Efficacy of Surgically Extracted Sperm in Art
Abstract
It is feasible to beat extreme occurrences of male element fruitlessness, for example, azoospermia, with the utilization of intracytoplasmic sperm infusion. The chance for beset guys to become natural guardians is made accessible by the recovery of the male gamete from the epididymis or the testis. The reason for this study is to analyze the latest discoveries about the careful sperm recovery methods that might be utilized related to in vitro treatment (ICSI). We present a verifiable survey of the development of careful sperm recovery techniques, remembering its sign for both azoospermic and non-azoospermic men, as well as the specialized elements of each methodology. What's more, we present and basically look at the information about the viability of in vitro treatment (ICSI) using non-discharged sperm, as well as the repercussions of this strategy to the soundness of the children that are effectively delivered. In the event that the PESA technique is fruitless, a needle yearning biopsy (Seize) is performed to recover testicular sperm. The epididymis of guys who have non-obstructive azoospermia will be without any trace of sperm, and recovering sperm from the gonads during this condition will be vital. Catch might be utilized to do a percutaneous recovery as an initial step. From that point onward, testicular sperm extraction (TESE) from open microsurgical biopsies is done utilizing either the single seminiferous tubule (SST) or the microdissection TESE methods. This is finished if they are ineffective. The technique that is the most un-convoluted and meddlesome ought to be endeavored first.
Keywords
Sperm retrieval, Assisted reproductive techniques, Male infertility, Azoospermia, DNA fragmentation
Introduction
Barren guys who are tormented by azoospermia have the choice of getting intracytoplasmic sperm infusion (ICSI), which is a treatment that is both viable and compelling. Through the most common way of collecting sperm from the epididymis or testis, it is feasible to embrace in vitro preparation (ICSI) and offer distressed men with the chance to become natural guardians. Disregarding the way that reconstructive medical procedure can possibly fix obstructive azoospermia (OA), the couple might find that careful rebuilding isn't generally practical or wanted [1]. As opposed to constant obstructive pneumonic sickness (COPD), non-obstructive azoospermia (NOA) is portrayed by shifting levels of spermatogenic disappointment and is entirely difficult to fix. In circumstances like these, the best way to become an organic dad is to go through testicular sperm recovery, which is then trailed by in vitro treatment (ICSI). In this review, we give a rundown of the data that relates to the techniques that are utilized for careful sperm recovery (SR). Initial, a verifiable survey of the careful sperm recovery strategies, its sign in both azoospermic and non-azoospermic guys, and the specialized elements of each approach are examined. This is trailed by a conversation of the specialized qualities of every methodology. We will address the outcome of in vitro preparation (ICSI) utilizing precisely extricated sperm, as well as the consequences for the children that are created because of this system.
At the point when it came to treating azoospermia previously, the main decisions accessible were either contributor insemination or reconstructive medical procedure (if there was blockage). A rising number of azoospermic men are currently ready to become natural fathers by means of the utilization of sperm gathered from their epididymis or testis because of the advancement of intracytoplasmic sperm infusion, frequently known as ICSI. Regardless of whether discharged, epididymal, or testicular sperm are used in vitro treatment (ICSI), the pregnancy rate continues as before [1]. The methodology of recovering sperm from the epididymis with the end goal of in vitro preparation (IVF) was first revealed for an optional obstructive patient azoospermania. The creators used a micropipette to suction sperm from the uncovered epididymal ductule after they had penetrated it with the instrument. In this manner, Silber made a promotion of an open strategy for guys who had vas aplasia. This method incorporated the microsurgical analyzation of the epididymal ductule, trailed by the opening, goal, and ensuing conclusion of the ductule with stitches (Plateau) [2]. We adjusted this methodology by utilizing a clear 26-G needle to suction sperm from the uncovered epididymis straightforwardly. This was managed with practically no microsurgical entry point or stitching (OFNA) [3,4]. Another and more clear technique was accounted for by Shrivastava, et al., in which the goal was done through the skin (PESA) [5].
A Historical Overview of Sperm Retrieval Methods
The treatment known as microepididymal sperm goal (Plateau), which was powerful in accomplishing pregnancy, was first detailed by Sanctuary Smith and partners in the year 1985 [6]. The primary series of in vitro treatment (ICSI) utilizing sperm recuperated from the epididymis was accounted for by Tournaye, et al. in 1994. The review was led on a gathering of twelve men who were determined to have osteoarthritis (OA) inferable from innate reciprocal shortfall of the vas deferens (CBAVD) [7]. Plateau was performed on the male life partners while they were under broad sedation. The creators utilized a careful technique that has remained for the most part steady up till the current day. 21 point four percent of the ones who had sperm desire methodology proceeded to get pregnant.
Testicular sperm recovery by the utilization of an open biopsy (TESE; testicular sperm extraction) was done by Devroey, et al. around the same time and at similar foundation concerning guys who had encountered ineffective epididymal goal [8]. The ripeness rate after in vitro preparation (ICSI) utilizing testicular sperm was 45.5%; be that as it may, there were no pregnancies gotten. Despite this, the main pregnancies after TESE-ICSI were recorded in a bunch of twelve men who were experiencing osteoarthritis because of CBAVD one year later. At around similar time, similar gathering of examiners likewise completed TESE on fifteen male patients who were determined to have NOA and had differing levels of testicular brokenness [9]. Testicular recovery was powerful as a rule, and in vitro preparation (ICSI) utilizing testicular sperm extricated from this gathering of guys with NOA brought about a treatment pace of 47.8% and an undeveloped organism implantation pace of 18.7% following new exchanges [10]. Make and Shrivastav were quick to report a more simple choice for sperm recovery in guys who had irreversible osteoarthritis (OA) in the year 1994 [11]. Following the system known as percutaneous epididymal sperm goal (PESA), spermatozoa were treated with pentoxifylline prior to being infused into the oocytes. The method was performed on 21 guys. 89% of the sperm was effectively removed, and simply four men expected to have salvage Plateau after PESA was fruitless [12].
After two years, in 1996, Lewin, et al. distributed the principal effective occurrence of testicular sperm yearning (TESA) to recover suitable sperm for in vitro treatment (ICSI) from a man who had NOA inferable from development arrest [8]. Cryptorchidism was the explanation of this patient's set of experiences of five orchidopexy medical procedures that were performed on them when they were kids. As well as having an atrophic left testis, he accompanied a high blood level of follicle invigorating chemical (FSH), and his right testis was of an ordinary size. Furthermore, confined scarring and development capture were seen during the goal biopsy. After a fruitful pregnancy, the couple brought forth a sound youngster at the full term [13]. In 1999, Schlegel and partners distributed a paper that definite the primary microdissection of seminiferous tubules with the end goal of straightforwardly distinguishing dynamic spermatogenic regions. This strategy was alluded to as the microdissection testicular sperm extraction procedure (miniature TESE). The standard TESE was contrasted with this technique, which brought about a lessening in sperm recovery from 45% to 63%. This methodology was finished on guys who had NOA [14]. In 56% (15/27) of the examples, it was possible to effectively recognize tubules that were amplified and obscure. Besides, miniature TESE was viable in recovering sperm from six men who had recently been not able to do so utilizing the ordinary TESE approach [15].
These days, in vitro treatment (ICSI) has turned into the most famous sort of preparation used for helped conceptive innovation (Workmanship), and sperm recovery (SR) has turned into an ordinary activity for barren men who have azoospermia. Ten to evade the DNA fracture that is brought about by oxidative pressure during the course of sperm travel and development through the epididymis, testicular sperm extraction has likewise been utilized in men who don't have azoospermia. Despite these endeavors, the pregnancy rates (PR) and conveyance rates that have been distributed for in vitro treatment (ICSI) keep on being fairly low. In 2008, these rates came to 28.7% and 18.9%, separately; in 2009, they came to 27.7% and 19.9%, and in 2010, they came to 26.8 and 20.0%. It is critical to take note of that the results of in vitro treatment (ICSI) are much of the time unfortunate when the amount or nature of the sperm is decreased. This is the kind of thing that is generally found in occasions of azoospermia, particularly those that incorporate spermatogenic disappointment [16].
Indications for Sperm Retrieval and Available Surgical Methods
The signs for sperm recovery shift contingent upon the restorative setting in which they are performed. The epididymis or the gonad may both give mature gametes to in vitro treatment (ICSI) in patients with OA. Since sperm are not distinguished in that frame of mind of these men, the testis is the sole organ that is focused on in NOA-related cases. Both percutaneous epididymal sperm desire (PESA) and microsurgical epididymal sperm goal (Plateau) are the methods that are many times utilized in the expulsion of epididymal sperm from patients who have osteoarthritis (OA) [17].
An essential outline of PESA is that a direct procedure might be performed under either intravenous (IV) sedation or general sedation (Figure 1). A ten milliliter arrangement of lidocaine with a grouping of two percent is regulated near the outer ring to impede the spermatic line. To forestall harm to the small veins that are apparent through the skin, loupe amplification might be utilized [18]. The recovery of sperm from the epididymis was first achieved with the utilization of a butterfly needle with a measure going from 29 to 33 [19]. The most well-known technique that is right now being utilized is the usage of a 23-30-check needle that is connected to a 1 mL tuberculin needle. This needle is then brought through the skin into the epididymis [5]. To work with the pull of epididymis liquid, which is then inspected in the embryology research center, a slight negative strain is managed once the cut techniques have been finished. A further PESA might be performed at an alternate area, moving from the tail or body to the caput epididymis, until adequate motile sperm is recuperated for in vitro preparation (ICSI) or freezing. TESA ought to be attempted if neither of the epididymis has any motile sperm that can be recovered [20].
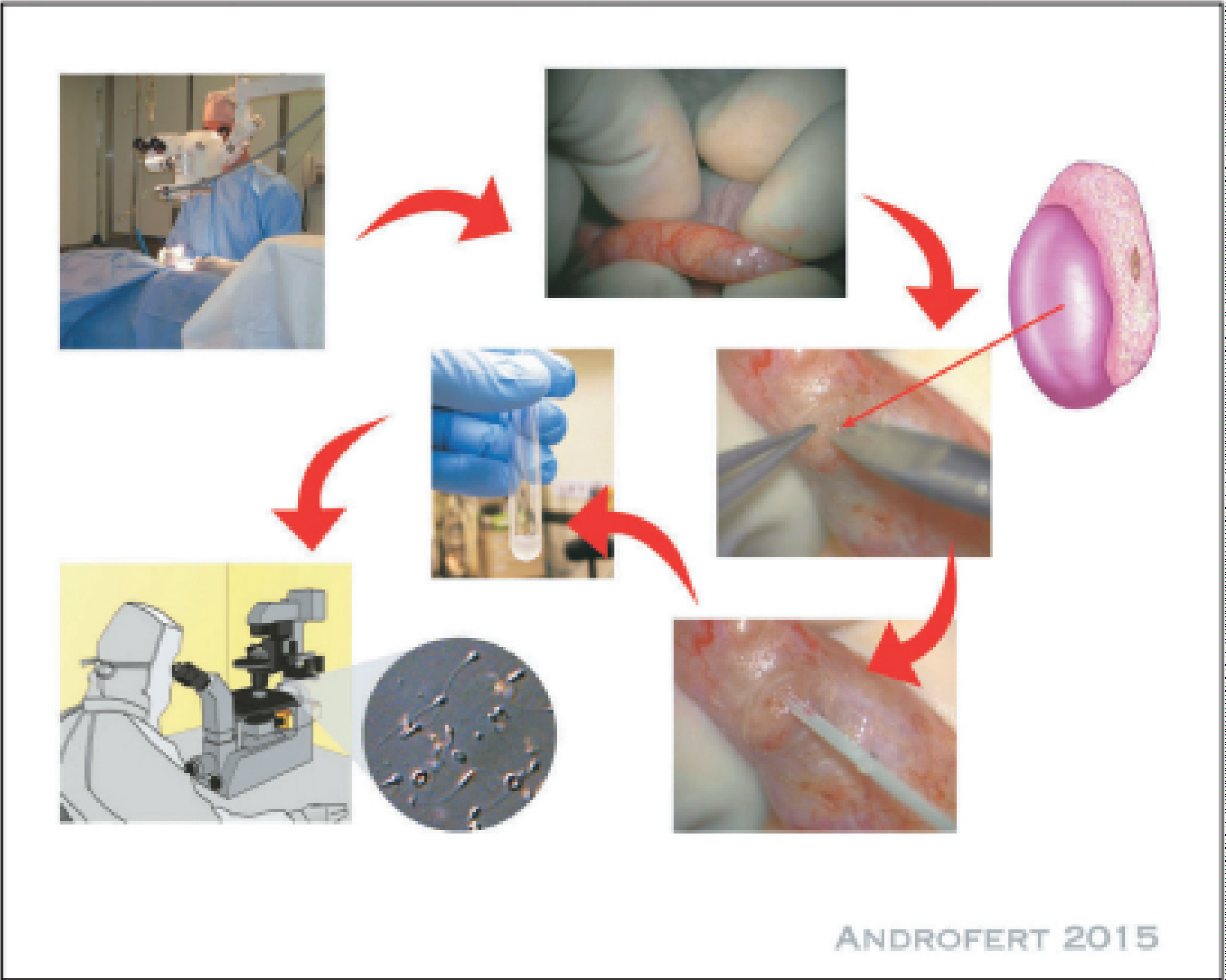
Much of the time, the Plateau activity (Figure 2) involves conveying the testis through a cross over scrotal cut that is somewhere in the range of two and three centimeters long. Analyzation of the epididymal tunica is performed under amplification going from 16 to multiple times, and a solitary hazy bigger tubule is picked for opening utilizing a sharp edge intended for use with a magnifying instrument. Goal of liquids and recuperation of sperm are both made conceivable with the expansion of culture medium drop by drop. Like the technique utilized in PESA, further desires ought to be performed nearer to the top of the epididymis than the principal endeavor. This is finished if suitable example can't be acquired. Since the distal sperm is more senescent and, accordingly, has more DNA sperm discontinuity, the proximal epididymal sperm is many times the one that is utilized for mesenchymal erythrocyte adenocarcinoma (Plateau) [21].
As a salvage choice, either TESA or TESE might be utilized if Plateau can't effectively recover motile sperm. Disregarding this, a solitary Plateau medical procedure yields a sizeable amount of great sperm that might be utilized for guaranteed in vitro treatment (ICSI) or cryopreserved for additional ICSI endeavors, subsequently diminishing the requirement for extra surgeries [22]. TESA and open testicular sperm extraction (TESE) are the strategies that are utilized for the recovery of testicular sperm [23]. In a circumstance that doesn't require hospitalization, these medicines might be performed without risk. Either the separated sperm is immediately used for in vitro treatment (ICSI) or it is cryopreserved for use sometime in the future [24]. Testicular sperm gathering is expected in circumstances of non-ovulatory adenomas (NOA), however it might likewise be expected in instances of cryptozoospermia, when discharged sperm is deficient or lacking for in vitro preparation (ICSI), and in instances of typical or odd sperm with high DNA fracture [25].
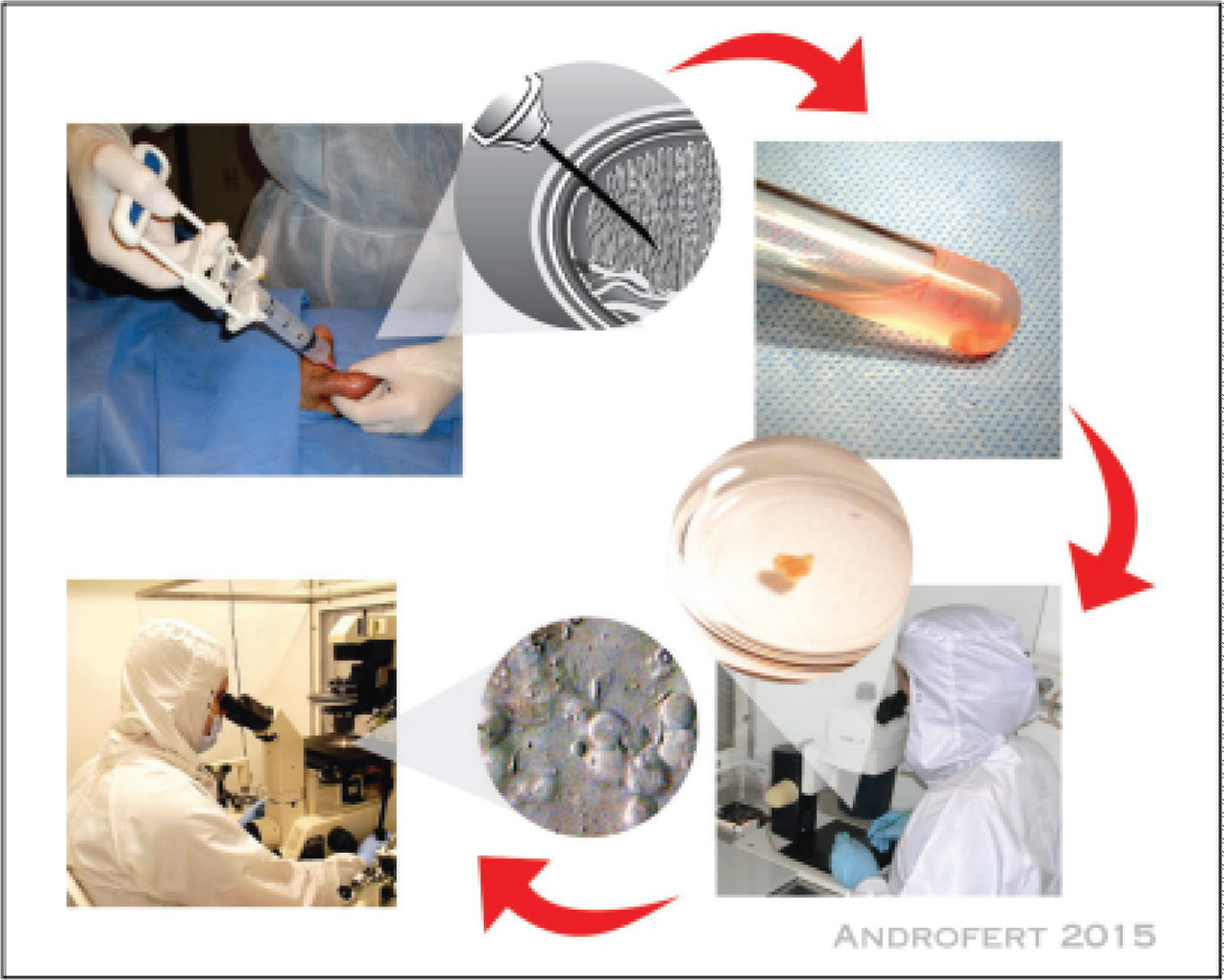
To sum up, TESA may likewise be performed under broad sedation or intravenous sedation, and it tends to be matched with spermatic string blockage, much as PESA (Figure 3). A needle with a 18-check measure is connected to a needle that has a limit of 20 milliliters, and the percutaneous cut is completed under bad tension. Under loupe amplification, the needle is embedded to forestall any harm to the skin's veins. It is then pushed this way and that to disturb the tubules and make the extraction cycle more straightforward. A very smart arrangement would be one in which all testicular regions would be examined; the needle would be put at a point of slant in the front part of the upper testicular post. A TESA might be directed on the contralateral gonad following the material has been examined at the embryology research facility. Assuming the example is considered to be lacking regarding the sum and additionally nature of sperm, then, at that point, TESA can be performed quickly [24].
Anesthesia for Sperm Retrieval
The vaginal locale is the essential focal point of sperm recovery since it is a bound and delicate piece of the male life systems. Besides, there are elevated requirements related with the entire course of helped conceptive innovation (Workmanship), and it is notable that these patients are exceptionally stressed upon the arrival of the treatment. Along these lines, one shouldn't believe this strategy to be a cutting edge sedation method, in spite of the way that it is feasible to give neighborhood sedation to most of cases through a locoregional sedative block. This is on the grounds that it may not give adequate solace to a critical extent of patients, especially the people who are going through careful intercession (PESA, TESA, TESE, Miniature TESE, Plateau). In place of reality, 33% of patients revealed feeling extremely stressed before the activity; patients who fall into this class might acquire benefits from the utilization of both locoregional sedation and sedation, or even from the usage of general sedation [26].
Since propofol has antiemetic impacts, the utilization of complete intravenous sedation with propofol (2,6-diisopropylphenol) imbuement assists patients with staying away from the most ridiculously feared postoperative side effect, which is queasiness and retching. This is as opposed to general sedation, which is led utilizing inhalational drugs [10,27]. A delicate arousing is connected with propofol mixture in contrast with breathed in halogenated drugs. This is because of the way that patients awaken with a sensation of prosperity and a reasonable mental state in the wake of getting propofol imbuement. These patients frequently endure considerably less postoperative bewilderment and tend to perceive the environmental elements and individuals around them effortlessly. They appear to be not so much unsettled but rather more helpful than they were before [28]. Patients who have general sedation with propofol prone to have a deferred recuperation than patients who get propofol alone for intravenous sedation. This is regardless of the way that patients who go through broad sedation with propofol frequently experience less postoperative issues. To wrap things up, the blend of intravenous propofol sedation under unconstrained ventilation and neighborhood sedation gives patients the pain relieving impact of nearby sedatives as well as the solace and powerful control of tension that is given by intravenous sedation. This mix empowers patients to encounter amazing fulfillment, quick recuperation, and insignificant antagonistic impacts [32-50].
Sperm Retrieval Techniques
With regards to men who have obstructive azoospermia, extricating sperm from either the epididymis or the testis is conceivable. Then again, with regards to men who don't have obstructive azoospermia, the main tasks that are gainful are those that include the gonads.
Microsurgical epididymal sperm aspiration technique
Using a scrotal entry point, the epididymis is made visible. Inside the limits of a working magnifying lens, a cut is made in the epididymal tunica, and an epididymal ductule is assembled. It is then important to suction the spermatic liquid that is removed once the ductule has been opened. Following the recuperation of an adequate number of sperm, the ductule is then shut utilizing microsutures. One more ductule is analyzed if no sperm are found (Table 1).
Percutaneous epididymal sperm aspiration
The most effective method to do it the scrotum is first purified with germ-free, and afterward it is flushed fastidiously with saline to eliminate any disinfectant that might have been abandoned. The top of the epididymis is touched and balanced out between the thumb and the index finger while the patient is affected by neighborhood sedation [20]. From that point forward, a 26-G needle that is connected to a tuberculin needle that contains 0.1 ml of sperm-washing media is utilized to puncture it straight through the skin of the scrotum. To keep the elastic from coming into direct touch with the medium, an air bubble is kept up with between the media and the elastic plug utilized by the unclogger. The partner makes a pull force by pulling the unclogger the entire way to the highest point of the needle, which is the finish of the system. During the strategy, the needle is traveled through the epididymal ductule in a quiet and delicate way. To keep up with its situation inside the epididymis, the needle is first turned through 180 degrees and afterward halfway removed. From that point onward, it is moved toward another path while as yet keeping the attractions it was before in. The pull is halfway delivered, and the needle is taken out from the epididymis; the system is finished. There are occurrences when the epididymal suction might be so slight and inadequate that there is no apparent suction, however there are different times when drops of liquid might be seen entering the needle. To decide if sperm are available, the items in the needle are painstakingly depleted onto a dish and afterward contemplated. In the event that motile sperm are not seen, the strategy is rehashed at a marginally unique site on the epididymal head. In instances of obstructive azoospermia, it is more compelling to extricate sperm from the proximal epididymis rather than the distal body or tail. The way that this is a visually impaired technique intends that there might be a requirement for a few attempts before an excellent sperm result is found. While doing PESA, a few essayists utilize a greater butterfly needle [5] and a needle with a limit of 20 milliliters. It is easy to see the drops of liquid that have been suctioned inside the cylinder.
Role of Special Tissue Processing Methods
In circumstances when there are only a couple of sperm present in the testicular tissue, the tubules must be lysed in the suitable way to help their ID. To start with, this might be achieved utilizing mechanical means simply by mincing the tissue and putting it through a progression of goes through a slim needle that is associated with a syringe [12]. Furthermore, collagenase might be utilized to do an enzymatic analyzation of the tubules [51]. Likewise, erythrocyte lysing support might be utilized to make the example more clean [52-55]. It has been accounted for by various examinations that brooding the testicular tissue for a while going from 24 to 72 hours will improve sperm motility and may likewise improve the probability of origination, particularly in men who have testicular disappointment.
ICSI Outcomes
Table 2, Table 3 and Table 4
Complications of Sperm Retrieval Procedures
Hematoma, disease, testicular parenchymal fibrosis, and testicular decay are the most well-known issues that could emerge because of sperm recovery medicines. Both percutaneous and open medicines can possibly bring about these difficulties. Following a TESE, testosterone levels might decline by a fifth or more, and it might require over a year for them to recover [33]. Hypogonadism is bound to happen in men who have minuscule testicles that are disabled and who have continuous biopsies. These men ought to be followed for the improvement of hypogonadism [34].
Conclusion
It is possible to undertake sperm retrieval treatments as an outpatient intervention under intravenous sedation and local anaesthesia when they are performed percutaneously. These procedures are free of risk. The epididymis (PESA/MESA) or testis (TESA/TESE) may be punctured or dissected in order to collect sperm in situations of OA. This process is simple and straightforward. It seems that the SR method and the reason of OA have very little influence on the results of SRR or ICSI as well as LBR. Testicular histology is a factor that determines success in instances of NOA, with better outcomes favouring hypospermatogenesis. The micro-TESE treatment is the preferred SR procedure in these specific circumstances. When processing sperm after either epididymal or testicular extraction, it is necessary to handle and prepare the sperm in an appropriate manner for both immediate usage and preservation. For subsequent usage, it is necessary to maximise the survival of post-thaw sperm, and the selection of non-motile sperm may need the use of certain laboratory procedures for accurate identification. The current research reveals that there is no significant difference in the results of in vitro fertilisation (ICSI) procedures in males who have osteoarthritis (OA) when comparing the sperm source (epididymis vs. testis), the reason of blockage (congenital versus acquired), or the sperm status (fresh versus frozen). The reproductive results of individuals with NOA seem to be less favourable when compared with those of patients with OA and ejaculated sperm. It seems that the testicular source produces superior results from in vitro fertilisation (ICSI) and a greater LBR in men who do not have azoospermia when DNA fragmentation is significant. In vitro fertilisation (ICSI) does not seem to have any adverse effects on the health of the babies who are produced as a result of assisted reproductive technology (ART). Instead, it is more probable that the gametes taken from azoospermic infertile males are of lesser quality. There is some data that suggests that the use of TESE in ICSI therapy for NOA may increase the incidence of autism and mental impairment; nevertheless, further study is required to arrive at a conclusive conclusion.
References
- Nagy Z, Liu J, Cecile J, et al. (1995) Using ejaculated, fresh, and frozen-thawed epididymal and testicular spermatozoa gives rise to comparable results after intracytoplasmic sperm injection. Fertil Steril 63: 808-815.
- Temple-Smith PD, Southwick GJ, Yates CA, et al. (1985) Human pregnancy by in vitro fertilization (IVF) using sperm aspirated from the epididymis. J In Vitro Fert Embryo Transf 2: 119-122.
- Patrizio P, Silber S, Ord T, et al. (1988) Two births after microsurgical sperm aspiration in congenital absence of vas deferens. Lancet 2: 1364.
- Shah RS (2001) Operative sperm retrieval for ART. In: Goenka ML, Goenka D, Recent advances in infertility management. Guwahati: Goenka, 179-183.
- Shrivastav P, Nadkarni P, Wensvoort S, et al. (1994) Percutaneous epididymal sperm aspiration for obstructive azoospermia. Hum Reprod 9: 2058-2061.
- Craft I, Bennett V, Nicholson N (1993) Fertilising ability of testicular spermatozoa. Lancet 342: 864.
- Schoysman R, Vanderzwalmen P, Nijs M, et al. (1993) Pregnancy after fertilisation with human testicular spermatozoa. Lancet 342: 1237.
- Devroey P, Liu J, Nagy Z, et al. (1994) Normal fertilization of human oocytes after testicular sperm extraction and intracytoplasmic sperm injection. Fertil Steril 62: 639-641.
- Devroey P, Liu J, Nagy Z, et al. (1995) Pregnancies after testicular sperm extraction and intracytoplasmic sperm injection in non-obstructive azoospermia. Hum Reprod 10: 1457-1460.
- Schlegel PN, Su LM (1997) Physiological consequences of testicular sperm extraction. Hum Reprod 12: 1688-1692.
- Ron-El R, Strauss S, Friedler S, et al. (1998) Serial sonography and colour flow Doppler imaging following testicular and epididymal sperm extraction. Hum Reprod 13: 3390-3393.
- Schlegel PN, Li PS (1998) Microdissection TESE: Sperm retrieval in nonobstructive azoospermia. Hum Reprod Update 4: 439.
- Belenky A, Avrech O, Bachar G, et al. (2001) Ultrasound-guided testicular sperm aspiration in azoospermic patients: A new sperm retrieval method for intracytoplasmic sperm injection. J Clin Ultrasound 29: 339-343.
- Herwig R, Tosun K, Schuster A, et al. (2007) Tissue perfusion-controlled guided biopsies are essential for the outcome of testicular sperm extraction. Fertil Steril 87: 1071-1076.
- Verheyen G, Joris H, Crits K, et al. (1997) Comparision of different hypo-osmotic swelling solutions to select viable immotile spermatozoa for potential use in intracytoplasmic sperm injection. Hum Reprod Update 3: 195-203.
- Tournaye H, Liu J, Nagy Z, et al. (1996) The use of testicular sperm for intracytoplasmic sperm injection in patients with necrozoospermia. Fertil Steril 66: 331-334.
- Silber SJ, Nagy ZP, Liu J, et al. (1994) Conventional in-vitro fertilization versus intracytoplasmic sperm injection for patients requiring microsurgical sperm aspiration. Hum Reprod 9: 1705-1709.
- Girardi SK, Schlegel P (1996) MESA: Review of techniques, preoperative considerations and results. J Andrology 17: 5-9.
- Shah RS (2002) Surgical and non-surgical methods of sperm retrieval. In: Hansotia M, Desai S, Parihar M, advanced infertility management. New Delhi: Jaypee Brothers, 253-258.
- Gorgy A, Meniru GI, Naumann N, et al. (1998) The efficacy of local anaesthesia for percutaneous epididymal sperm aspiration and testicular sperm aspiration. Hum Reprod 13: 646-650.
- Craft I, Tsirigotis M (1995) Simplified recovery, preparation and cryopreservation of testicular sperm. Hum Reprod 10: 1623-1627.
- Tournaye H, Clasen K, Aytoz A, et al. (1998) Fine needle aspiration versus open biopsy for testicular sperm recovery: A controlled study in azoospermic men with normal spermatogenesis. Hum Reprod 13: 901-904.
- Turek PJ, Givens CR, Schriock ED, et al. (1999) Testis sperm extraction and intracytoplasmic sperm injection guided by prior fine-needle aspiration mapping in patients with non-obstructive azoospermia. Fertil Steril 71: 552-557.
- Friedler S, Raziel A, Strassburger D, et al. (1997) Testicular sperm retrieval by percutaneous fine needle aspiration compared with testicular sperm extraction by open biopsy in men with NOA. Hum Reprod 12: 1488-1493.
- Marmar JL, Benoff S (2005) The safety of ultrasonically guided testis aspiration biopsies and efficacy of use to predict varicocelectomy outcome. Hum Reprod 20: 2279-2288.
- Ezeh UI, Moore HD, Cooke ID (1998) A prospective study of multiple needle biopsies versus a single open biopsy for testicular sperm extraction in men with non-obstructive azoospermia. Hum Reprod 13: 3075-3080.
- Morey AF, Deshon GEJ, Rosanski TA, et al. (1993) Technique of biopty gun testis needle biopsy. Urology 42: 325-326.
- Manning M, Junemann KP, Alken P (1998) Decrease in testosterone blood concentrations after testicular sperm extraction for intracytoplasmic sperm injection in azoospermic men (letter). Lancet 352: 37.
- Schlegel PN (2009) Nonobstructive azoospermia: A revolutionary surgical approach and results. Semin Reprod Med 27: 165-170.
- Schlegel PN (1999) Testicular sperm extraction: Microdissection improves sperm yield with minimal tissue excision. Hum Reprod 14: 131-135.
- Colpi GM, Colpi EM, Piediferro G, et al. (2009) Microsurgical TESE versus conventional TESE for ICSI in non-obstructive azoospermia: A randomized controlled study. Reprod Biomed Online 18: 315-319.
- Ramasamy R, Schlegel PN (2007) Microdissection testicular sperm extraction: Effect of prior biopsy on success of sperm retrieval. J Urol 177: 1447-1449.
- Ramasamy R, Yagan N, Schlegel PN (2005) Structural and functional changes to the testis after conventional versus microdissection testicular sperm extraction. Urology 65: 1190-1194.
- Schill T, Bals-Pratsch M, Küpker W, et al. (2003) Clinical and endocrine follow-up of patients after testicular sperm extraction. Fertil Steril 79: 281-286.
- Altay S, Hekimgil M, Cikili N, et al. (2001) Histopathological mapping of open testicular biopsies in patients with unobstructed azoospermia. Br J Urol Int 87: 834-837.
- Tournaye H, Liu J, Nagy PZ, et al. (1996) Correlation between testicular histology and outcome after intracytoplasmic sperm injection using testicular spermatozoa. Hum Reprod 11: 127-132.
- Donoso P, Tournaye H, Devroey P (2007) Which is the best sperm retrieval technique for non-obstructive azoospermia? A systematic review. Hum Reprod Update 13: 539-549.
- Su LM, Palermo GD, Goldstein M, et al. (1999) Testicular sperm extraction with intracytoplasmic sperm injection for nonobstructive azoospermia: Testicular histology can predict success of sperm retrieval. J Urol 161: 112-116.
- Seo JT, Ko WJ (2001) Predictive factors of successful testicular sperm recovery in non-obstructive azoospermia patients. Int J Androl 24: 306-310.
- Carpi A, Sabanegh E, Mechanick J (2009) Controversies in the management of nonobstructive azoospermia. Fertil Steril 91: 963-970.
- Stahl PJ, Masson P, Mielnik A, et al. (2010) A decade of experience emphasizes that testing for Y microdeletions is essential in American men with azoospermia and severe oligozoospermia. Fertil Steril 94: 1753-1756.
- Vernaeve V, Staessen C, Verheyen G, et al. (2004) Can biological or clinical parameters predict testicular sperm recovery in 47, XXY Klinefelter's syndrome patients? Hum Reprod 19: 1135-1139.
- Ramasamy R, Lin K, Gosden LV, et al. (2009) High serum FSH levels in men with nonobstructive azoospermia does not affect success of microdissection testicular sperm extraction. Fertil Steril 92: 590-593.
- Tournaye H, Merdad T, Silber S, et al. (1999) No differences in outcome after intracytoplasmic sperm injection with fresh or with frozen-thawed epididymal spermatozoa. Hum Reprod 14: 90-95.
- Ben Rhouma K, Marrakchi H, Khouja H, et al. (2003) Outcome of intracytoplasmic injection of fresh and frozenthawed testicular spermatozoa. A comparative study. J Reprod Med 48: 349-354.
- Verheyen G, Nagy Z, Joris H, et al. (1997) Quality of frozen-thawed testicular sperm and its preclinical use for intracytoplasmic sperm injection into in vitro-matured germinal-vesicle stage oocytes. Fertil Steril 67: 74-80.
- Friedler S, Raziel A, Soffer Y, et al. (1997) Intracytoplasmic injection of fresh and cryopreserved testicular spermatozoa in patients with nonobstructive azoospermia - a comparative study. Fertil Steril 68: 892-897.
- Verheyen G, Vernaeve V, Van Landuyt L, et al. (2004) Should diagnostic testicular sperm retrieval followed by cryopreservation for later ICSI be the procedure of choice for all patients with non-obstructive azoospermia? Hum Reprod 19: 2822-2830.
- Vernaeve V, Tournaye H, Osmanagaoglu K, et al. (2003) Intracytoplasmic sperm injection with testicular spermatozoa is less successful in men with nonobstructive azoospermia than in men with obstructive azoospermia. Fertil Steril 79: 529-533.
- Kanto S, Sugawara J, Masuda H, et al. (2008) Fresh motile testicular sperm retrieved from nonobstructive azoospermic patients has the same potential to achieve fertilization and pregnancy via ICSI as sperm retrieved from obstructive azoospermic patients. Fertil Steril 90: 2010.e5-e7.
- Crabbé E, Verheyen G, Silber S, et al. (1998) Enzymatic digestion of testicular tissue may rescue the intracytoplasmic sperm injection cycle in some patients with nonobstructive azoospermia. Hum Reprod 13: 2791-2796.
- Nagy ZP, Verheyen G, Tournaye H, et al. (1997) An improved treatment procedure for testicular biopsy specimens offers more efficient sperm recovery: Case series. Fertil Steril 68: 376-379.
- Morris DS, Dunn RL, Schuster TG, et al. (2007) Ideal culture time for improvement in sperm motility from testicular sperm aspirates of men with azoospermia. J Urol 178: 2087-2091.
- Proctor M, Johnson N, van Peperstraten AM, et al. (2008) Techniques for surgical retrieval of sperm prior to intra-cytoplasmic sperm injection (ICSI) for azoospermia. Cochrane Database Syst Rev 2: CD002807.
- Houwen J, Lundin K, Söderlund B, et al. (2008) Efficacy of percutaneous needle aspiration and open biopsy for sperm retrieval in men with non-obstructive azoospermia. Acta ObstetGynecol Scand 87: 1033-1038.
Corresponding Author
Rajendra Kumar Sahoo, IVF Lab Director of Future Fertility Pvt. Ltd. Bhubaneswar, Odisha, India.
Copyright
© 2024 Sahoo RK. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.