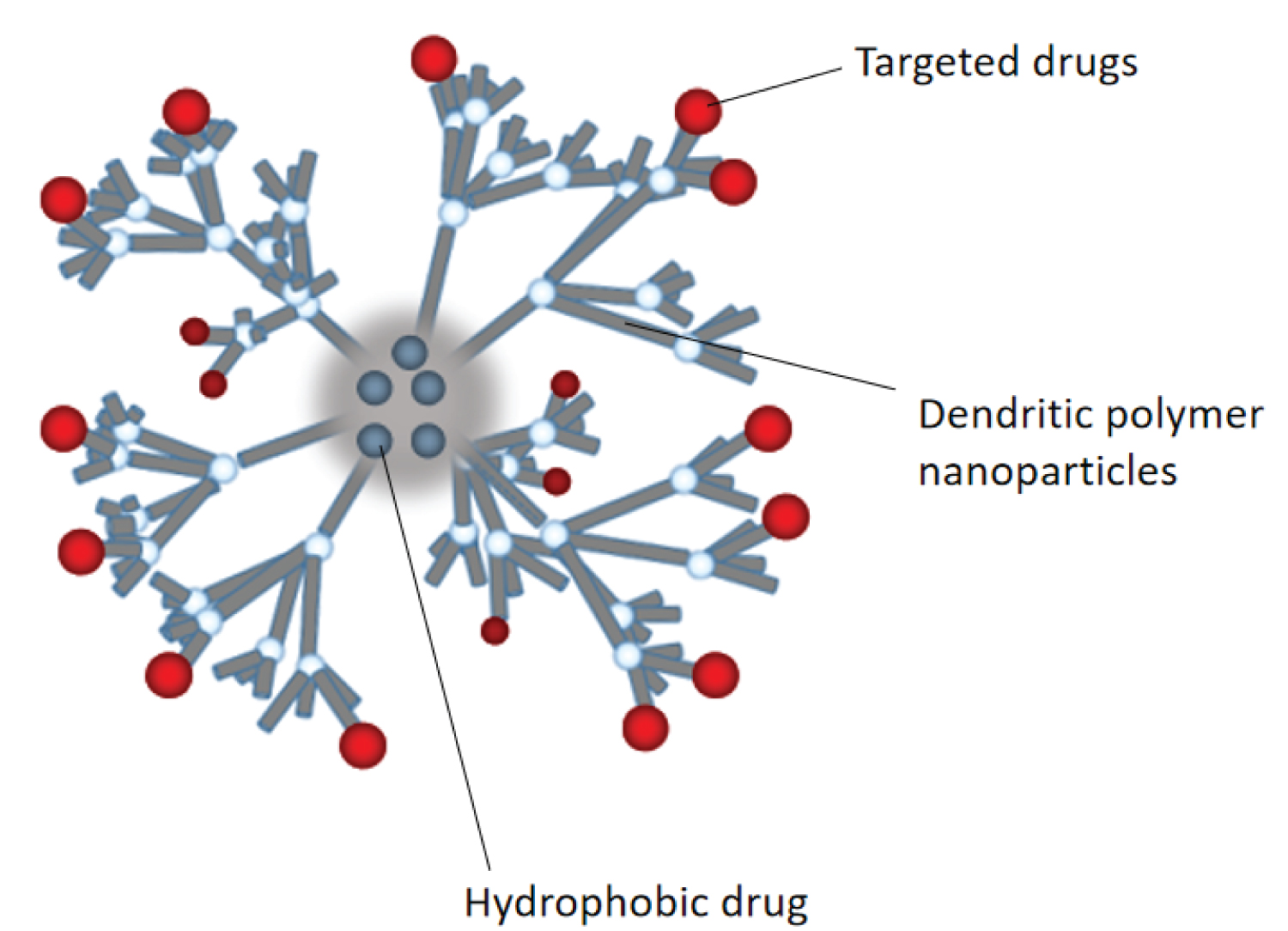
In tumor chemotherapy regimens, using only one drug may cause various problems such as insufficient efficacy, high toxicity, and easiness in leading to drug resistance, and thus more than two drugs are often used in combination. The combination strategy of antineoplastic agents is to improve efficacy without increasing overall toxicity and reducing the potential for resistance. A large number of clinical studies and treatment results provide strong evidence for better tumor treatment effects with combined drugs [65-70]. However, excessive toxicity, drug interaction, and unsatisfactory synergy due to different pharmacokinetic properties of different drugs limit the practice of combined drugs in clinical chemotherapy. As a tiny drug-carrying system with rich drug-carrying space, nanoparticles can complete the joint delivery of a variety of antitumor drugs with its excellent permeability, targeting and accurate control of the drug content ratio. Figure 3 is a structural diagram of the dendrimer PAMAM loaded with multiple drugs simultaneously.
Paclitaxel resistance often occurs, leading to chemotherapy failure. Combined use of P-gp inhibitors can reverse paclitaxel resistance. Liang Zou, et al., [71] used PEG-PAMAM dendritic polymer nanoparticles to jointly deliver paclitaxel and borneol with P-gp inhibition. The same fat-soluble paclitaxel and borneol were loaded into the hydrophobic core of PAMAM by nano-precipitation. Compared with PAMAM loaded with paclitaxel alone, the nanoparticles loaded with the combination drug showed more significant tumor inhibition. Due to the encapsulation effect of the nanoparticles, the pharmacokinetic behaviors of paclitaxel and borneol in A2780/ PTX tumor-bearing nude mice were similar, which was conducive to the synergistic antitumor effect of the two.
Resistance in some cancers is associated with specific signaling pathways. Studies have shown that the resistance of 5-FU to colorectal cancer is related to the down-regulation of human DNA MutS homologue 2 (hMSH2) by miR-21 [72], and this resistance can be overcome by co-delivery of the restored dysregulated miRNA with anticancer drugs. Gaofeng Liang, et al., [73] co-packaged 5-FU and miR-21i into engineered exosomes by electroporation to evaluate in vitro reverse resistance of HCT-116 human colon cancer resistant cell line. The combination group showed a higher rate of apoptosis (42.3%) compared to the delivery of miR-21i alone (12.6%) or 5-FU alone (26.2%). The overexpression of T-type Ca2+ channel has been confirmed to be the key factor for drug resistance in HepG2 hepatoma cells. To solve this problem, Jie Zhao, et al., [74] constructed silicon dioxide (LSC) nanoparticles to co-deliver Ca2+ channel siRNA and doxorubicin (Adr). The results of cell mechanism research have shown that compared with the single delivery of ADRs, the introduction of siRNA effectively reduced the level of Ca2+ in G0/G1 cells, thus increasing the intracellular accumulation of ADRs. The results of antitumor experiments in vitro confirmed this mechanism.
Tumor micro environment has an important impact on drug chemotherapy. Heterogeneity of the micro environment of some tumors, such as hypoxia and insufficient blood supply, will hinder the transport of drugs in the blood vessels of tumor tissues, leading to drug resistance. Therefore, normalizing tumor blood vessels with nanoparticles is one of the mechanisms for reversing drug resistance. The antimalarial drug chloroquine (CQ) has been shown to normalize blood vessels. CQ can reduce tumor hypoxia, enhance intra-tumor chemotherapy drug delivery, and inhibit tumor invasion and diffusion [75]. Tingting Lv, et al., [76] coadministered the CQ and the anti-EGFP agent ERL and co-encapsulated the two by a PAMAM dendrimer, thereby reversing the resistance to EGFR mutations in NSCLC.
Lipid rafts are microstructural domains rich in cholesterol and sphingomyelin on the plasma membrane. It was about 70 nm in size, and it was a dynamic structure, located in the outer small page of the plasma membrane. Studies have shown that drug-resistant cancer cells have high levels of cholesterol and sphingomyelin on their plasma membrane, which are extremely easy to form lipid rafts. This high-density structure hinders the drugs from entering the cells [77,78]. Targeted, combined use of lipid-lowering drugs may help chemotherapy drugs to break through this obstacle. In order to ensure that chemo therapy drugs can pass the "road" opened by lipid-lowering drugs in time, the use of nanoparticles to deliver the two in the same time and space is an ideal strategy. Bin Du, et al., [79] studied the therapeutic effect of doxorubicin (DOX) and simvastatin (SV)-co-loaded poly (lactic-co-glycolic acid) nanoparticles (SV/DOX@TPGS2k-PLGA nanoparticles) on drug-resistant colon cancer. SV can consume cholesterol used for the synthesis of lipid rafts and destroy the lipid raft structure, increasing the intracellular accumulation of DOX.
Analysis of major tumor tissues excised from tumor-bearing nude mice after 14 days of treatment showed that after 14 days of treatment with SV/DOX@TPGS2k-PLGA nanoparticles, tumor growth rates in nude mice decreased to 0.5%, significantly lower than those in the DOX group (7.1%), the SV group (5.6%), the DOX@TPGS2k-PLGA group (2.7%), the SV@TPGS2k-PLGA group (4.2%), the TPGS2k-PLGA group (7.6%), and the saline group (8.1%). This indicates that the combined delivery of anti-lipid rafts and chemotherapeutic drugs with nanoparticles has the potential to reverse drug resistance of tumors.
Increase the solubility and stability of insoluble drugs
Some natural or synthetic drugs have anti-tumor activity, but many of them have unsatisfactory chemotherapy effect due to poor water solubility and stability. Nanoparticles can improve their solubility and stability by encapsulating the drug. Take the insoluble compound paclitaxel as an example. Free paclitaxel has low solubility and poor stability in aqueous solution system, and may undergo aggregation, settlement and other phenomena. Paclitaxel is delivered encapsulated in liposomes or polymeric micelles, and the drug is encapsulated in the hydrophobic core of the nanoparticles, thereby increasing its solubility and stability. Huan Yang, et al., [80] constructed paclitaxel-loaded ethylene glycol chitosan nanoparticles. As a polymer shell, ethylene glycol chitosan had high water solubility and could stably carry paclitaxel to a target site, where it was dissolved to release the drug.
Making poorly soluble drugs into amorphous nanocrystals is another strategy to improve their solubility [81,82]. Miriam, et al., [83] adopted the water-wet bead milling method to prepare amorphous indomethacin nanoparticles, and the prepared nanosuspension reduced the crystallinity of the drug particles, thereby improving the saturated solubility. 5.2-fold increase in amorphous indomethacin nanoparticles compared to original indomethacin. Correspondingly, the solubility of indomethacin nanocrystals was only 2.6 times higher than that of the original drug.
The nanoparticles can firmly encapsulate the drug in it by virtue of its tight structure, to ensure that the drug is stable on the way of transportation to the target site, and does not leak, be hydrolyzed, enzymatically inactivated, or undergo aggregation and settlement. There is evidence that the nanoparticles with crosslinked structure have a stronger ability to improve drug stability [84-86], which is related to the rigid and dense space formed by the crosslinked structure. Nieves Iglesias, et al., [87] showed that the core crosslinked nanocarriers had excellent drug retention capacity (> 90%) and could significantly increase the stability of camptothecin in physiological media. The covalent bonds formed during the reticulation between the hydrophobic segments of the polymer in the core and the crosslinking agent DMDOO are the key to the stability of the nanoparticles in the high dilution solution.
Increase drug targeting
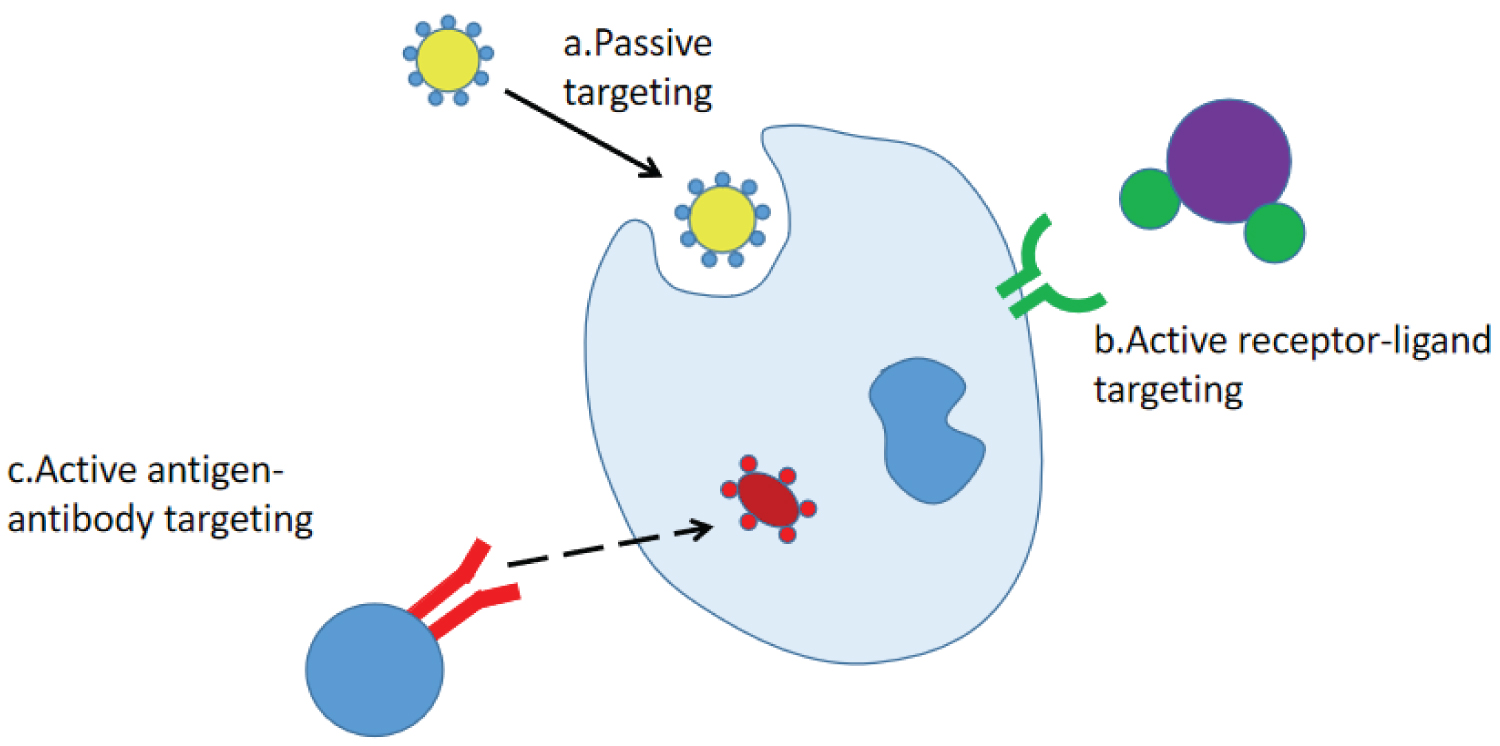
Another important cause of tumor resistance is the failure of targeted therapy. Poor targeting directly results in reduced accumulation of drugs in cells, and the resulting poor targeting is related to such factors as pharmacokinetic properties as drug absorption and distribution, increase in the content of drug-targeting enzymes, and change or increase in the action targets of drugs. Compared with common preparations, drug-loaded nanoparticles could easily cross various biological barriers such as the blood-brain barrier and the blood-retina barrier and allow the targeted enrichment of drugs. There are two ways for nanoparticles to achieve the targeting effect: Passive targeting and active targeting. Passive targeting refers to the targeting achieved due to physiological endocytosis after the drug or drug delivery system enters the body. The distribution of the passively targeted nanoparticles in vivo is mainly dependent on the particle size. The internal diameter of the smallest capillary in the human body is about 5 μm, and the drug-loaded nanoparticles are in the nanometer level. Therefore, the nanoparticles administered by injection are not easy to block blood vessels, and can be targeted to liver, spleen, and bone marrow. However, the accurate interaction between drugs and tumor tissue targets by passive targeting is limited, so the current targeting of nanoparticles focuses on active targeting. Active targeting refers to the structural modification of nanoparticles so that they can specifically identify target cells, so that drugs can be directionally delivered to the target area to play a role. According to the different recognition methods, there are two main targeting mechanisms for nanoparticles: Receptor-mediated [88-91] and antigen-antibody mediated [92-95]. Figure 4 shows the passive and active targeting of nanoparticles.
Folate receptor (FR) is overexpressed in tumor cells, and its expression in some types of cancer cells is even 100-300 times higher than that in normal cells [96], so it is considered as a biomarker of tumors. The modification of folic acid on the surface of nanoparticles enables them to obtain the ability to target the folate receptor. Dandan Wang, et al., [97] investigated the targeting ability of folic acid modified albumin nanoparticles loaded with siRNA to leukemia cancer cells. To enhance the targeting effect, all-trans retinoic acid was also administered to selectively enhance the expression of tumor cell FRβ. The fluorescence intensity of tumor areas treated with FA-targeted NPs or non-targeted NPs was compared, and it was found that the fluorescence intensity of the targeted group was significantly higher than that of the non-targeted group, and the fluorescence intensity of the targeted group in other non-tumor tissues was very weak, indicating that folate-modified albumin nanoparticles had a high degree of tumor targeting under the coordination of all-trans retinoic acid, and achieving this effect was related to both size-dependent passive targeting and folate receptor-mediated active targeting.
Somatostatin receptor is highly expressed in neuroendocrine tissue tumors, and lanreotide (LT) has been found to specifically bind to the somatostatin receptor SSTR [98,99]. Based on the receptor-mediated mechanism in active targeting, Hanh, et al., [100] designed methotrexate-lanreotide hybrid polymer nanoparticles. Lanreotide on the surface of the nanoparticles could induce the nanoparticles to specifically bind to tumor cells with positive somatostatin receptor SSTR. The results of the in vivo distribution study showed that the fluorescence intensity of LT-MTX nanoparticles in tumor sites was brighter than that of non-targeted nanoparticles, which verified this point.
Under the stimulation of antigen, the body produces antibodies from B lymphocytes-an immunoglobulin that can specifically bind to antigen. The antibodies have the characteristics of strong specificity, high sensitivity, and easy preparation. Therefore, the nanoparticles modified with antibodies can be loaded with drugs to target and enrich in tumor cells. Bis ((S)-3- methylmorpholino) pyrido [2,3-D] pyrimidin -7- yl) -n-methylbenzamide (AZD-2014) is a potent competitive inhibitor of ATP with inhibitory potential on tumor cells, but a targeted nanodelivery system is needed to transport it to tumor areas to avoid normal tissue damage. Xiaolong Tang, et al., [101] designed an anti-CD20 antibody (rituximab)-coated AZD-2014-PLGA-PEG (AB-NPS AZD-2014) nanoparticle drug loading system. Rituximab can specifically bind to CD20 highly expressed experimental tumor cells. Flow cytometry results show that the uptake rate of rituximab-modified nanoparticles is about four times that of unmodified nanoparticles, which can significantly improve the anti-tumor effect.
Changing the pharmacokinetic behavior of drugs in vivo
The development of tumor resistance is largely related to the pharmacokinetic behavior of drugs in vivo. Less drug absorption, insufficient or unstable release, short retention time, and rapid elimination will all reduce the effective drug amount acting on tumor cells. As ideal drug delivery vehicles for new strategies to reverse drug resistance, such as gene therapy and targeted therapy, nanoparticles can improve the pharmacokinetics of drugs in vivo.
Nanoparticles can increase drug oral acceptance [102-105]. The traditional chemotherapeutic agents are mainly administrated by intravascular route. Compared with oral administration, oral administration can make patients have better compliance and reduce adverse reactions. However, poor oral absorption and low oral bioavailability must be solved. Nanoparticles can cross the intestinal mucosal barrier by virtue of their small size, and their mechanisms include either permeation from the intercellular space or passive transport. In addition, the fat-soluble nanoparticles easily adhere to the cell membrane and internalize by endocytosis to increase cellular uptake of the drug. Eun Suh Kim, et al., [106] designed quercetin-loaded nanoparticles with mucoadhesive properties, and the intestinal cell model formed by coculture of Caco-2 and HY-29 cells proved that the nanoparticles promoted the uptake of quercetin into intestinal epithelial cells. Enhance the ability of the drug to transport across the membrane and across the intestinal barrier; Xiaomin Qian, et al., [107] attached a cell-penetrating peptide TAT to granzyme B, and then encapsulated the granzyme B in polymer nanocapsules, thereby improving its transmembrane transport efficiency. The shape of nanoparticles is also an important factor affecting the ability of nanoparticles to cross the physiological barrier, Cheng Bao, et al., [108] demonstrated that adjusting the shape and rigidity of α-lactalbumin nanotubes could enhance their ability to cross the intestinal mucus and cell barriers.
Nanoparticles have sustained and controlled release effects [109-112]. After some pharmaceutical preparations enter the body, the blood drug concentration is unstable or the minimum effective concentration cannot be reached due to the unstable and continuous release. Therefore, nanoparticles can provide a drug storage space and achieve slow and controlled drug release through a specific response mechanism. Some excipients are almost not affected by the slight changes in blood pH. The drugs encapsulated by nanoparticles can be released continuously, stably and slowly in tumor microenvironment simulation solutions with different pH [113]. The release of some nanoparticles is related to the environment, which can be used to design the drug-loaded nanoparticles with pH-responsive release. G. Deepa, et al., [114] explored the in vitro release of cytarabine nanoparticles loaded with chitosan as the scaffold. They found that the release rate of chitosan nanoparticles was related to pH: Under low pH conditions, the protonation of amino groups in chitosan polymers would lead to the enhanced repulsion between chains and the increase in pore size, thus accelerating the release of drug. On the contrary, under alkaline conditions, the release of drug was slow, and the sustained release could reach 72 h.
Nanoparticles can prolong the retention time and half-life of drugs in vivo [115-118]. Pulmonary drug delivery can prolong the retention time of drug in blood due to its large absorption surface area and high permeability of epithelium. Taking advantage of the fact that microparticles with a size of 1-5μm can be deposited at the bottom of the lung and reach the alveolar, it is possible to design nanoparticles of corresponding size to encapsulate the drug and extend the time of circulation in the blood through lung absorption. In the research by Kang Liu, et al., [119] compared with single and combined intravenous administration of paclitaxel (PTX) and (QUE), nanoparticles with the particle size of 1-5 μm can significantly increase the blood circulation time of PTX and QUE, with the half-lives of PTX and QUE extending from 0.32h and 1.13h to 3.58h, respectively.
As the nanocarrier, liposome has the characteristics of non-toxicity, no immunogenicity, degradation, and sustained release, which have been mentioned above, However, the traditional liposome composed of lecithin and cholesterol is a thermodynamically unstable system, which limits its application. There were many destructive factors to liposomes in blood: High density lipoprotein (BCD) was the main component that destroyed liposomes; apolipoprotein was easy to fall off from BCD and bind to liposome phospholipid; BCD and liposome were easy to occur, and they exchanged with phospholipid, and liposome membrane formed pores. At the same time, the liposome activates the complement system in blood and finally forms a tapping membrane complex, and hydrophilic channels appear in the liposome membrane, causing drug leakage and massive entry of water and electrolyte, and finally permeating and cracking the liposome. Serum albumin and liposome phospholipid combine to form a complex to reduce the stability; Phospholipases in blood can hydrolyze phospholipids, and the strength of this reaction is determined by the structure of phospholipids. After entering the circulatory system, most of the unmodified liposomes were transferred to the liver and spleen and other regions rich in mononuclear phagocyte system, and a small amount was taken up by the lung, bone marrow and kidney. The hepatocyte membrane receptor recognizes the negatively charged groups of phospholipids directly exposed to the surface, so that the liposomes are first swallowed by the hepatocytes. These factors combined to make the half-life of traditional liposomes only ten minutes, but the birth of long-circulating liposomes to make up for this defect. Long-circulating liposomes can prevent many different components in the blood, especially opsonins, from binding to it due to the hydrophilic groups, thus reducing the affinity with the mononuclear phagocyte system MPS, and it can stably exist in the circulatory system and prolong the half-life to increase the uptake of tumor tissue by itself. Jibin Guan, et al., [120] designed a cation long-circulation liposome, in which the cation was located in the liposome, and PEG coverage played a protective role with the outer layer of the liposome. The liposome had an asymmetric structure, which was conducive to covering the surface negative charge with the liposome, avoiding the removal of RES, and improving its in vivo circulation time. The in vivo anti-tumor result show that that siRNA and PTX delivered by the liposome can effectively reverse the drug resistance of Hela tumor-bearing mice.
Discussion
This paper reviews the various types of nanoparticles used in the field of drug delivery and their role in reversing drug resistance in tumors. It should be noted that although nanotechnology is an emerging discipline, and most of the examples mentioned above are in the laboratory research stage, with the increased attention, enrichment and improvement of research methods, some representative nano-preparations have been approved for marketing (Table 2). The development of these pharmaceutical preparations is largely associated with the lack of clinical demand for treatment of drug-resistant tumors. At the same time, it should be noted that these preparations also had some side effects, which required further research and improvement (Table 3).
Although some problems inevitably occur in the research and development of nanoparticles, based on the basic characteristic of extremely small particle size, nanoparticles show many advantages in the delivery of drugs to reverse tumor drug resistance: The nano drug carrier can enter capillaries through blood circulation, and can also penetrate through the intercellular space of endothelial cells, enter lesions, and be absorbed by cells in a pinocytosis manner, thus realizing targeted drug administration and improving the bioavailability of drugs. The nano carrier has smaller particle size and higher specific surface area, can embed hydrophobic drugs, improves the solubility of the hydrophobic drugs, and reduces the side effect of cosolvent in conventional medicine application. Target drug administration can be realized aft that nano drug carrier is modified by the targeting group, so that the drug dosage can be reduce, and the side effects can be reduced, such as folic acid modified drug-loaded nanoparticles, magnetic drug-loaded nanoparticles and the like. The nanocarrier can penetrate through the restrictions imposed by body barriers on the action of drugs, such as the blood-brain barrier, the blood-eye barrier, and the cell biofilm barrier, to allow the drugs to reach the lesions and improve the efficacy.
From the point of view of the mechanism of tumor drug resistance, the transport of drugs by drug efflux transporters is still the most important factor leading to drug resistance [47-50]. In response to one of the mechanisms, researchers are constantly developing new strategies to reverse drug resistance from multi-pathway, multi-pathway inhibition of drug efflux transporters. Representative signaling pathways that mediate drug efflux transporters are NF-κB61, PKC, and PI3 [121]. Therefore, the delivery of small biological molecules such as mRNA, miRNA, and siRNA that can directly act on these pathways has come into the attention of researchers [122-125]. Compared with chemical drugs, these bioactive small molecules have the following characteristics: 1) Small molecular weight, easy to be encapsulated; 2) Having good biocompatibility and weak immunogenicity; 3) Direct localization to cells for more accurate targeting; 4) It is more susceptible to environmental factors such as temperature, enzyme, and pH.
With the promotion of "precision medicine", small bioactive molecules as drugs targeted treatment of drug-resistant cancer undoubtedly has great potential and advantages. Just as good ammunition needs a good gun to fire, so good drugs need the most appropriate delivery vehicles to carry them to tumor sites to exert their effects. And nanoparticles are undoubtedly the right "guns". In conclusion, we have reason to believe that, with the deepening of more research, drug-loaded nanoparticles will surely play its unique and irreplaceable role in reversing tumor drug resistance.
Conclusion
In conclusion, as a relatively emerging idea for drug delivery, the research on drug loading by nanoparticles has shown a sharp upward trend in the research on the reversal of tumor drug resistance, and some studies have achieved preliminary results. However, tumor resistance has long been a problem, and its occurrence and development depend on complex physiological mechanisms. Therefore, reversing tumor resistance cannot be achieved over night, but the development of nanoparticle as a drug carrier provides a reliable method for achieving this goal. Looking into the future, nanoparticles will surely play a greater role in reversing drug resistance of tumors, and nanoparticles will also assume more important tasks in the struggle between human beings and cancer.
References
- Bukowski K, Kciuk M, Kontek R (2020) Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci 21: 3233.
- Boshra Tinoush, Iman Shirdel, Michael Wink (2020) Phytochemicals: Potential lead molecules for MDR reversal. Front Pharmacol 11: 832.
- Paskeviciute M, Petrikaite V (2019) Overcoming transporter-mediated multidrug resistance in cancer: Failures and achievements of the last decades. Drug Deliv Transl Res 9: 379-393.
- Yuhong Cao, Yiwei Shi, Ying Cai, et al. (2020) The effects of traditional chinese medicine on p-glycoprotein-mediated multidrug resistance and approaches for studying the herb-p-glycoprotein interactions. Drug Metab Dispos 48: 972-979.
- Peggy Liu-Kreyche, Hong Shen, Anthony M Marino, et al. (2019) Lysosomal p-gp-mdr1 confers drug resistance of brentuximab vedotin and its cytotoxic payload monomethyl auristatin e in tumor cells. Front Pharmacol 10: 749.
- Su-Fern Tan, Wendy Dunton, Xin Li, et al. (2019) Acid ceramidase promotes drug resistance in acute myeloid leukemia through NF-?B-dependent P-glycoprotein upregulation. J Lipid Res 60: 1078-1086.
- Jayshree Hirpara, Jie Qing Eu, Joanna Kia Min Ta, et al. (2019) Metabolic reprogramming of oncogene-addicted cancer cells to OXPHOS as a mechanism of drug resistance. Redox Biol 25: 101076.
- Ayman N Abunimer, Heba Mohammed, Katherine L Cook, et al. (2018) Mitochondrial autophagosomes as a mechanism of drug resistance in breast carcinoma. Ultrastruct Pathol 42: 170-180.
- Lina Al-Akra, Dong-Hun Bae, Lionel Y W Lec, et al. (2019) The biochemical and molecular mechanisms involved in the role of tumor micro-environment stress in development of drug resistance. Biochim Biophys Acta Gen Subj 1863: 1390-1397.
- Min Gao, Jian Denga, Fang Liu, et al. (2019) Triggered ferroptotic polymer micelles for reversing multidrug resistance to chemotherapy. Biomaterials 223: 119486.
- Chenwei Wu, Li Xu, Leilei Shi, et al. (2018) Supramolecularly self-assembled nano-twin drug for reversing multidrug resistance. Biomaterials Science 6: 2261-2269.
- Ruiling Chen, Gangyang Wang, Ying Zheng, et al. (2019) Drug resistance-related microRNAs in osteosarcoma: Translating basic evidence into therapeutic strategies. J Cell Mol Med 23: 2280-2292.
- Hyoung Kim, Haineng Xu, Erin George, et al. (2020) Combining PARP with ATR inhibition overcomes PARP inhibitor and platinum resistance in ovarian cancer models. Nat Commun 11: 3726.
- Leilei Guo, Yurui Xu, Anwei Zho, et al. (2020) A stimuli-responsive combination therapy for recovering p53-inactivation associated drug resistance. Materials science & engineering. C, Materials for biological applications 108: 110403.
- Hongyue Jin, Yang He, Pengfei Zhao, et al. (2019) Targeting lipid metabolism to overcome EMT-associated drug resistance via integrin beta3/FAK pathway and tumor-associated macrophage repolarization using legumain-activatable delivery. Theranostics 9: 265-278.
- Ilya Yakavets, Henri-Pierre Lassalle, Dietrich Scheglmann, et al. (2018) Temoporfin-in-cyclodextrin-in-liposome-a new approach for anticancer drug delivery: The optimization of composition. Nanomaterials (Basel) 8: 847.
- Kamel S Ahmed, Sun Changling, Xiaotian Shan, et al. (2020) Liposome-based codelivery of celecoxib and doxorubicin hydrochloride as a synergistic dual-drug delivery system for enhancing the anticancer effect. J Liposome Res 30: 285-296.
- Ji-Yeon Lee, Dae Hwan Shin, Jin-Seok Kim (2019) Anticancer effect of metformin in herceptin-conjugated liposome for breast cancer. Pharmaceutics 12: 11.
- Anran Cai, Chunhua Wang, Miaomiao Yan, et al. (2018) A liposome preparation based on ß-CD-LPC molecule and its application as drug-delivery system. Nanomedicine (Lond) 13: 2777-2789.
- Prachi Desai, Anjana Venkataramanan, Rebecca Schneider, et al. (2018) Self-assembled, ellipsoidal polymeric nanoparticles for intracellular delivery of therapeutics. J Biomed Mater Res A 106: 2048-2058.
- Shaoqiong Liu, Robert J Ono, Chuan Yan, et al. (2018) Dual ph-responsive shell-cleavable polycarbonate micellar nanoparticles for in vivo anticancer drug delivery. ACS Appl Mater Interfaces 10: 19355-19364.
- E Judy, Moumita D, N Kishore (2020) Partitioning of anticancer drug 5-fluorouracil in micellar media explored by physicochemical properties and energetics of interactions: Quantitative insights for implications in drug delivery. Colloids Surf B Biointerfaces 187: 110730.
- Petr Chytil, Milada Šírová, Júlia Kudlácová, et al. (2018) Bloodstream stability predetermines the antitumor efficacy of micellar polymer-doxorubicin drug conjugates with ph-triggered drug release. Mol Pharm 15: 3654-3663.
- Sylwia Michlewska, Maksim Ionov, Marta Maroto-Díaz, et al. (2018) Ruthenium dendrimers as carriers for anticancer siRNA. Journal of Inorganic Biochemistry 181: 18-27.
- Ugutz Unzueta, Paolo Saccardo, Joan Domingo-Espín, et al. (2014) Sheltering DNA in self-organizing, protein-only nano-shells as artificial viruses for gene delivery. Nanomedicine 10: 535-541.
- Sudarat Khadsai, Noppadol Seeja, Nunthiya Deepuppha, et al. (2018) Poly(acrylic acid)-grafted magnetite nanoparticle conjugated with pyrrolidinyl peptide nucleic acid for specific adsorption with real DNA. Colloids Surf B Biointerfaces 165: 243-251.
- Juan Zhao, Yangyang Liu, Lulu Wang, et al. (2018) Functional and modified nanocrystals technology for target drug delivery. J Nanosci Nanotechnol 18: 5207-5221.
- Lydia Alvarez-Erviti, Yiqi Seow, HaiFang Yin, et al. (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature Biotechnology 29: 341-345.
- Yang Yang, Ze Zhao, Changwei Xie, et al. (2020) Dual-targeting liposome modified by glutamic hexapeptide and folic acid for bone metastatic breast cancer. Chem Phys Lipids 228: 104882.
- Yan Lyu, Jianfeng Zeng, Yuyan Jiang, et al. (2018) Enhancing both biodegradability and efficacy of semiconducting polymer nanoparticles for photoacoustic imaging and photothermal therapy. ACS Nano 12: 1801-1810.
- Xiao-Yun Lu, Dao-Cheng Wu, Zheng-Jun Li, et al. (2011) Polymer nanoparticles. Prog Mol Biol Transl Sci 104: 299-323.
- Leopoldo Sitia, Raffaele Ferrari, Martina B Violatto, et al. (2016) Fate of PLA and PCL-based polymeric nanocarriers in cellular and animal models of triple-negative breast cancer. Biomacromolecules 17: 744-755.
- Qinyue Chen, Huihui Liang, Yali Sun, et al. (2019) A carbohydrate mimetic peptide modified size-shrinkable micelle nanocluster for anti-tumor targeting and penetrating drug delivery. Int J Nanomedicine 14: 7339-7352.
- Hong-Lin Mao, Feng Qian, Shun Li, et al. (2019) Delivery of doxorubicin from hyaluronic acid-modified glutathione-responsive ferrocene micelles for combination cancer therapy. Mol Pharm 16: 987-994.
- Qi Shuai, Yue Cai, Guangkuo Zhao, et al. (2020) Cell-penetrating peptide modified PEG-PLA micelles for efficient PTX delivery. Int J Mol Sci 21: 1856.
- Jasbir Singh, Sapna Desai, Snehlata Yadav, et al. (2016) Polymer drug conjugates: Recent advancements in various diseases. Curr Pharm Des 22: 2821-2843.
- Feng Q, Tong R (2016) Anticancer nanoparticulate polymer-drug conjugate. Bioeng Transl Med 1: 277-296.
- Chauhan AS (2015) Dendrimer nanotechnology for enhanced formulation and controlled delivery of resveratrol. Ann N Y Acad Sci 1348: 134-140.
- Strong LE, West JL (2015) Hydrogel-coated near infrared absorbing nanoshells as light-responsive drug delivery vehicles. ACS Biomater Sci Eng 1: 685-692.
- Toni Nunes, Thomas Pons, Xue Hou, et al. (2019) Pulsed-laser irradiation of multifunctional gold nanoshells to overcome trastuzumab resistance in HER2-overexpressing breast cancer. J Exp Clin Cancer Res 38: 306.
- Rita Mendes, Pedro Pedrosa, Joao C Lima, et al. (2017) Photothermal enhancement of chemotherapy in breast cancer by visible irradiation of gold nanoparticles. Scientific Reports 7: 10872.
- Lakshmi Pallavi Ganipineni, Bernard Ucakar, Nicolas Joudiou, et al. (2018) Magnetic targeting of paclitaxel-loaded poly(lactic-co-glycolic acid)-based nanoparticles for the treatment of glioblastoma. Int J Nanomedicine 13: 4509-4521.
- Ling Chen, Yang Wu, Haoan Wu, et al. (2019) Magnetic targeting combined with active targeting of dual-ligand iron oxide nanoprobes to promote the penetration depth in tumors for effective magnetic resonance imaging and hyperthermia. Acta Biomater 96: 491-504.
- Christopher J Legge, Helen E Colley, Michelle A Lawson, et al. (2019) Targeted magnetic nanoparticle hyperthermia for the treatment of oral cancer. J Oral Pathol Med 48: 803-809.
- Flavia Fontanaa, Patrícia Figueiredo, Pei Zhang, et al. (2018) Production of pure drug nanocrystals and nano co-crystals by confinement methods. Advanced Drug Delivery Reviews 131: 3-21.
- Sierra Walker, Sara Busatto, Anthony Pham, et al. (2019) Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics 9: 8001-8017.
- Gu KS, Chen Y (2012) Mechanism of P-glycoprotein expression in the SGC7901 human gastric adenocarcinoma cell line induced by cyclooxygenase-2. Asian Pac J Cancer Prev 13: 2379-2383.
- Meltem DK, Ozlem DI, Ufuk G, et al. (2006) Development of rational in vitro models for drug resistance in breast cancer and modulation of MDR by selected compounds. Anticancer Res 26: 4559-4568.
- Yancai Liu, Xuegang Liu, Shan Yang (2021) microRNA-221 upregulates the expression of P-gp and Bcl-2 by activating the Stat3 pathway to promote doxorubicin resistance in osteosarcoma cells. Biol Pharm Bull 44: 861-868.
- Lee FY, Sciandra J, Siemann DW (1989) A study of the mechanism of resistance to Adriamycin in vivo. Glutathione metabolism, P-glycoprotein expression, and drug transport. Biochem Pharmacol 38: 3697-3705.
- Battistella C, Klok HA (2017) Reversion of p-gp-mediated drug resistance in ovarian carcinoma cells with phpma-zosuquidar conjugates. Biomacromolecules 18: 1855-1865.
- Yu Gao, Lingli Chen, Zhiwen Zhang, et al. (2011) (Reversal of multidrug resistance by reduction-sensitive linear cationic click polymer/iMDR1-pDNA complex nanoparticles. Biomaterials 32: 1738-1747.
- Shafiei-Irannejad V, Samadi N, Salehi R, et al. (2018) Reversion of multidrug resistance by co-encapsulation of doxorubicin and metformin in poly(lactide-co-glycolide)-d-alpha-tocopheryl polyethylene glycol 1000 succinate nanoparticles. Pharmaceutical Research 35: 119.
- Jiaqi Wang, Xinyi Tao, Yufei Zhan, et al. (2010) Reversion of multidrug resistance by tumor targeted delivery of antisense oligodeoxynucleotides in hydroxypropyl-chitosan nanoparticles. Biomaterials 31: 4426-4433.
- Cong Wu, Meng-Qing Gong, Bo-Ya Liu, et al. (2017) Co-delivery of multiple drug resistance inhibitors by polymer/inorganic hybrid nanoparticles to effectively reverse cancer drug resistance. Colloids Surf B Biointerfaces 149: 250-259.
- Xu Cheng, Dapeng Li, Min S, et al. (2019) Co-delivery of DOX and PDTC by pH-sensitive nanoparticles to overcome multidrug resistance in breast cancer. Colloids Surf B Biointerfaces 181: 185-197.
- Bao-an Chen, Pei-pei Mao, Jian Chen, et al. (2010) Reversal of multidrug resistance by magnetic Fe3O4 nanoparticle copolymerizating daunorubicin and MDR1 shRNA expression vector in leukemia cells. Int J Nanomedicine 5: 437-444.
- Nana Guo, Chen Gao, Jing L, et al. (2018) Reversal of ovarian cancer multidrug resistance by a combination of lah4-l1-simdr1 nanocomplexes with chemotherapeutics. Mol Pharm 15: 1853-1861.
- Haijun Yu, Zhiai Xu, Xianzhi Chen, et al. (2014) Reversal of lung cancer multidrug resistance by pH-responsive micelleplexes mediating co-delivery of siRNA and paclitaxel. Macromol Biosci 14: 100-109.
- Bharti AC, Aggarwal BB (2002) Nuclear factor-kappa B and cancer: its role in prevention and therapy. Biochem Pharmacol 64: 883-888.
- Huanan Li, Yong Sun, Jie Liang, et al. (2015) pH-Sensitive pullulan-DOX conjugate nanoparticles for co-loading PDTC to suppress growth and chemoresistance of hepatocellular carcinoma. Journal of Materials Chemistry B 41: 8070-8078.
- Yuhua W, Haihua X, Jinchao Zhang, et al. (2019) RNAi therapeutic and its innovative biotechnological evolution. Biotechnol Adv 37: 801-825.
- Sandeep Kumar Vishwakarma, Priyanka Sharmila, Avinash Bardi, et al. (2017) Use of biocompatible sorafenib-gold nanoconjugates for reversal of drug resistance in human hepatoblatoma cells. Sci Rep 7: 8539.
- Jing Miao, Yong-Zhong Du, Hong Yuan, et al. (2013) Drug resistance reversal activity of anticancer drug loaded solid lipid nanoparticles in multi-drug resistant cancer cells. Colloids Surf B Biointerfaces 110: 74-80.
- Meng-Dan Zhao, Jun-Qin Li, Feng-Ying Che, et al. (2019) Co-delivery of curcumin and paclitaxel by "core-shell" targeting amphiphilic copolymer to reverse resistance in the treatment of ovarian cancer. Int J Nanomedicine 14: 9453-9467.
- Jia-Hui Sun, Chong Ye, En-He Bai, et al. (2019) Co-delivery nanoparticles of doxorubicin and chloroquine for improving the anti-cancer effect in vitro. Nanotechnology 30: 085101.
- Sameer SK, Eameema M, Towseef AR, et al. (2016) Co-delivery of rapamycin- and piperine-loaded polymeric nanoparticles for breast cancer treatment. Drug Deliv 23: 2608-2616.
- Wei He, Annie Turkeshi, Xiaotong Li, et al. (2020) Progress in systemic co-delivery of microRNAs and chemotherapeutics for cancer treatment by using lipid-based nanoparticles. Ther Deliv 11: 591-603.
- Maofan Zhang, C Tilden Hagan, Yuangzeng Min, et al. (2018) Nanoparticle co-delivery of wortmannin and cisplatin synergistically enhances chemoradiotherapy and reverses platinum resistance in ovarian cancer models. Biomaterials 169: 1-10.
- Yong-E Gao, Shuang Bai, Xiaoqian Ma, et al. (2019) Codelivery of doxorubicin and camptothecin by dual-responsive unimolecular micelle-based beta-cyclodextrin for enhanced chemotherapy. Colloids Surf B Biointerfaces 183: 110428.
- Liang Zou, Di Wang, Yichen Hu, et al. (2017) Drug resistance reversal in ovarian cancer cells of paclitaxel and borneol combination therapy mediated by PEG-PAMAM nanoparticles. Oncotarget 8: 60453-60468.
- Nicola Valeri, Pierluigi Gasparini, Chiara Braconi, et al. (2010) MicroRNA-21 induces resistance to 5-fluorouracil by down-regulating human DNA MutS homolog 2 (hMSH2). Proc Natl Acad Sci USA 107: 21098-22103.
- Gaofeng L, Yanliang Z, Doulathunnisa JA, et al. (2020) Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. Journal of Nanobiotechnology 18: 10.
- Jie Zhao, Bin Wen, Zhengbing Tan, et al. (2020) IRGD-targeted hybrid nanoparticles reverses multi-drug resistant to effectively combat liver cancer. J Drug Target 28: 1063-1070.
- Hannelore M, Anna K, Aleksandar P, et al. (2014) Tumor vessel normalization by chloroquine independent of autophagy. Cancer Cell 26: 190-206.
- Tingting Lv, Ziying Li, Liang Xu, et al. (2018) Chloroquine in combination with aptamer-modified nanocomplexes for tumor vessel normalization and efficient erlotinib/Survivin shRNA co-delivery to overcome drug resistance in EGFR-mutated non-small cell lung cancer. Acta Biomater 76: 257-274.
- Simons K, Sampaio JL (2011) Membrane organization and lipid rafts. Cold Spring Harb Perspect Biol 3: a004697.
- Simons K, Toomre D (2000) Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1: 31-39.
- Bin Du, Wanying Zhu, Lili Yu, et al. (2021) TPGS2k-PLGA composite nanoparticles by depleting lipid rafts in colon cancer cells for overcoming drug resistance. Nanomedicine 35: 102307.
- Huan Y, Tang C, Yin C (2018) Estrone-modified pH-sensitive glycol chitosan nanoparticles for drug delivery in breast cancer. Acta Biomater 73: 400-411.
- Manish Kumar, Nithya Shanthia, Arun Kumar Mahato, et al. (2019) Preparation of luliconazole nanocrystals loaded hydrogel for improvement of dissolution and antifungal activity. Heliyon 5: e01688.
- Jean-Baptiste Coty, Cédric Martin, Isabella Telò, et al. (2020) Use of spray flash evaporation (sfe) technology to improve dissolution of poorly soluble drugs: Case study on furosemide nanocrystals. International Journal of Pharmaceutics 589: 119827.
- Miriam Colombo, Clara Minussi, Steven Orthma, et al. (2018) Preparation of amorphous indomethacin nanoparticles by aqueous wet bead milling and in situ measurement of their increased saturation solubility. European Journal of Pharmaceutics and Biopharmaceutics 125: 159-168.
- Ying Zhang, Lin Lina, Liang Liu, et al. (2018) Ionic-crosslinked polysaccharide/PEI/DNA nanoparticles for stabilized gene delivery. Carbohydrate Polymers 201: 246-256.
- Duy Toan Pham, Nuttawut Saelim, Waree Tiyaboonchai (2019) Alpha mangostin loaded crosslinked silk fibroin-based nanoparticles for cancer chemotherapy. Colloids Surf B Biointerfaces 181: 705-713.
- Chuanfen Pu, Wenting Tang, Mengyao Liu, et al. (2020) Resveratrol-loaded hollow kafirin nanoparticles via gallic acid crosslinking: An evaluation compared with their solid and non-crosslinked counterparts. Food Research International 135: 109308.
- Nieves Iglesias, Elsa Galbis, M Jesús Díaz-Blanco, et al. (2018) Loading studies of the anticancer drug camptothecin into dual stimuli-sensitive nanoparticles. Stability scrutiny. Int J Pharm 550: 429-438.
- Shi Xu, Bogdan Z Olenyuk, Curtis T Okamoto, et al. (2013) Targeting receptor-mediated endocytotic pathways with nanoparticles: Rationale and advances. Adv Drug Deliv Rev 65: 121-138.
- Kumari S, Kondapi AK (2018) Receptor-mediated targeted delivery of DNA using lactoferrin nanoparticles. Int J Biol Macromol 108: 401-407.
- Wen-Long Wang, Zhengxi Guo, Yu Lu, et al. (2019) Receptor-mediated and tumor-microenvironment combination-responsive Ru nanoaggregates for enhanced cancer phototheranostics. ACS Appl Mater Interfaces 11: 17294-17305.
- Sahoo AK, Bose B, Mahesh A (2017) Receptor-mediated enhanced cellular delivery of nanoparticles using recombinant receptor-binding domain of diphtheria toxin. Mol Pharm 14: 23-30.
- Crous A, Abrahamse H (2020) Effective gold nanoparticle-antibody-mediated drug delivery for photodynamic therapy of lung cancer stem cells. Int J Mol Sci 21: 3742.
- Niladri Chattopadhyay, Humphrey Fonge, Zhongli Cai, et al. (2012) Role of antibody-mediated tumor targeting and route of administration in nanoparticle tumor accumulation in vivo. Mol Pharm 9: 2168-2179.
- Luis J Cruza, Paul J Tacken, Remco Fokkink, et al. (2011) The influence of PEG chain length and targeting moiety on antibody-mediated delivery of nanoparticle vaccines to human dendritic cells. Biomaterials 32: 6791-6803.
- Rong Zhu, Chun-ge Zhang, Yang Liu, et al. (2015) CD147 monoclonal antibody mediated by chitosan nanoparticles loaded with alpha-hederin enhances antineoplastic activity and cellular uptake in liver cancer cells. Sci Rep 5: 17904.
- Lu Sun, Qinjie Wu, Feng Peng, et al. (2015) Strategies of polymeric nanoparticles for enhanced internalization in cancer therapy. Colloids and Surfaces B: Biointerfaces 135: 56-72.
- Dandan Wang, Hui Li, Weiliang Chen, et al. (2021) Efficient tumor-targeting delivery of siRNA via folate-receptor mediated biomimetic albumin nanoparticles enhanced by all-trans retinoic acid. Mater Sci Eng C Mater Biol Appl 119: 111583.
- Nan Zheng, Wenbing Dai, Hua Zhang, et al. (2015) Lanreotide-conjugated PEG-DSPE micelles: An efficient nanocarrier targeting to somatostatin receptor positive tumors. J Drug Target 23: 67-78.
- Daniel Putzer, Alexander Kroiss, Dietmar Waitz, et al. (2013) Somatostatin receptor PET in neuroendocrine tumours: 68Ga-DOTA0, Tyr3-octreotide versus 68Ga-DOTA0-lanreotide. Eur J Nucl Med Mol Imaging 40: 364-372.
- Hanh Thuy Nguyen, Cao Dai Phung, Raj Kumar Thapa, et al. (2018) Multifunctional nanoparticles as somatostatin receptor-targeting delivery system of polyaniline and methotrexate for combined chemo-photothermal therapy. Acta Biomater 68: 154-167.
- Xiaolong Tang, Chunmei Xie, Zhenyou Jiang, et al. (2018) Rituximab (anti-CD20)-modified AZD-2014-encapsulated nanoparticles killing of B lymphoma cells. Artif Cells Nanomed Biotechnol 46: 1063-1073.
- Stewart AM, Grass ME (2020) Practical approach to modeling the impact of amorphous drug nanoparticles on the oral absorption of poorly soluble drugs. Mol Pharm 17: 180-189.
- Ramesh Soundararajan, George Wang, Asya Petkova, et al. (2020) Hyaluronidase coated molecular envelope technology nanoparticles enhance drug absorption via the subcutaneous route. Mol Pharmaceutics 17: 2599-2611.
- Chiaki Yoshioka, Yoshimasa Ito, Noriaki Nagai (2018) An oral formulation of cilostazol nanoparticles enhances intestinal drug absorption in rats. Exp Ther Med 15: 454-460.
- Zhonghui Xie, Zhenhai Zhang, Huixia Lv (2019) Rapamycin loaded tpgs-lecithins-zein nanoparticles based on core-shell structure for oral drug administration. Int J Pharm 568: 118529.
- Eun Suh Kim, Da Young Kim, Ji-Soo Lee, et al. (2019) Mucoadhesive chitosan-gum arabic nanoparticles enhance the absorption and antioxidant activity of quercetin in the intestinal cellular environment. J Agric Food Chem 67: 8609-8616.
- Xiaomin Qian, Zhendong Shi, Hongzhao Qi, et al. (2019) A novel Granzyme B nanoparticle delivery system simulates immune cell functions for suppression of solid tumors. Theranostics 9: 7616-7627.
- Cheng Bao, Bin Liu, Bin Li, et al. (2020) Enhanced transport of shape and rigidity-tuned alpha-lactalbumin nanotubes across intestinal mucus and cellular barriers. Nano Lett 20: 1352-1361.
- Xiaoxiao Sun, Dongyan Yu, Zhuoyang Ying, et al. (2019) Fabrication of ion-crosslinking aminochitosan nanoparticles for encapsulation and slow release of curcumin. Pharmaceutics 11: 584.
- Giulia Orlandi, Elia Bari, Laura Catenacci, et al. (2020) Polyphenols-loaded sericin self-assembling nanoparticles: A slow-release for regeneration by tissue-resident mesenchymal stem/stromal cells. Pharmaceutics 12: 381.
- Marwa Hamdi, Rim Nasri, Suming Li, et al. (2020) Design of blue crab chitosan responsive nanoparticles as controlled-release nanocarrier: Physicochemical features, thermal stability and in vitro pH-dependent delivery properties. Int J Biol Macromol 145: 1140-1154.
- Yoshinori Morikawa, Tatsuaki Tagami, Akihiro Hoshikawa, et al. (2018) The use of an efficient microfluidic mixing system for generating stabilized polymeric nanoparticles for controlled drug release. Biol Pharm Bull 41: 899-907.
- Sofia Moura, Jennifer Noro, Patrícia Cerqueira, et al. (2020) Poloxamer 407 based-nanoparticles for controlled release of methotrexate. Int J Pharm 575: 118924.
- Deepa G, Sivakumar KC, Sajeevan TP (2018) Molecular simulation and in vitro evaluation of chitosan nanoparticles as drug delivery systems for the controlled release of anticancer drug cytarabine against solid tumours. 3 Biotech 8: 493.
- Shaojun Pan, Lijia Pei, Amin Zhang, et al. (2020) Passion fruit-like exosome-PMA/Au-BSACe6 nanovehicles for real-time fluorescence imaging and enhanced targeted photodynamic therapy with deep penetration and superior retention behavior in tumor. Biomaterials 230: 119606.
- Peipei Jin, Rui Sha, Yunjiao Zhang, et al. (2019) Blood circulation-prolonging peptides for engineered nanoparticles identified via phage display. Nano Lett 19: 1467-1478.
- Jialiang Zhang, Zhihong Liu, Chun Tao, et al. (2020) Cationic nanoemulsions with prolonged retention time as promising carriers for ophthalmic delivery of tacrolimus. European Journal of Pharmaceutical Sciences 144: 105229.
- Haiwei Ye, Liping Zhou, Haili Jin, et al. (2020) Sorafenib-loaded long-circulating nanoliposomes for liver cancer therapy. Biomed Res Int 2020: 1351046.
- Kang Liu, Weijuan Chen, Tingting Yang, et al. (2017) Paclitaxel and quercetin nanoparticles co-loaded in microspheres to prolong retention time for pulmonary drug delivery. Int J Nanomedicine 12: 8239-8255.
- Jibin Guan, Jin Sun, Feilong Su, et al. (2017) Hypoxia-induced tumor cell resistance is overcome by synergistic GAPDH-siRNA and chemotherapy co-delivered by long-circulating and cationic-interior liposomes. Nanoscale 9: 9190-9201.
- Richard Callaghan, Emily Crowley, Simon Potter, et al. (2008) P-glycoprotein: So many ways to turn it on. J Clin Pharmacol 48: 365-378.
- Zhdanov VP (2019) Intracellular RNA delivery by lipid nanoparticles: Diffusion, degradation, and release. Biosystems 185: 104032.
- Ziying Yi, Yunhai Li, Yushen Wu, et al. (2020) Circular RNA 0001073 attenuates malignant biological behaviours in breast cancer cell and is delivered by nanoparticles to inhibit mice tumour growth. Onco Targets Ther 13: 6157-6169.
- Min Sang Lee, Nak Won Kim, Jung Eun Lee, et al. (2018) Targeted cellular delivery of robust enzyme nanoparticles for the treatment of drug-induced hepatotoxicity and liver injury. Acta Biomater 81: 231-241.
- Suresh Kumar Gulla, Bonda Rama Rao, Gopikrishna Mok, et al. (2019) In vivo targeting of DNA vaccines to dendritic cells using functionalized gold nanoparticles. Biomaterials Science 7: 773-788.
Corresponding Author
Marine College, Shandong University, Weihai 264209, China.
Copyright
© 2022 Jinqian M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.