Protective Effects of Arbuscular Mycorrhizae against Fusarium Oxysporum F. Sp. Ranunculi in Ranunculus Asiaticus Cultivations for Flower Crop
Abstract
The protective effects of arbuscular mycorrhizal colonization provided by use of Glomus sp. commercial formulates in Ranunculus asiaticus against Fusarium oxysporum f. sp. ranunculi have been evaluated. Soil parcels infected with the pathogen spores (1 × 108 CFU/ml) were used as substrates for Ranunculus asiaticus plants: half of them with mycorrhized roots, and the other half without the fungal symbionts. Two different cultivars were used, each represented by 240 plants, set according to a completely randomized experimental design. The statistical analysis of the data showed that mycorrhizal colonization exerts an important protective effect on plants towards Fusarium oxysporum f. sp. ranunculi during the first three months of cultivation: at the end of this period, 40% of mycorrhizal plants were still full healthy, in comparison to the only 5% of the plants without symbiont. Three months later that means six months from the beginning of the cultivation, the plants without mycorrhizal were all dead while 20% of plants with mycorrhizae were still alive. From a practical point of view, since the Ranunculus cultivation period lasts from 6 to 8 months, the utilization of mycorrhizal plants could help to reduce the need and frequency of pesticide treatments during the culture exploitation, according to a more environmentally friendly strategy.
Keywords
Mycorrhizal colonization, Ranunculus, Arbuscular mycorrhizal fungi, Protection, Fusarium wilt
Introduction
Fusarium oxysporum f. sp. ranunculi (F.o.r.) is the agent of one of the most severe diseases affecting the Persian Buttercup, Ranunculus asiaticus, which causes important crop losses in Ranunculus cultivations [1]. The disease manifests itself through the development of small, necrotic cortical patches, spread along the epigeous stems and leaf petioles, and vase injuries and rottenness at the level of plant collar and roots. The affected plant undergoes a gradual decay, beginning with yellowing and dieback of leaves and flowers. Generally, this fungal pathology takes place in autumn and spring, when plants are in full vegetative growth, and the severity of the attacks is enhanced by rainy seasons and especially when associated with mild temperatures (18-25 ℃). The control of this pest has been until now carried out by the use of soil fumigants such as methyl bromide, chloropicrin, metam sodium, and other soil sterilants. The use of these chemicals, however, has been recently forbidden due both to the environmental damages they produce and their high toxicity to animals, people and soils.
In the search of an environmentally-friendly alternative, the natural protective effect of mycorrhizae against soil-borne pathogens was investigated [2,3]. Mycorrhizal fungi have played an important role in the reduction of plant pathogen attacks in several crops, such as tomatoes, cotton, palm and other species of economic interest [4,5]. Several recent studies indicate that the presence of Vesicular-Arbuscular Mycorrhiza (VAM), induced by using fungal strains belonging to Glomus sp., may display a protective effect against infections by soil-borne pathogenic fungi [6,7]. Based on these considerations, a trial has been carried out to assess the effect of mycorrhization with three Arbuscular Mycorrhizal Fungal (AMF) species (i.e. Rhizophagus irregularis, Funelliformis mosseae and Glomus sp.) in the control of the Fusarium blight of Ranunculus. Once determined and quantified this possible defensive role, an analysis of the aromatic metabolites released by roots into the rhizosphere will be carried out. Based on the obtained data, a hypothesis will be likewise formulated about the possible defensive role of root metabolites elicited as a consequence of the presence of both mycorrhizal symbionts and telluric fungal pathogens in soil.
Materials and Methods
Plant material
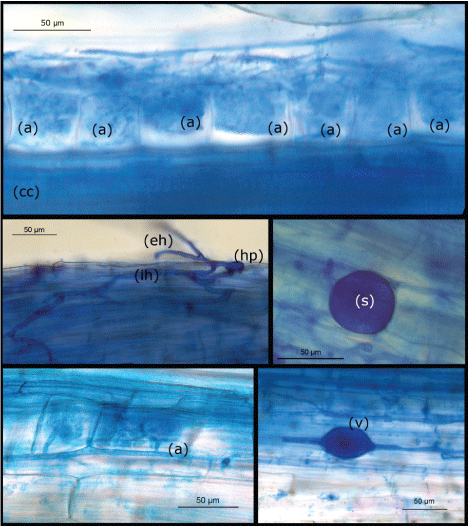
The experiments were performed on Ranunculus (Ranunculus asiaticus) plants belonging to 2 commercially important cultivars, both susceptible towards F.o.r., named "Elegance" and "New White", respectively. The commercial dry plants, consisting of a short stem bearing tuberous roots [8], were rehydrated by immersion for one week into sterile water, then harvested and kept under moist atmosphere conditions (100% R.H.) and allowed to sprout along a 20-day period. Half of the sprouted plants were then mycorrhized by transfer into Jiffy pots (10 cm diameter) containing a sterilized soil substrate (Universal Soil, Vigorplant, Italy) and a granular commercial formulation (Myc Agro Lab., Technopole Agro Environment, Bretenière - France) of three species of VAM fungal species: Rhizophagus irregularis (Syn Glomus intraradices), Funelliformis mosseae (Syn Glomus mosseae) and Glomus spp. [9]. Twenty days later, the mycorrhizal colonization of the roots was checked by sampling roots of the Ranunculus population and microscopically observing the harvested roots (Figure 1). In particular, each plant's root system was washed free of soil with tap water and immediately used for morphological analyses. Roots were cleared with 10% KOH for 30 min at 90 ℃. After rinsing several times with tap water, root fragments were transferred to alkaline water (H2O:NH4OH:H2O2 198:1:1) for 60 min, rinsed again in tap water several times and then placed in a solution of HCl 0.2 N. The clarified samples were stained with 0.1% cotton blue in 80% lactic acid for about 18 h and then destained several times with 80% lactic acid washes. The roots were cut into small fragments (about 1 cm each) and mounted onto microscope slides with some drops of lactic acid. About 60-90 fragments were observed for each treatment per sampling. AMF colonization, was assessed as described by Trouvelot, et al. [10].
Both mycorrhizal and not mycorrhizal plants were then transferred into steam-sterilized soil when had been infected through incorporation and milling with kernels of wheat inoculated with F.o.r., till to obtain an average concentration of 5 × 104 chlamydospores/ml of soil. Mycorrhizal and non-mycorrhizal plants placed on sterilized soil not inoculated with the pathogen served as controls. Soil composition features were: Turf: 15%; humus: 30%; perlite: 10%; bovine manure: 5%; sand, silt and clay: 40%; pH: 6.5 to 7.0. The cultivation was carried out in open air, under the overhang protection in a warm temperature climate, typical for Sanremo: a hot mediterranean/dry-summer subtropical climate (Köppen-Geiger classification: Csa). San Remo, Liguria, Italy is at 43°47'N, 7°46'E, 9 m (30 ft).
Experimental settings
The experiments were set according to the schema presented in Table 1. Twelve treatments were planned, each one consisting of 4 replicates of 10 plants; a total of 480 plants of Ranunculus were then used, 240 belonging to the cultivar "Elegance" and 240 to the cultivar "New White". One-half of plants of each cultivar had been previously mycorrhized. To ensure an optimal development of the mycorrhization process, a further addition of a tablespoon of the VAM commercial formulation (Myc Agro Lab. Technopole Agro Environment, Bretenière - France) at the bottom of each hole where the individuals were planted was carried out. The plant material had a density of 20 rhizomes∙square meter-1 and the experimental design was planned according to a complete randomization scheme. The treatments "Soil inoculated with F.o.r. + Enovit metil®" are meant as monthly administrations of this systemic fungicide in its commercial formulate Tiofanate™ at the dose of 1 g∙l-1 and 2 liters∙square meter-1. The inclusion of this additional chemical treatment was motivated by the need of proposing a management condition typical of commercial cultivation of Buttercup, which involves the use of chemical treatments aimed at counteracting the development of soil fusaria. Plants were considered hit by the pathogen whenever stem collapsed and microscopical observation evidenced typical vase browning and necrosis.
The statistical analysis of the data was carried out through the Student-Newman-Keuls method, a stepwise multiple comparisons procedure. The percentages of healthy plants obtained for each different treatment were put in comparison after having been transformed in arcsin and the probability level chosen to test a significant difference was P = 0.05.
HPLC analysis
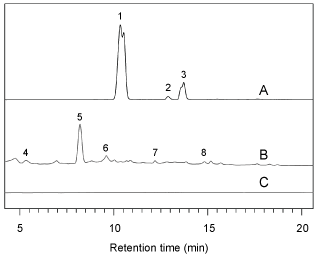
To check the possible presence of metabolites released into soil by roots, the thin soil portion in which roots are embedded, i.e. the rhizosphere [11], was collected with a thickness of about 0.5-1.0 cm. Soil samples were extracted after boiling in MeOH for half an hour and then analysed through HPLC (High Performance Liquid Chromatography) according to the protocol of Cangelosi, et al. [12]. The separated molecules were identified through the comparison of their respective retention times with those of known reference samples run under the same analytical conditions. The detector lamp was set at 280 and 350 nanometer wavelengths, to monitor the presence of aromatic compounds, from now on called simply "metabolites" (Figure 2).
Results and Discussion
The results of the trials, obtained by visual and microscopic observations performed every 20 days from November to May and summarized in global values, refer to periods of three and six months, respectively, and are shown in Table 2. From the results concerning the percentages of plants hit by the pathogen in the different experimental groups, it appears that the induced mycorrhizal colonization in plants of Ranunculus displays an appreciable effect of protection against F.o.r.. It was noted that approximately 40% of mycorrhizal individuals grown in the infected soil (compared to only 6% of plants free from symbiont) were found healthy after the first 3 months from the starting of cultivation. After 6 months of culture, however, because the pathogen, which develops mycelium in the soil according to an exponential rate, the mycorrhizal plants displayed an increased percentage of F.o.r. infection: the percentage of healthy plants was reduced to approximately 20% of the total cultivated individuals. This percentage however. Greatly exceeds that of the plants not containing the endophyte symbiont AMF and grown on infected soil. Treatments with Enovit-methyl ensured protection against the disease only slightly higher than that offered by mycorrhiza and in the long term and for the New White-cultivar, while for Elegance cultivar no statistically appreciable differences in comparison with the chemical treatments were observed. Interestingly, after three months, the effect of symbiosis provided a protection statistically not lower than that offered by the chemical.
It has long been known in many species of the protective effect of VAM against telluric pathogenic fungi [6,13], which is expressed through competition for resources and induction of metabolic changes in the host plant, though the presence of antifungal molecules has not yet been fully demonstrated [4]. The induction of metabolic changes in plant tissues by mycorrhizae might lead to the release of molecules with allelopathic effects on plants and microorganisms. Total extracts of R. asiaticus plants have been already reported to have an important allelopathic effect against wheat [14], evidencing a natural content of some metabolites with biological activities. These molecules, in some cases fungitoxic just toward Fusarium oxysporum [15], were isolated as endogenous constituents of plant tissues, however, and until now, their release in soil wasn't demonstrated.
The production of root exudates able to inhibit microbial terrestrial pathogens has been proposed as one of the mechanisms involved in defensive processes induced by VAM fungi [16,17]. However, few studies have been carried out concerning the issuing of such compounds in the soil and their direct or indirect effect on pathogenic microorganisms. During our trials, it has been observed, only in mycorrhizal Ranunculus plants grown in F.o.r.-infected soil, a root release of metabolites in soil, likely induced and mediated by arbuscular mycorrhizal fungi as a response to the presence of F.o.r.
The chemical analysis of the rhizosphere soil related to mycorrhizal plants revealed the presence of significant amounts of metabolites-that are the same in both the considered Ranunculus cultivars as shown in Table 2. In particular, syringic acid (3,5-Dimethoxy-4-hydroxybenzoic acid), ferulic acid (trans-4-Hydroxy-3-methoxycinnamic acid) and isorutin (Quercetin derivative) issued in the rhizosphere only by mycorrhizal plants roots in presence of F.o.r. These molecules were found to undergo a rapid degradation, once released into the soil, as a result of soil microbial activity, with formation of syringic acid-derived products of decomposition. The same mycorrhizal plants grown on soil not infected by F.o.r. appeared instead to release a different group of metabolites (unidentified), with a HPLC retention time different from those related to the former detected compounds. This seems to indicate that the mycorrhizal fungi promote in the Ranunculus colonized roots the release into soil of an array of different metabolites depending on the presence or on the absence of the telluric pathogen F.o.r. The non-mycorrhizal Ranunculus plants cultivated in uninfected soil did not free into soil rhizosphere any appreciable amount of root metabolites. The observed resistance of Ranunculus mycorrhizal plant to F.o.r. comparable to that induced by the chemical treatments with Enovit metil®, could be ascribed to the possible protective effects of plant metabolites issued in the rhizosphere. Further researches should be necessary to shed light on the role of these molecules, possibly defensive, against telluric pathogens such as F.o.r.
Acknowledgements
Work carried out with the financial support from the International Project no. 178, Alcotra 2007, Fioribio 2. The authors thank Mrs Fulvia Rebecchi, Mr Mino Graniglia and Mr Massimo Serra for the technical support.
References
- Garibaldi A, Gullino ML (1985) Wilt of Ranunculus asiaticus caused by Fusarium oxysporum F. Sp. Ranunculi, forma specialis nova. Phytopathologia Mediterranea 24: 213-214.
- Jung SC, Martinez-Medina A, Lopez-Raez JA, et al. (2012) Mycorrhiza-induced resistance and priming of plant defenses. J Chem Ecol 38: 651-664.
- Tahat MM, Kamaruzaman, Saini, et al. (2010) Mycorrhizal fungi as a biocontrol agent. Plant Pathol J 9: 198-207.
- Whipps JM (2004) Prospects and limitations for mycorrhizas in biocontrol of root pathogens. Can J Bot 82: 1198-1227.
- Jaiti F, Meddich A, El Hadrami I (2007) Effectiveness of arbuscular mycorrhizal fungi in the protection of date palm (Phoenix dactylifera) against bayoud disease. Physiol Mol Plant Pathol 71: 166-173.
- Sikes BA (2010) When do arbuscular mycorrhizal fungi protect plant roots from pathogens? Plant Sign Behav 5: 763-765.
- Yanan W, Xusheng Z, Baozhong Y, et al. (2015) Biochemical defenses induced by mycorrhizae fungi Glomus mosseae on controlling strawberry Fusarium wilt. Open Biomed Eng J 9: 301-304.
- Kamenetsky R, Peterson RL, Melville LH, et al. (2005) Seasonal adaptations of the tuberous roots of Ranunculus asiaticus to desiccation and resurrection by changes in cell structure and protein content. New Phytol 166: 193-204.
- Knopf E, Blaschke H, Munch JC, et al. (2016) Impact on soil of arbuscular mycorrhizal fungi: growth responses of Moringa spp., plant sampled from Lake Victoria basin. J Biol Sci 16: 12-21.
- Trouvelot A, Kough JL, Gianinazzi-Pearson V (1986) Mésure du taux de mycorhization VA d'un système radiculaire. Récherche de methodes d'estimation ayant une signification fonctionnelle. In: Gianinazzi-Pearson V, Gianinazzi S, Physiological and genetical aspects of mycorrhizae. INRA, Paris, France, 217-221.
- McNear Jr DH (2013) The rhizosphere - Roots, soil and everything in between. Nature Education Knowledge 4: 1-9.
- Cangelosi B, Clematis F, Monroy F, et al. (2015) Filiferol, a chalconoid analogue from Washingtonia filifera possibly involved in the defense against the red palm weevil Rhynchophorus ferrugineus Olivier. Phytochemistry 115: 216-221.
- Azcòn-Aguilar C, Barea JM (1996) Arbuscular mycorrhizas and biological control of soil-borne plant pathogens - An overview of the mechanisms involved. Mycorrhiza 6: 457-464.
- Qasem JR (1993) Allelopathic effects of some arable land weeds on wheat. Pakistan J Weed Sci Res 6: 46-55.
- Qasem JR (1996) Fungicidal activity of Ranunculus asiaticus and other weeds against Fusarium oxysporum f.sp. lycopersici. Ann Appl Biol 128: 533-540.
- Hooker JE, Jaizme-Vega M, Atkinson D (1994) Biocontrol of plant pathogens using arbuscular mycorrhizal fungi. In: Gianinazzi S, Schiiepp H, Impact of Arbuscular Mycorrhizas on Sustainable Agriculture and Natural Ecosystems. Birkhiiuser Verlag Basel, Switzerland, 191-200.
- Barea JM, Jeffries P (1995) Arbuscular mycorrhizae in sustainable soil plant systems. In: Varma A, Hock B, Mycorrhiza Structure, Function, Molecular Biology and Biotechnology. Springer-Verlag Heidelberg, Germany, 521-560.
Corresponding Author
Margherita Beruto, Regional Institute for Floriculture, Via Carducci, 12, 18038 Sanremo, Italy.
Copyright
© 2016 Cangelosi B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.