Maternal History and First Trimester Biochemical and Biophysical Markers in the Prediction of Spontaneous Preterm Delivery in Singleton Pregnancies
Abstract
Background
Preterm delivery (PTD) is a major cause of perinatal mortality and delayed psychomotor development in children. The application of new biochemical and biophysical markers can improve the accuracy of the prediction of spontaneous PTD.
Objectives
To examine the performance of screening for spontaneous PTD by a combination of maternal history, biochemical and biophysical markers at 11 + 0 - 13 + 6 weeks' (wks) gestation.
Material and methods
This was a case-control study of 180 pregnant women between 11-13 + 6 weeks' gestation. Study group consisted of 100 healthy participants and 80 participants with at least one risk factor of spontaneous PTD in their medical history. Following parameters were recorded: Maternal history, cervical length (Cx), uterine artery pulsatility index (UtA PI), pregnancy-associated plasma protein A (PAPP-A), free beta subunit of human chorionic gonadotropin (β-hCG), alpha-fetoprotein (AFP). Based on the gestational age at delivery the study group was retrospectively divided into three groups: 1. Patients who delivered at term (control group); 2. Patients who delivered before 34 weeks' gestation (early PTD); 3. Patients who delivered between 34 and 37 weeks' gestation (late PTD). In these groups potential biomarkers for spontaneous PTD were analysed.
Results
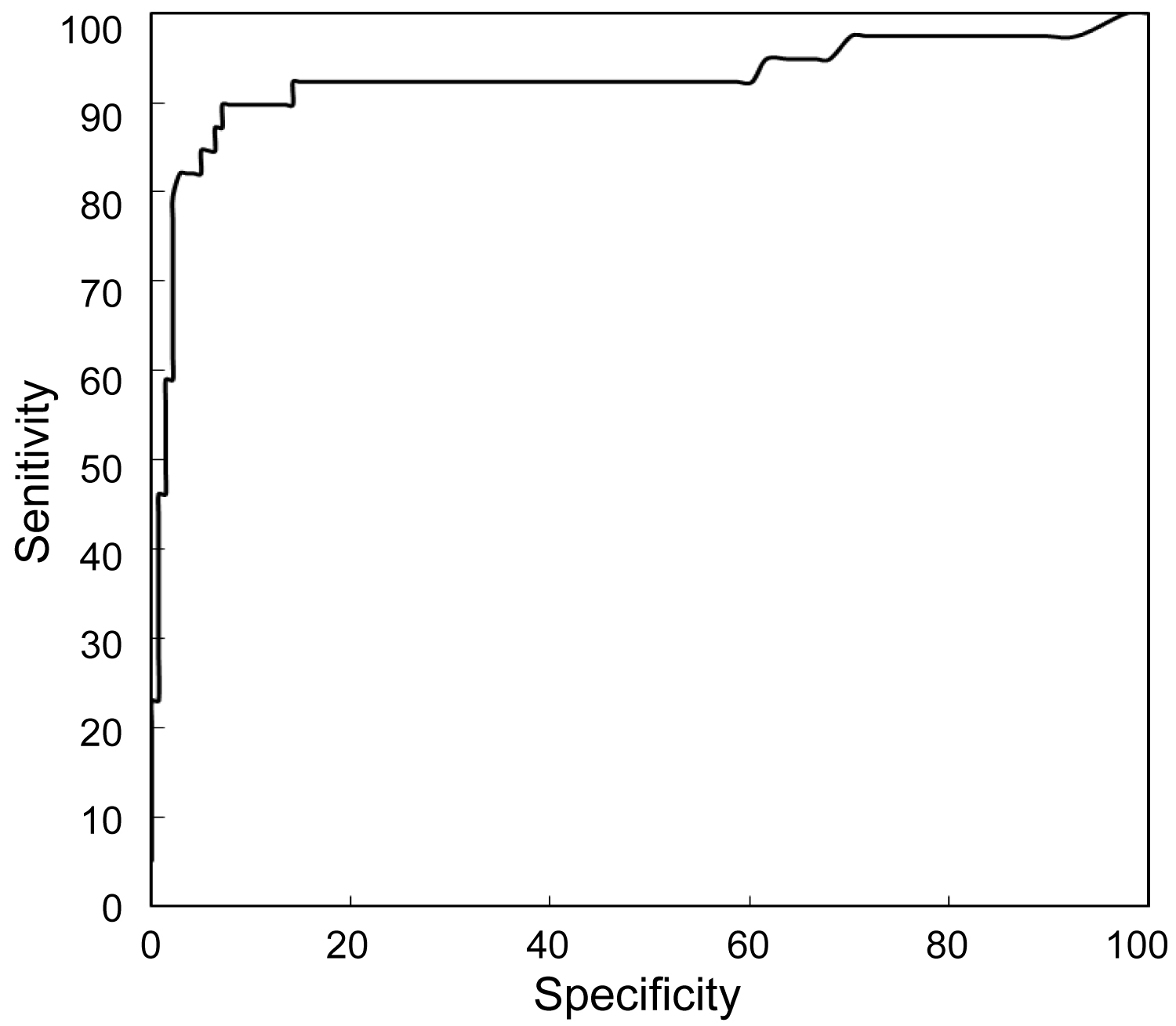
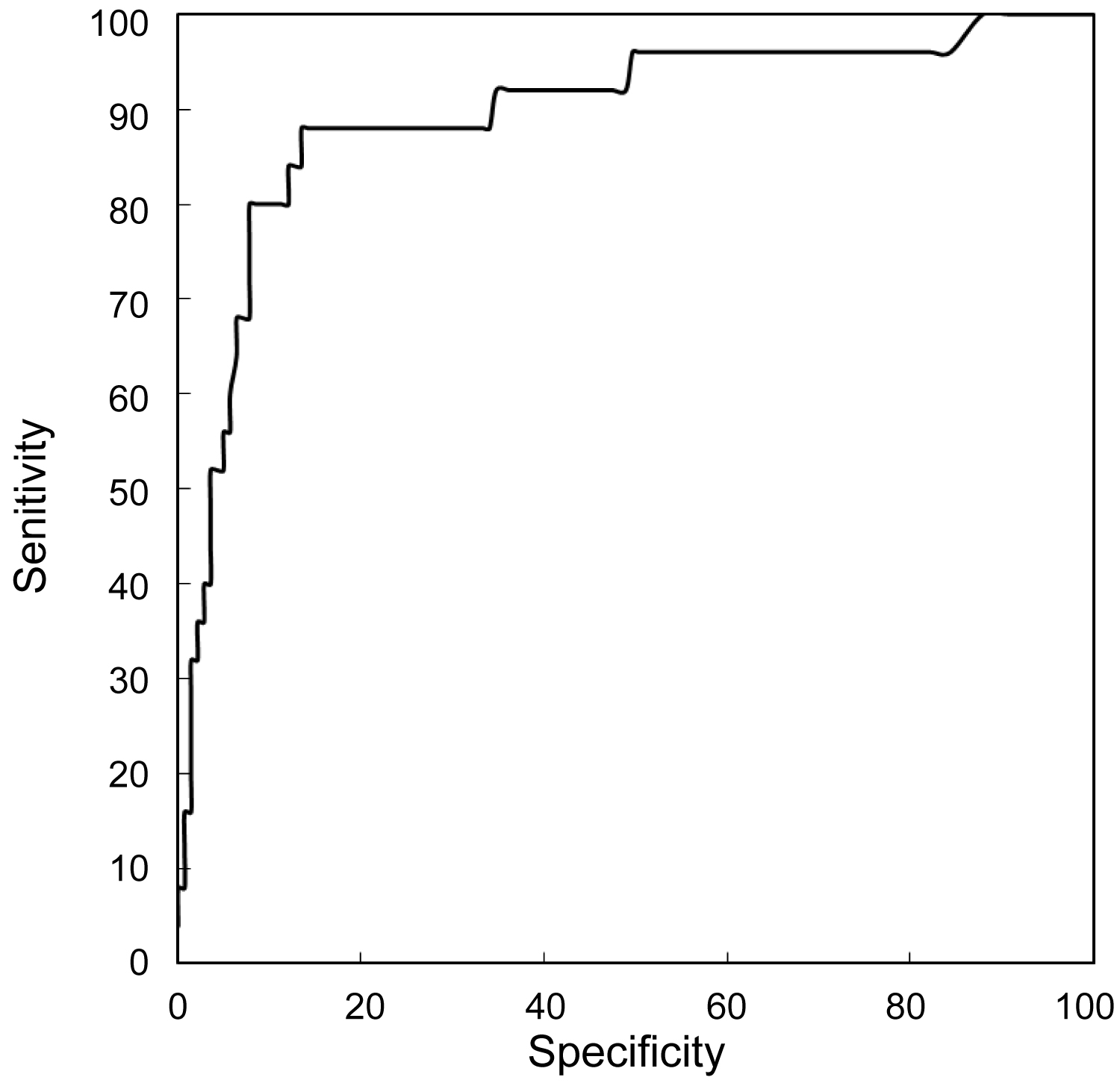
A multivariable stepwise logistic regression analysis indicated that early PTD can be predicted based on a combined analysis of maternal history, cervical length and AFP and PAPP-A concentrations: Detection rate (DR): 93%; false positive rate (FPR): 10%. The performance of screening for late PTD is less effective: Combined analysis of maternal risk factors, cervical length and AFP and PAPP-A concentrations allows to detect 88% of cases at a FPR of 10%.
Conclusions
The best fitted model for the prediction of spontaneous preterm delivery before 34 weeks is based on a combined analysis of maternal risk factors, measurement of the cervical length and measurement of AFP and PAPP-A concentrations. The best fitted model for the prediction of preterm delivery between 34-37 weeks' gestation is based on a combined analysis of maternal factors, measurement of the cervical length and measurement of AFP concentrations.
Introduction
Preterm delivery (PTD) is a major cause of perinatal mortality and delayed psychomotor development in children [1]. According to the recommendations adopted by the World Health Organization (WHO) every birth that takes place between 23 and 37 weeks' gestation should be regarded as premature. However, the vast majority of complications are associated with delivery before 34 weeks [2,3]. Currently, patients at increased risk of spontaneous preterm delivery are identified based on their clinical factors, among which the most important is previous preterm delivery [4,5]. Important maternal risk factors also include the black race, age under 18 years and over 40 years, BMI below 19 kg/m2 before pregnancy and a short interval between subsequent pregnancies [6].
Incorporation of risk factors listed above enables to detect only 38% multiparous who will go into preterm delivery. Moreover, this strategy is inefficient in nulliparous women without other risk factors [7]. The application of new biochemical and biophysical markers can improve the accuracy of prediction of spontaneous preterm birth.
The objective of this study is firstly, to evaluate the efficiency of combined analysis of maternal risk factors, biochemical markers: alfa-fetoprotein (AFP), pregnancy-related plasma protein (PAPP-A), free-beta human chorionic gonadotropin (free β-hCG) and biophysical markers: Cervical length (Cx) and uterine artery dopplers (UtA PI) at 11 + 0 - 13 + 6 weeks' gestation in prediction of spontaneous preterm birth and secondly, to establish a diagnostic algorithm based on the conducted analysis.
Materials and Methods
This was a case-control study of 180 pregnant women attending a routine prenatal visit at 11 + 0 to 13 + 6 weeks' gestation in the years 2013-2018. The study was performed in the local outpatient clinics. Study group consisted of 100 healthy participants and 80 participants with at least one risk factor of spontaneous PTD in their medical history (maternal age > 40 years, smoking, BMI < 20 kg/m2, history of PTD, assisted conception). Written informed consent was obtained from women agreeing to participate in the study. The study was approved by Polish Mother's Memorial Research Hospital Ethics Committee. Patients in multiple pregnancies, in the presence of significant fetal abnormalities, abortion or termination of pregnancy were excluded from the study. The presence of risk factors did not indicate the occurrence of spontaneous PTD in all the cases. On the basis of the occurrence of spontaneous PTD the study group was retrospectively divided into three groups: 1. Patients who delivered at term (control group) n = 141; 2. Patients with early PTD (< 34 weeks) n = 14; 3. Patients with late PTD (34-37 weeks) n = 25. In these groups potential risk markers for spontaneous PTD were analysed.
In the first antenatal visit a detailed interview with each patient was conducted, blood sample was collected for analysis of AFP, PAPP-A, free β-hCG and ultrasound examination of cervical length and uterine artery pulsatility index (UtA PI) according to the Fetal Medicine Foundation recommendations was performed (Voluson E6, GE Healthcare). Cevical length was measured using vaginal approach with empty bladder and patient placed in dorsal lithotomy position. The ultrasound probe is introduced in the vagina and directed in the anterior fornix. Care is taken to avoid exerting undue pressure on the cervix. A sagittal view of the cervix is obtained and the endocervical mucosa is used as a guide to the true position of the internal os. The calipers are used to measure the linear distance between the triangular area of echodensity at the external os and the V-shaped notch at the internal os.
The 5 mL blood samples taken during the first visit were collected in plastic tubes with ethylenediaminetetraacetic acid (EDTA). Following centrifugation, the serum was stored at -80 ℃. AFP, PAPP-A and free β-hCG concentrations were evaluated using equipment that provides reproducible results (Delfia Xpress System, PerkinElmer, Waltham, USA).
The collected data were archived in the ViewPoint system. Prediction models were developed using multivariable stepwise logistic regression. A p value < 0.05 was considered significant.
For the following markers: Cx, UtA PI, AFP, PAPP-A, β-hCG values of multiples of median were calculated (MoM). Values were adjusted to fetal crown-rump length (CRL), maternal age, body mass index (BMI), smoking status, parity and type of conception.
The results of examined parameters were converted into Log10. In order to compare values in early and late PTD following statistical tests were used: Chi-square test with adequate adjustments and U Mann-Whitney test. In order to compare two continuous coefficients, Spearmann correlation was calculated. In order to evaluate correlation coefficients, following levels were established: 0-0.1 - very weak correlation; 0.1-0.3 - weak correlation; 0.3-0.6-middle strong correlation; > 0.6 - strong correlation.
Logistic regression analysis was used to calculate the risk for early and late PTD based on maternal risk factors only and based on a combination of maternal risk factors together with sonographic markers (Cx, UtA PI), biochemical markers (AFP, PAPP-A), anthropometric data (birth weight) and gestational age at delivery. Results were presented as ROC curves, area under the curve (AUC), and detection rates (DR) for a 10% false positive rate (FPR).
Results
We analysed individual prognostic values of the following parameters: Cx, UtA PI, AFP, PAPP-A, β-hCG. For each marker the expected normal median (MoM) was calculated and values were converted into Log10.
Characteristics of the study group is presented in Table 1. The groups were compared using U Mann Whitney test and Chi-square test with necessary adjustments. A p value < 0.05 was considered significant.
Table 2 presents the comparison of analysed biochemical and biophysical markers between groups of patients with early PTD (< 34 wks), late PTD (34-37 wks) and patients delivering before 37 wks. Groups were compared using U Mann-Whitney test.
Logistic regression analysis was performed to evaluate the impact of UtA PI MoM, Cx, AFP MoM, PAPP-A MoM, β-hCG MoM Mean Log10 on the occurrence of early PTD (< 34 wks), late PTD (34-37 wks) and birth before 37 wks.
Table 3 presents the performance of screening by single biomarkers and a combination of risk factors and biomarkers for a 10% false positive rate in the groups of early PTD (< 34 wks), late PTD (34-37 wks) and birth before 37 wks.
Table 4 presents the comparison of the area under the curve for different combinations of screening studies for PTD < 34 wks., 34-37 wks. oraz < 37 wks.
Discussion
In the presented case-control study we aimed to establish best-fitted models for the prediction of spontaneous PTD in the first trimester based on maternal risk factors and biochemical and biophysical markers. The analysis comprised patients attending their routine pregnancy visit in the first trimester of pregnancy. Appropriately trained doctors collected medical history and performed ultrasound scans that included measurement of the mean UtA PI and cervical length according to the Fetal Medicine Foundation guidelines. A blood sample was collected to evaluate the concentrations of AFP, PAPP-A, free β-hCG. After performing statistical analysis we concluded that screening studies incorporating above mentioned markers enable to identify the majority of pregnancies complicated by preterm delivery.
Detailed medical and obstetric history is the cheapest and easily accessible screening tool. In the presented analysis, screening studies based only on the medical and obstetric history had a sensitivity of only 36% at a false positive rate of 10%. This results are in agreement with previous studies. Beta, et al. examined a population of 33,000 patients and found that medical interview had a DR of 27.5% at a FPR of 10% [7]. Similar results were presented by Parra-Cordero, et al. In this study, authors found that the most significant risk factors for early PTD are previous PTD and smoking. These risk factors were predictive of 25% of cases of PTD [8].
The history of previous preterm delivery is the most significant risk factor of preterm delivery in the next pregnancy. In our cohort, the history of PTD was significantly more prevalent in the group of patients that delivered before 34 weeks. Numerous studies confirmed an increased risk of repeating occurrence of PTD in the next pregnancy [9,10]. In a cohort study of 154.809 singleton pregnancies, Ananth, et al. found that the percentage of deliveries before 35 weeks is significantly higher in patients who delivered prematurely compared to patients who delivered at term (13% vs. 3%) [11].
We found that smoking is an important risk factor of PTD. In the group of patients who experienced PTD, 38% of women were smokers. In other studies an association between smoking and increased risk of preterm delivery was also confirmed [7,12,13]. The mechanism of increased risk probably results from the immunologic factors and activation of inflammation cascade [14].
Maternal age above 40 years is another risk factor of preterm delivery. We found that in nulliparous, who do not smoke with no induction of ovulation, the risk is increasing a linear way. Similar association was found in other studies. In a cohort study of 172,715 singleton pregnancies, Cnattingius, et al. found an increased incidence of PTD (< 32 wks) from 1% in woman aged 20-24 years to 2.4% in patients above 40-years-old [15].
The prognostic value of the measurement of cervical length remains controversial [16-18]. Celik, et al. confirmed the usefulness of cervical length measurement in the second trimester in the prediction of PTD [6], however the value of the measurement in the first trimester is uncertain. In population studies conducted in two separate centres, significant shortening of the cervix at 11 + 0 to 13 + 6 was found in patients who experienced early PTD. In a prospective screening study of 10,000 patients with singleton pregnancies in the first trimester in London, Greco, et al. found a statistically significant shortening of the cervix in woman who experienced both early and late PTD. Authors created an algorithm that incorporated maternal risk factors and measurement of cervical length that enabled to identify 55% of patient that experience PTD at a FPR of 10% [19-20]. Similar results were presented by Souka, et al. In a prospective study of 978 patients in singleton pregnancies they proved that the cervical length of patients who delivered before 34 weeks was significantly shorter than in woman who delivered at term [17]. Our results are in agreement with those presented by Greco, et al. and Souka, et al. However, equally numerous are studies which show no significant differences in the cervical length in woman who deliver prematurely in comparison to those who deliver at term [18,21]. The most recent analysis by Parra-Cordero, et al. pointed out a possible reason for these results addressing technical differences in the methodology of the measurement. Additionally, there were differences in racial origins between patients [8].
Doppler studies of uterine arteries blood flows provide indirect evidence of impaired placental perfusion in pregnancies with preterm delivery [8,22]. Presented study analysed the potential value of the measurement of the UtA PI as a possible biophysical marker of PTD. Numerous histological and Doppler analyses in the second and third trimesters of pregnancy indicated abnormal placentation and placental ischemia as a potential cause of preterm delivery [23,24]. In a screening study by Fonseca, et al., in which the pulsatility index value was analysed in the second trimester in 33,629 patients in singleton pregnancies, a significant increase in the pulsatility index was observed in 237 patients, who had a spontaneous delivery before 33 weeks' gestation. However, based on a multiple logistic regression analysis, it was found that the prediction of preterm delivery did not significantly improve compared to a model that only includes maternal demographic and obstetric factors [25]. In the analysis by Soares, et al., no significant differences were found in the UtA PI at 11 - 13 + 6 weeks' gestation in patients who delivered before 34 and those who delivered at term [26]. Similar results were obtained in a population study conducted by the Fetal Medicine Foundation [7]. In the presented study, analysis of UtA PI showed no statistical differences in individual groups of patients.
Measurement of placental biochemical markers such as PAPP-A and β-hCG in screening for chromosomal abnormalities allows to increase the sensitivity of ultrasound scan up to 90% at a false positive rate of 5% [27,28]. In a preliminary study by Ong, et al. the usefulness of these proteins, in particular PAPP-A, was found also in screening for preterm delivery [29]. According to the researchers of the FASTER group, the PAPP-A concentration below the 5th percentile was associated with an increased risk of intrauterine fetal demise before 24 weeks of pregnancy, preterm delivery, gestational hypertension, pre-eclampsia and low birth weight. A population study by Spencer, et al. that included 54,772 pregnant patients, showed a statistically significant lower levels of PAPP-A in the first trimester in patients who delivered prematurely. This was particularly significant in the group of patients who delivered before the 32 weeks' gestation [30]. These results are consistent with the data obtained in the presented study. Current reports in the literature on the correlation between the concentration of the free β-hCG and the occurrence of obstetric complications are contradictory. In a multicentre study conducted by Smith, et al. that included 8839 pregnant women, β-hCG below the 5th percentile in the first trimester was associated with increased risk of intrauterine growth restriction [31]. The analysis by Ong, et al., showed a significant correlation between β-hCG below the 10th percentile and the occurrence of miscarriage, gestational induced hypertension, intrauterine growth restriction and gestational diabetes [29]. In the presented study, no correlation was found between the level of β-hCG and the occurrence of both early and late forms of preterm labour.
Numerous studies confirmed the role of the AFP measurment in the first and second trimester of pregnancy in screening for chromosomal abnormalities, central nervous system defects and obstetric complications such as preeclampsia and intrauterine growth restriction [32,33]. In an uncomplicated pregnancy, AFP concentration is influenced by gestational age, ethnicity and smoking status [34,35]. Therefore, in order to effectively use the measurements of AFP concentrations numerous coefficients need to be taken into consideration in order to calculate the multiples of median [36]. In the presented analysis, we proved that AFP concentration level at 11 + 0 to 13 + 6 weeks' gestation is increased in pregnancies complicated by PTD. Additionally, incorporation of AFP improves the sensitivity of the PTD prediction model. These results are in agreement with findings of Beta, et al. In a case-control study that examined 33 patients with PTD and 99 patients that delivered at term, the authors found a significantly increased level of AFP is pregnancies complicated by PTD in comparison with patients in the control group [37].
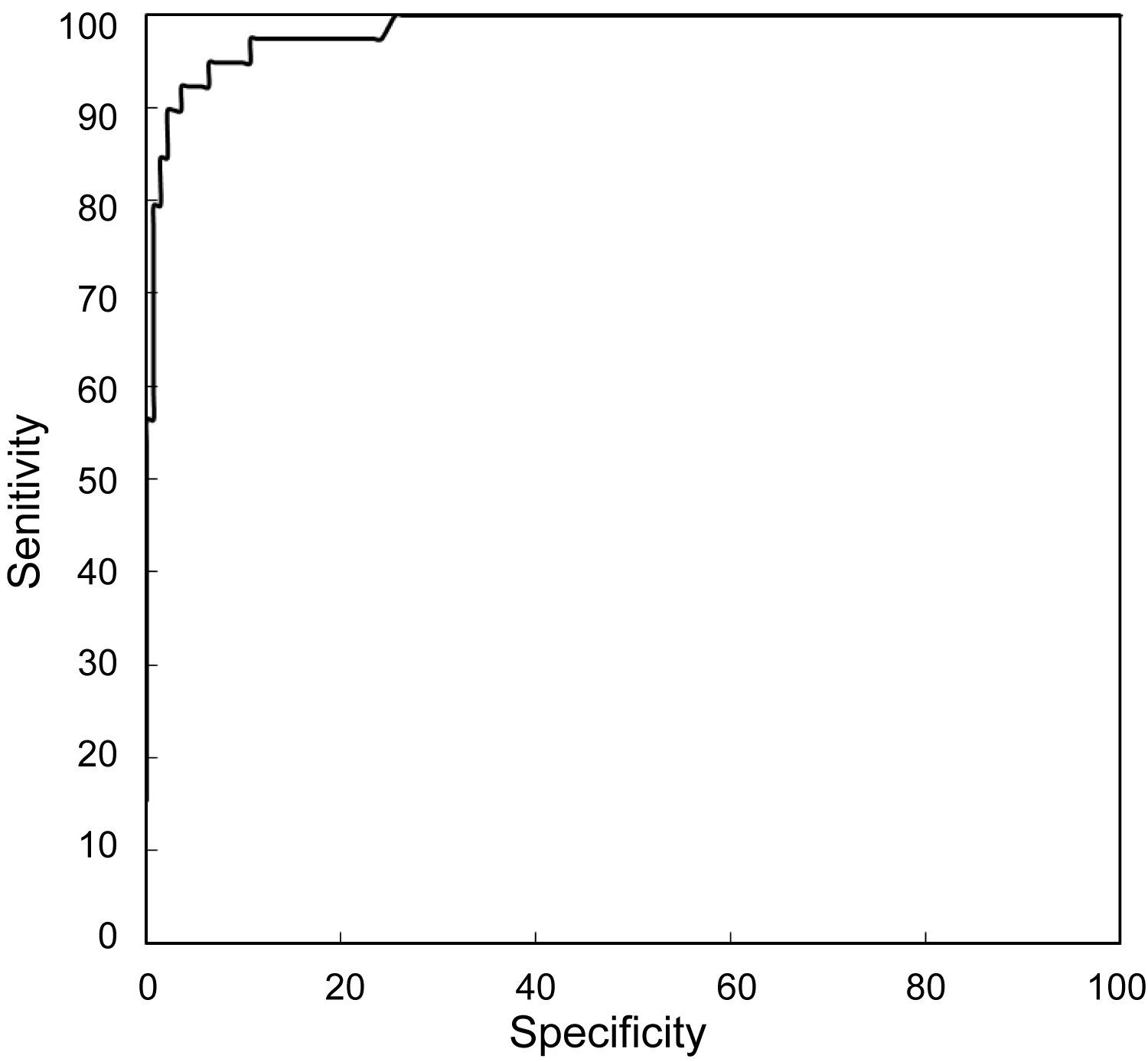
Due to numerous associations between described biochemical and biophysical markers, it was necessary to create an integrated model of screening studies for PTD. In the presented analysis, incorporating an integrated model of screening studies, a significant improvement in sensitivity of the prediction test especially for early form of PTD was achieved. Finally, after taking into consideration all markers it was found that the best prediction model for early PTD incorporates evaluation of maternal factors, cervical length, and concentrations of PAPP-A and AFP (Figure 1). In the prediction of late PTD, the best fitted model incorporates evaluation of maternal risk factors, cervical length, and AFP concentration (Figure 2). Screening studies for early PTD are very important because birth before 34 weeks is the main cause of perinatal mortality and psychomotor delay in children. The best fitted model for the prediction of PTD defined as PTD before 37 weeks comprises analysis of maternal risk factors, cervical length and PAPP-A and AFP concentrations (Figure 3).
Incorporation of presented algorithm in the routine practice could potentially result in better selection of woman who could benefit from preventive methods of PTD. Extensive studies have shown that vaginal progesterone decreases the risk of preterm birth and improves perinatal outcomes in singleton gestations with risk factors of PTD. Validation of presented algorithm reqiures further studies.
Conclusions
The best screening tool for the prediction of early spontaneous PTD is based on a combined analysis of maternal risk factors, measurement of the cervical length and measurement of the AFP and PAPP-A. The best fitted model for the prediction of late form of PTD is based on a combined analysis of maternal risk factors, measurement of the cervical length and evaluation of the AFP levels. The presented model of combined analysis of maternal risk factors, biochemical and biophysical markers in the first trimester is characterized by greater efficiency in detecting early form (< 34 weeks' gestation) compared to the late form (34-37 weeks' gestation) of PTD.
References
- Government Statistical Service for the Department of Health. NHS Maternity Statistics, England, 2002-2003.
- Veen S, Ens-Dokkum MH, Schreuder AM, et al. (1991) Impairments, disabilities, and handicaps of very preterm and very-low-birthweight infants at five years of age. The collaborative project on preterm and small for gestational age infants (POPS) in The Netherlands. Lancet 338: 33-36.
- Gardosi J, Kady SM, Francis A (2004) Fetal growth, maturity and preterm birth. In: Critchley HO, Bennet P, Thornton S, Preterm Birth. RCOG Press, London, 66-67.
- Centre for Maternal and Child Enquiries (CMACE) (2010) Perinatal Mortality 2008. CMACE, London.
- Saigal S, Doyle LW (2008) An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 371: 261-269.
- Celik E, To M, Gajewska K, et al. (2008) Cervical length and obstetric history predict spontaneous preterm birth: development and validation of a model to provide individualized risk assessment. Ultrasound Obstet Gynecol 31: 549-554.
- Beta J, Akolekar R, Ventura W, et al. (2011) Prediction of spontaneous preterm delivery from maternal factors, obstetric history and placental perfusion and function at 11-13 weeks. Prenat Diagn 31: 75-83.
- Parra-Cordero M, Sepulveda-Martinez, Munoz H (2014) Is there a role for cervical assessment and uterine artery Doppler in the first trimester of pregnancy as a screening test for spontaneous preterm delivery? Ultrasound Obstet Gynecol 43: 291-296.
- Yamashita M, Hayashi S, Endo M, et al. (2015) Incidence and risk factors for recurrent spontaneous preterm birth: A retrospective cohort study in Japan. J Obstet Gynaecol Res 41: 1708-1714.
- Drassinower D, Obican SG, Siddiq Z, et al. (2015) Does the clinical presentation of a prior preterm birth predict risk in a subsequent pregnancy? Am J Obstet Gynecol 213: 686.
- Ananth CV, Getahun D, Peltier MR, et al. (2006) Recurrence of spontaneous versus medically indicated preterm birth. AmJObstet Gynecol 195: 643-650.
- Shah NR, Bracken MB (2000) A systematic review and meta-analysis of prospective studies on the association between maternal cigarette smoking and preterm delivery. Am J Obstet Gynecol 182: 465-472.
- Aliyu MH, Lynch O, Saidu R, et al. (2010) Intrauterine exposure to tobacco and risk of medically indicated and spontaneous preterm birth. Am J Perinatol 27: 405-410.
- French JI, McGregor JA (1996) The pathobiology of premature rupture of membranes. Semin Perinatol 20: 344-368.
- Cnattingius S, Forman MR, Berendes HW, et al. (1992) Delayed childbearing and risk of adverse perinatal outcome. A population-based study. JAMA 268: 886-890.
- Carvalho MH, Bittar RE, Brizot ML, et al. (2003) Cervical length at 11-14 weeks' and 22-24 weeks' gestation evaluated by transvaginal sonography, and gestational age at delivery. Ultrasound Obstet Gynecol 21: 135-139.
- Souka AP, Papastefanou I, Michalitsi V, et al. (2011) Cervical length changes from the first to second trimester of pregnancy, and prediction of preterm birth by first-trimester sonographic cervical measurement. J Ultrasound Med 30: 997-1002.
- Antsaklis P, Daskalakis G, Pilalis A, et al. (2011) The role of cervical length measurement at 11-14 weeks for the prediction of preterm delivery. J Matern Fetal Neonatal Med 24: 465-470.
- Greco E, Lange A, Ushakov F, et al. (2011) Prediction of spontaneous preterm delivery from endocervical length at 11 to 13 weeks. Prenat Diagn 31: 84-89.
- Greco E, Gupta R, Syngelaki A, et al. (2012) First-trimester screening for spontaneous preterm delivery with maternal characteristics and cervical length. Fetal Diagn Ther 31: 154-161.
- Tsikouras P, Galazios G, Zalvanos A, et al. (2007) Transvaginal sonographic assessment of the cervix and preterm labor. Clin Exp Obstet Gynecol 34: 159-162.
- Conoscenti G, Meir YJ, D'Ottavio G, et al. (2003) Does cervical length at 13-15 weeks' gestation predict preterm delivery in an unselected population? Ultrasound Obstet Gynecol 21: 128-134.
- Agarwal N, Suneja A, Arora S, et al. (2004) Role of uterine artery velocimetry using color-flow Doppler and electromyography of uterus in prediction of preterm labor. J Obstet Gynecol Res 30: 402-408.
- Kim YM, Bujold E, Chaiworapongsa T, et al. (2003) Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol 189: 1063-1069.
- Fonseca E, Yu CK, Singh M, et al. (2006) Relationship between second-trimester uterine artery Doppler and spontaneous early preterm delivery. Ultrasound Obstet Gynecol 27: 301-305.
- Soares SC, Fratelli N, Prefumo F, et al. (2007) First-trimester uterine artery Doppler and spontaneous preterm delivery. Ultrasound Obstet Gynecol 29: 146-149.
- Avgidou K, Papageorghiou A, Bindra R, et al. (2005) Prospective first-trimester screening for trisomy 21 in 30,564 pregnancies. Am J Obstet Gynecol 192: 1761-1767.
- Nicolaides KH, Spencer K, Avgidou K, et al. (2005) Mutlitcenter study of first-trimester screening for trisomy 21 in 75 821 pregnancies: Results and estimation of the potential impact of individual risk-orientated two-stage first-trimester screening. Ultrasound Obstet Gynecol 25: 221-226.
- Ong CYT, Liao AW, Spencer K, et al. (2000) First trimestermaternal serum free ß human chorionic gonadotrophin and pregnancy associated plasma protein A as predictors of pregnancy complications. BJOG 107: 1265-1270.
- Spencer K, Cowans NJ, Molina F, et al. (2008) First-trimester ultrasound and biochemical markers of aneuploidy and the prediction of preterm or early preterm delivery. Ultrasound Obstet Gynecol 31: 147-152.
- Smith GCS, Stenhouse EJ, Crossley JA, et al. (2002) Early pregnancy levels of pregnancy associated plasma protein A and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. J Clin Endocrinol Metab 87: 1762-1767.
- Bredaki FE, Wright D, Matos P, et al. (2011) First-trimester screening for trisomy 21 using alpha-fetoprotein. Fetal Diagn Ther 30: 215-218.
- Nicolaides KH, Wright D, Poon LC, et al. (2013) First-trimester contingent screening for trisomy 21 by biomarkers and maternal blood cell-free DNA testing. Ultrasound Obstet Gynecol 42: 41-50.
- Bredaki FE, Wright D, Akolekar R, et al. (2011) Maternal serum alpha-fetoprotein in normal pregnancy at 11-13 weeks' gestation. Fetal Diagn Ther 30: 274-279.
- Crossley JA, Aitken DA, Waugh SM, et al. (2002) Maternal smoking: Age distribution, levels of alpha-fetoprotein and human chorionic gonadotrophin, and effect on detection of Down syndrome pregnancies in second-trimester screening. Prenat Diagn 22: 247-255.
- Bredaki FE, Sciorio C, Wright A, et al. (2015) Serum alpha-fetoprotein in the three trimesters of pregnancy: Effects of maternal characteristics and medical history. Ultrasound Obstet Gynecol 46: 34-41.
- Beta J, Bredaki FE, Calvo JR, et al. (2011) Maternal serum - alpha-fetoprotein at 11-13 weeks' gestation in spontaneous early preterm delivery. Fetal Diagn Ther 30: 88-93.
Corresponding Author
Ewelina Litwinska, Department of Perinatology and Gynecology, Polish Mother's Memorial Hospital Research Institute, Lodz, Poland, Tel: +48601636778.
Copyright
© 2020 Litwinska E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.