Classification of Biostimulants Origin Using Amino Acids Composition of Hydrolyzed Proteins
Abstract
The mechanisms of action of biostimulants are multiple and often work in synergy, and then it is not easy to find analytical methods for their characterization. This work provides a practical and fast approach to qualify and classify biostimulants in order to facilitate their control against fraudulent actions. The amino acid composition of different commercial biostimulants from animal and plant constituents, was used to prove the method. Results were processed by means of Partial Least Squares Discriminant Analysis (PLS-DA) demonstrating that classification of biostimulants can be done using amino acid composition with extremely good sensitivity and specificity in both calibration and cross-validation.
Keywords
Chemical fertilizers, Biostimulants, Animal and vegetal organic products, Multivariate classification analysis, Partial least squares discriminant analysis
Introduction
The plant development is influenced by both endogenous and exogenous factors. The use of exogenous preparations able to stimulate plant growth by acting on plant metabolism, and suitable to enhance the efficiency of chemical fertilizers has become very frequent [1-3]. A class of these compounds are biostimulants, which are products obtained by industrial processing of animal or vegetal constituents and containing amino acids, proteins, enzymes, carbohydrates, vitamins, humic substances and other biologically active elements [4,5].
Biostimulants are organic products with characteristics rather different from fertilizers, as their function is to stimulate natural processes to benefit nutrient uptake, nutrient use efficiency and crop quality (sugars, size, color, etc.), independently of their nutrient content [6,7].
Biostimulants are strategic products for modern agriculture, composed of very heterogeneous classes of compounds with a broad spectrum of action to improve quantitative and qualitative productions. The biostimulants used in agriculture are mainly amino acid mixtures. As their mechanisms of action are multiple and often work synergistically, it is not easy to find analytical methods for their characterization [8,9].
The protein sources are represented by residues of leather processing (eg. collagen) or by plant biomass. Products of animal origin are mainly obtained by chemical hydrolysis of collagen at high temperatures in a highly acidic or alkaline media, while the hydrolysates of vegetal origin are obtained through the use of specific enzymes at low temperatures. Hydrolysed proteins have different chemical characteristics depending on the origin of the raw material and the production process. Currently, there is no guidelines for these substances, but in the near future biostimulants will be regulated and commercialized worldwide exposing the market to fraudulent actions [10,11]. For the detection of biostimulating properties different methods of analysis have been developed, but, however, none of them is still able to characterize biostimulants in a comprehensive manner [4]. In order to facilitate the control of biostimulants, it is important to develop methodological approaches able to qualify and classify them. Very few studies on biostimulants were reported especially using multivariate analysis [12-14]. Most commonly, the samples were characterized by using multiple techniques especially NMR, infrared and other different spectroscopic methodologies, to highlight the biostimulants mechanism of action but never their classification.
This article reports a procedure, based on multivariate analysis, for the characterization of hydrolyzed proteins, a wide class of substances with biostimulating properties. The differences in the amino acid composition were used to identify the biostimulants origin. To evaluate the discriminatory capacities of this approach, PLS-DA was applied to the dataset divided in animal and vegetal products to find the linear subspace of those amino acid variables that best separated the biostimulant classes [15,16]. The PLS-DA is a well-known classification model applied in many scientific fields allowing straightforward control of data. PLS-DA was used as supervised deterministic classification technique capable of discriminating the observations on the basis of a class membership categorical matrix. Currently, there are no efficient methods for assessing biostimulant properties. To the author’s knowledge, this report is a first attempt to classify the biostimulants origin on the basis of their amino acids composition, thus simplifying the overall procedure to facilitate their control against fraudulent actions and limiting the negative effects on the commercialization.
Materials and Methods
Samples
Sampling of different hydrolyzed proteins were carried out following the European Standard sampling plans and methods during the years 2012-2016 (EN 2007) [17]. The samples were from Italian animal and plant products ((,41.9°N, 12.4°E). All samples had tracked origins, and were fertilizers with declared biostimulating properties, organo-mineral and organic fertilizers containing hydrolyzed proteins as organic source. A list with a description of the analyzed samples and biostimulants properties was reported in Table 1. The labels numbering was assigned to highlight the different matrices of the samples. As the research was done using samples taken in the context of official controls, for privacy it was not possible to provide information that goes beyond the legal wording of the products.
Analysis of amino acids
All reagents were of analytical grade and were supplied by Sigma-Aldrich (St. Louis, MO, USA). Amino acid standard solution and amino acid standards physiological basic (Sigma-Aldrich, St. Louis, MO, USA) were used for the amino acids recognition.
Samples were hydrolyzed before injection in the amino acid analyzer, in accordance with the method reported in the European Commission Regulation for feed [10]. Shortly, whole hydrolysis was carried out on samples by boiling with hydrochloric acid. The extract, diluted and adjusted to pH 2.20, was injected into the amino acid analyzer. After derivatization with ninhydrin, photometric detection was carried out at 570 nm for all amino acids except proline and hydroxyproline which were detected at 440 nm. The quantification was made using L-norleucine as internal standard. Amino acid composition was determined on an amino acid analyzer BioCHROM 30+ - (Biochrom Ltd Cambridge, UK). Each sample was analyzed in two replications carried out on different days, with a coefficient of variation less than 10%. The same methodology was used for liquid and solid products. The weighed sample portion had a nitrogen content of about 10 mg.
Statistical evaluation
Statistical analysis was performed using PLS-DA by means of MatLab R2011 (Mathworks, Natick, MA, USA) integrated with a classification toolbox for MATLAB from Milano Chemometrics and QSAR Research Group version 3.0. Data have been auto scaled (zero mean and unitary variance). A numerical evaluation of the models achieved was validated by cross-validation leave one out technique. Using confusion matrices, the reliability of the classification models achieved was studied in terms of recognition ability (percentage of the members of the training set correctly classified) and prediction ability (percentage of the members of the test set correctly classified by using the rules developed in the training step).
Results and Discussion
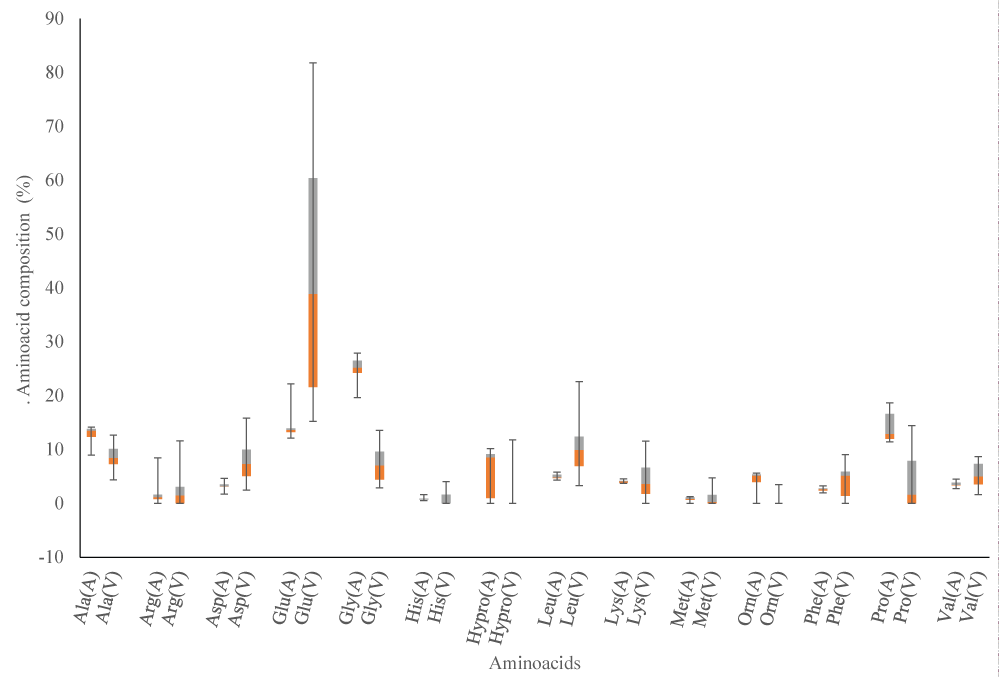
The composition of biostimulants depends on the origin and manufacturing processes. Proteins hydrolyzed from collagen show Glycine (Gly) and Proline (Pro) as dominant amino acids, while those from pulses contain Glutamic Acid (Glu) and Aspartic Acid (Asp) in large amount [18,19]. Using this information, fourteen most important amino acids were chosen in order to discriminate the biostimulants origin.
In order to have a statistically dataset, the amino acids of the 17 animals and 16 vegetal samples was quantitatively analyzed and resumed in Figure 1 in form of box and whistlers plot. The box and whistlers plot showed that the vegetal samples had higher concentration of Glutamic Acid (Glu) than the animal one. On the other hand, Glycine (Gly) and Proline (Pro) were more abundant in animal than vegetal hydrolyzed products. In particular animal class, the amount of glycine tended to double compared to Alanine (Ala), while in vegetal matrices the two amino acids were in the same amount. Histidine (His), Hydroxyproline (Hypro) and Ornithine (Orn), were absent in the majority of vegetal matrices while in the animal one was present in low quantities.
Since single parameters were not sufficient to provide a clear statistical discrimination, such variables were submitted to multivariate statistical procedures.
Amino acid composition dataset was analyzed by the PLS-DA. In this discriminant technique, the regression model is calculated relating an X block of instrumental signals to a Y block of sample classes in coded units. In this case the X block was the dataset of amino acid analysis of 33 samples (17 from animal and 16 from vegetal samples) and 14 variables (the amino acids) and Y block was a matrix of 33 rows and 2 columns related to the two classes selected (animal and vegetal).
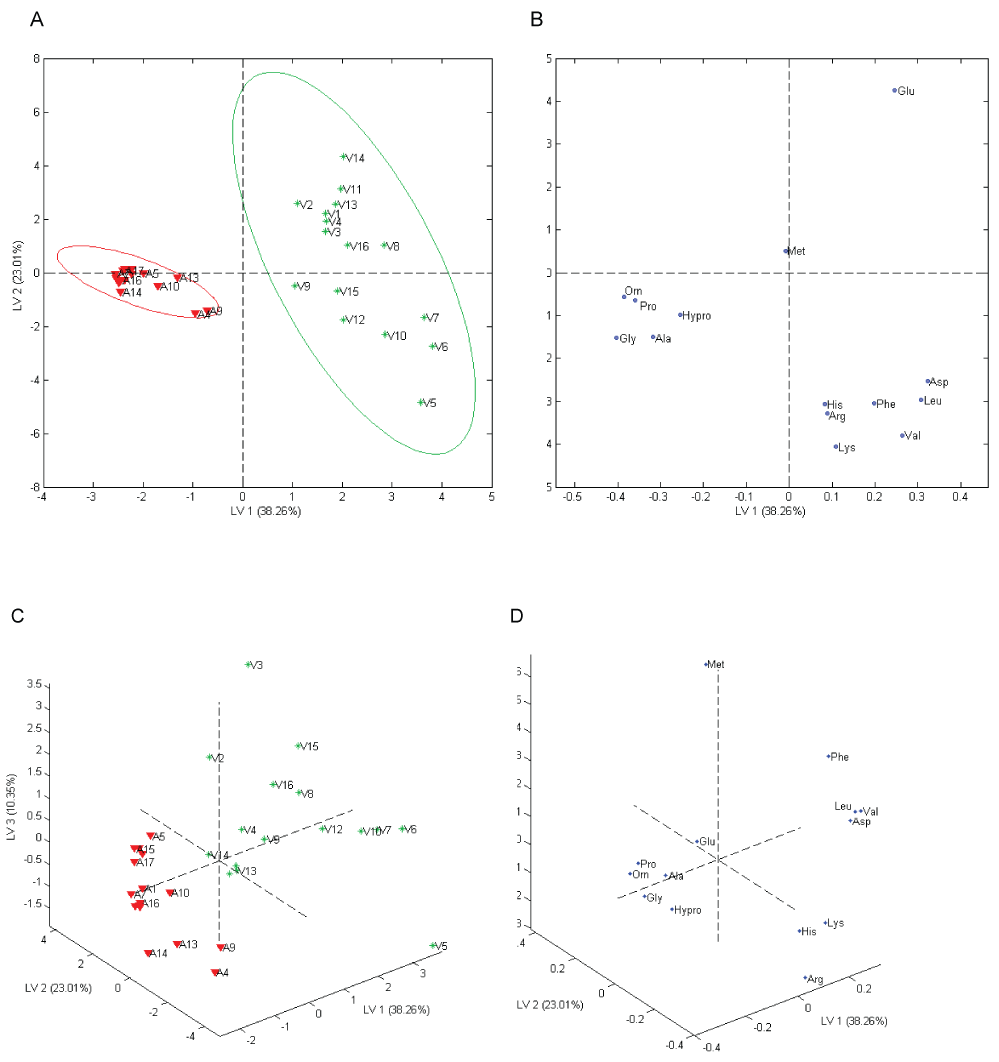
As reported in Table 1, the samples having same vegetal or animal matrix, were grouped using consecutive numerical order to check possible sub grouping classification, but according to the data obtained in Figure 2 no clusters within the two classes chosen was found.
Figure 2A depicted a strong separation between the data related to the two classes as emphasized by the confidence ellipse used as outline class group with a 95% confidence limit. In Figure 2C, the first three Latent Variables (LVs) explained 71.62% of the total variance of the data. The LV 1 (38.26% of variance) contributed to a clear discrimination between animal and vegetal hydrolyzed proteins highlighting a better clustering of animal than vegetal. On LV 1, the vegetal samples had a higher spatial dispersion than animal ones. This vegetal samples spreading was emphasized also by LV 2 and LV 3.
The contribution of the amino acids to the samples separation could be evaluated considering the position of the loadings with respect to the scores (Figure 2B). The amino acids Glycine (Gly), Ornithine (Orn), Alanine (Ala), Proline (Pro) and Hydroxyproline (Hypro) almost overlap in the third quadrant of the LV 1-LV 2 plane, contributing considerably to the separation of animal biostimulants from the vegetal ones. This result was in part showed also by the univariate analysis. Vegetal biostimulants were influenced by the amino acids Valine (Val), Leucine (Leu) and Aspartic acid (Asp) laid on the fourth quadrant of the LV 1-LV 2 plane along with glutamic acid in the first quadrant that greatly contribute in spreading the vegetal class. As shown by Figure 2D, both vegetal and animal classes were scattered on the LV 3 by the contribution of Arginine (Arg), Histidine (His), Lysine (lys), Phenylalanine (Phe) and Methionine (Met). These amino acids were rarely detected and they could be useful in a future sub-classification of the two classes. This trend could be explained by the protein composition of materials origin. Animal matrices had collagen as a common component which has amino acid composition constant regardless of their origin, while vegetal matrices came from a wide variety of sources. The results showed that amino acid composition, taken as analytical parameter, can be used to identify and classify biostimulants origin. A correlation between amino acid composition and biostimulant properties was beyond the scope of this study. Such dependency cannot be explained only by amino acids because biostimulant actions are given by multiple factors working in synergy.
Table 2 reported the statistical data of the PLS-DA model among the two biostimulant classes. Excellent sensitivity and specificity were obtained in both calibration and cross validation with a very low RMSEC, RMSECV and classification errors. A numerical evaluation of the classification properties was underlined in form of confusion matrices. According to the leave one out technique (by setting a maximum of twelve latent variables), in cross validation a total of 100% of the samples were correctly classified by amino acid variables in both animal and vegetal classes.
Conclusion
The present work opens a new view in the interpretation of the amino acid analysis of biostimulants. The univariate evaluation of amino acid composition showed a certain degree of correlation among each individual analyzed variable, but that was not sufficient to statistically discriminate biostimulants.
On the other hand, the multivariate approach was proved to be a valuable tool to readily discriminate the natural constituents of the hydrolyzed proteins. As demonstrated by PLS-DA results, correct classification could be done using this discriminant algorithm generating a robust method in term of sensitivity and specificity and proposing a criterion for the identification of biostimulants.
The raw materials used are one of the factors that significantly affect the economic value and the agronomic behavior of the end products. This can lead to unfair practices such as the commercialization of products with different origin from that declared. This method contributed effectively and concretely at the task to protect the market from possible fraudulent actions.
References
- Zhang X, Schmidt RE (2000) Hormone-containing products' impact on antioxidant status of tall fescue and creeping bentgrass subjected to drought. Crop Science 40: 1344-1349.
- Sánchez-Sánchez A, Sánchez-Andreu J, Juárezet M, et al. (2006) Improvement of iron uptake in table grape by addition of humic substances. Journal of Plant Nutrition 29: 259-272.
- Kirn A, Kashif SR, Yaseen M (2010) Using indigenous humic acid from lignite to increase growth and yield of okra (Abelmoschus esculentus L.). Soil & Environ 29: 187-191.
- Nardi S, Concheri G, Pizzeghello D, et al. (2000) Soil organic matter mobilization by root exudates. Chemosphere 41: 653-658.
- Eyheraguibel B, Silvestre J, Morard P (2008) Effects of humic substances derived from organic waste enhancement on the growth and mineral nutrition of maize. Bioresour Technol 99: 4206-4212.
- Khan W, Rayirath UP, Subramanian S, et al. (2009) Seaweed extracts as biostimulants of plant growth and development. Journal of Plant Growth Regulation 28: 386-399.
- Craigie JS (2011) Seaweed extract stimuli in plant science and agriculture. Journal of Applied Phycology 23: 371-393.
- Thomas J, Mandal AKA, Raj Kumar R, et al. (2009) Role of biologically active amino acid formulations on quality and crop productivity of Tea (Camellia sp.). International Journal of Agricultural Research 4: 228-236.
- Vranova V, Rejsek K, Skene KR, et al. (2011) Non-protein amino acids: plant, soil and ecosystem interactions. Plant Soil 342: 31-48.
- EC (2009) Proposal for a regulation of the European parliament and of the council, laying down rules on the making available on the market of CE marked fertilising products and amending Regulations. No 1069/2009 and EC No 1107/2009.
- Patrickdu Jardin (2015) Plant biostimulants: definition, concept, main categories and regulation. Scientia Horticulturae 196: 3-14.
- Tejada M, Benítez C, Gómez I, et al. (2011) Use of biostimulants on soil restoration: Effects on soil biochemical properties and microbial community. Applied soil Ecology 49: 11-17.
- Torpy FR, Irga PJ, Moldovan D, et al. (2013) Characterization and biostimulation of benzene biodegradation in the potting-mix of indoor plants. Journal of Applied Horticulture 15: 10-15.
- Ertani A, Pizzeghello D, Francioso O, et al. (2014) Capsicum chinensis L. growth and nutraceutical properties are enhanced by biostimulants in a long-term period: chemical and metabolomic approaches. Front Plant Sci 5: 375.
- Borràs E, Ferré J, Boqué R, et al. (2015) Data fusion methodologies for food and beverage authentication and quality assessment - a review. Anal Chim Acta 891: 1-14.
- Gromski PS, Muhamadali H, Ellis DI, et al. (2015) A tutorial review: Metabolomics and partial least squares-discriminant analysis - a marriage of convenience or a shotgun wedding. Anal Chim Acta 879: 10-23.
- EN (2007) Fertilizers and liming materials - Sampling and sample preparation - Part 1: Sampling 1482-1: 2008.
- Galili G, Höfgen R (2002) Metabolic engineering of amino acids and storage proteins in plants. Metab Eng 4: 3-11.
- Cavani L, Ciavatta C, Gessa C (2003) Determination of free l-and d-alanine in hydrolysed protein fertilisers by capillary electrophoresis. J Chromatogr A 985: 463-469.
Corresponding Author
Francesca Baroccio, MiPAAF, Department of Central Inspectorate for Quality Safeguarding and Anti-Fraud of Foodstuff and Agricultural Products (ICQRF), Rome Central Laboratory, 00149 Rome, Italy.
Copyright
© 2017 Baroccio F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.