Twin Pregnancy with a Complete Hydatidiform Mole and a Coexisting Normal Fetus
Abstract
Twins pregnancies associating both complete hydatidiform mole and normal pregnancies represent an exceptional obstetrical situation. It is a high risk gestation either for the mother or for the normal fetus. There are no specific clinical features. The ultrasonography examination, thanks to its recent evolution, is the key stone for an earlier diagnosis. Concerning the fetal prognosis, most of these pregnancies evolve spontaneously to abortion. Never the less, 40% of the cases end with the birth of a healthy normal child. The management of such pregnancies still remains controversial: When diagnosed should the gestation be continued despite all the potential maternal and fetal complications or should it be interrupted, considering that these pregnancies can be consecutive to an assisted reproductive technology? Through this new case and a review of the literature, we will discuss the specificities of the diagnosis and the management of this condition.
Keywords
Twin pregnancy, Hydatidiform mole, Triploidy, Ultrasonography
Introduction
Twin pregnancy with a complete mole and a coexisting normal pregnancy is an exceptional obstetrical situation, with significant maternal and fetal risks. In fact, the mother is a serious candidate for preeclampsia and gestational trophoblastic tumors, while the fetus is exposed to significant risks of chromosomal abnormalities and prematurity [1,2].
Most of such pregnancies reported in world, ends up with spontaneous termination with less than 40% chance of a live birth.
The dilemma for the physician arises in choosing between continuation or immediate termination of the pregnancy in view of the anticipated complications, particularly when the pregnancy has been long awaited.
Observation
A 29-year-old woman, gravida 2, para 1, with a previous normal delivery, was referred to our department of obstetrics and gynecology, with high blood pressure at 20 weeks gestation. She had a history of vaginal bleeding during the first trimester taking into account the threatened miscarriage. Hence, the blood loss is recurrent that it causes an iron deficiency anemia (Hb = 6.6 g/dl).
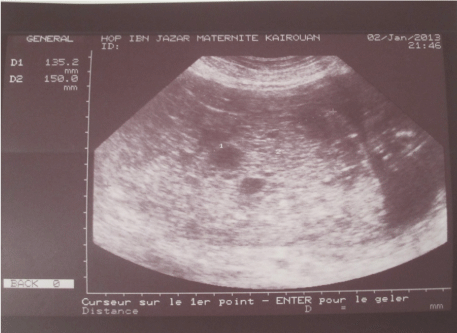
The patient was admitted for hypertension, edema of the lower extremities, the abdominal wall and proteinuria at 2.24 g/24 h. The uterus corresponded to 30 weeks size, otherwise examination was unremarkable. Ultrasound scan, practiced in the framework of a preeclampsia, showed a twin pregnancy; a 20 weeks size normal live foetus with multiple cystic areas in the placenta (Figure 1).
Subsequently, the patient developed a worsening clinical picture that urged the decision to terminate the pregnancy by caesarean section. She had an uneventful surgery and delivered a live male baby that weighed 450 g and discharged home on the 4th postoperative day.
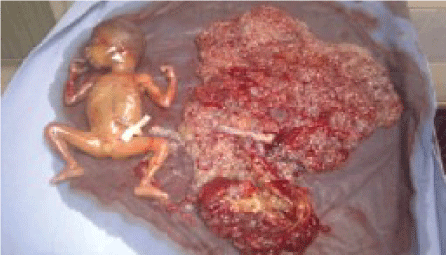
Macroscopic examination of the placenta in the fresh state showcases two separate and distinct masses (Figure 2a).
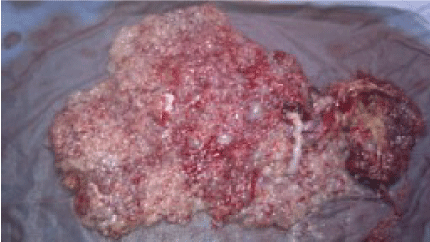
Indeed, the normal-looking placenta measuring 10 × 9 × 2 cm with a marginal insertion of umbilical cord was delivered and the molar tissue was distinct with irregularly shaped mass of necrotic tissue and multiple grape particularly vesicles adjacent to the normal placenta (Figure 2b).
Histological examination placenta consists of immature villi with regular trophoblastic coating without excessive nuclear clusters or trophoblastic hyperplasia. The intervillous chamber was normal and the truncal vessels were out of problems.
Examination of the molar tissue showed marked villous hydrops with central cistern formation, moderate focal trophoblast hyperplasia and stromal karyorrhectic debris. The P57 immunohistochemical marker is absent in both cytotraphoblast and mesenchymal cells Karyotyping of the foetus was normal, 46 XY. The diagnosis of twin pregnancy with complete hydatidiform mole and a co-existing live fetus was therefore retained.
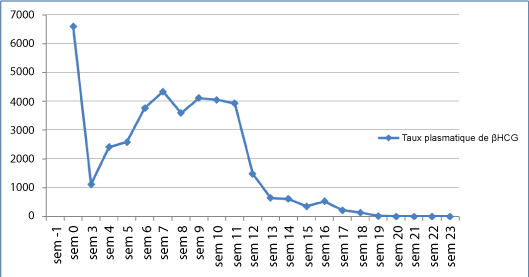
Our patient is followed with serial quantitative serum hCG assays every week. Blood levels of hCG had decreased through the first three weeks, and then increased suddenly (Figure 3). She had no symptoms and the pelvic ultrasound and thoracoabdominopelvic tomography were normal. That is the diagnosis of gestational trophoblastic tumor was confirmed. In the 2000 FIGO staging and classification, the risk score was 5. Our patient was classified as low risk and was treated with one of the single-agent methotrexate. In the first course of treatment, methotrexate (30 mg/m IM) was given on day 90 after evacuation and was well tolerated. The evolution was marked by a gradual decrease in HCG levels which became negative after the 11th course of treatment (160 days after evacuation). Two consolidation courses of methotrexate were administered. Following remission, hCG levels were performed every week for two months, then monthly for the rest of the first year and pelvic ultrasound in the 6th and 12th months. After three years of follow-up, the evolution was favorable.
Discussion
Twin pregnancy involving a complete hydatidiform mole (CHM) and a live fetus is a very rare entity. The reported incidence is 1 in 22,000-100,000 pregnancies [3]. Compared to complete hydatidiform moles which are more common at extreme ages, the association of CHM and a live fetus is seen mainly in patients between 21 and 41 years of age [3]. Some cases of twin pregnancy with complete hydatidifrorm mole and a healthy fetus have been reported following ovulation induction [3]. Indeed, Piura, et al., through a series of 30 cases of twin pregnancy with a CHM and a coexisting fetus, reported that 30% of these were preceded by ovulation induction [3]. However, no causal relationship between ovulation-inducing drugs and such pregnancies can be established [1,4]. This correlation may need further study and explanation. Dizygotic twin pregnancies were found more often. In very rare cases they can be monozygotic [5].
Clinically, there are no specific signs to predict the diagnosis of such pregnancies. That includes vaginal bleeding, hyperemesis gravidarum, early onset pre-eclampsia and a uterus larger than the period of gestation. All these symptoms were present in our patient.
Biologically, there are abnormally high levels of HCG and simultaneous elevation of HPL levels (usually lower in molar pathology). These results could predict the presence of twin pregnancy with complete hydatidiform mole and a coexisting live fetus.
For diagnostic ultrasonography, relative recent advances in technology have improved ultrasound's ability to diagnose these pregnancies at early gestation and provided a host of information regarding the fetus.
Massardier, et al. showed in their retrospective study that conventional diagnostic methods are adequate for diagnosis a twin pregnancy with a complete hydatidiform mole and a coexisting fetus (71% of cases) compared with a singleton complete molar pregnancy. This diagnosis is suspected in association with a normal growth fetus and a distinct heterogeneous placental mass known as "snowstorm" or "honeycomb" [1].
In antenatal, the principal differential diagnosis for twice pregnancy with hydatidiform mole is the partial mole [6]. Although a partial mole often coexists with a triploid fetus, there are case reports of twin pregnancies with fetuses of normal karyotype. Triploid fetuses tend to die before the end of the first trimester. The histological examination is useful for discriminating complete and partial moles showing the absence of membrane or fetal structure in the case of CHM. Moreover, the histological appearance of the villi, at an early term, is most often misleading because of the absence of sufficiently characteristic signs of the CHM, such the meandering appearance of the villi and focal trophoblastic hyperplasia.
However, the presence p57 nuclear in villous mesenchymal and cytotrophoblastic cells is useful for confirming the diagnosis of partial mole. Triploid foetuses have asymmetrical intrauterine growth restriction (IUGR), multiple malformations, a small hypotrophic and non molar placenta.
Finally, other differential diagnoses for molar changes in the placenta, such as mesenchymal dysplasia and hydropic changes in the placenta, were ruled out by clinical features as well as the histopathology of the placenta after delivery. In these cases, there are no clinical signs of mole or disruption of HCG levels, IUGR is common and about a quarter of these pregnancies are complicated by fetal death in utero [7].
In the present case the features were compatible with complete hydatidiform mole, while clinically it was believed to be a partial mole.
The management of twin pregnancy with complete hydatidiform mole and a coexisting healthy fetus is controversial. Indeed, the dilemma arises in choosing between TOP to avoid potential maternal complications or expectant management to save the fetus but endangering the mother with potential complications [4].
When detected early in the pregnancy, the management approach is generally to offer termination in view of the anticipated complications. But the pregnancy can be allowed to continue since it has a considerable chance to result in a normal live neonate. Nevertheless, it seems reasonable to opt for conservative management with the consent of the patient, after thorough counselling. As many of these molar pregnancies occur following the induction of ovulation, the desire to continue pregnancy often outweighs that of termination. However, one must be prepared for termination in the event of maternal complications [4].
In the study of sebire, et al. [8] on 77 Twins pregnancies associating both complete hydatidiform mole and normal fetus, 24 women had chosen the interruption of pregnancy against 53 parturients who had decided to continue this pregnancy. 20 Live births have been able to be obtained.
According to Vaisbuch, et al. a retrospective study of 130 cases of twin pregnancies involving CHM and normal fetus showed that the therapeutic interruption of pregnancy was recommended in 41% of cases in the face of high maternal risk [9].
Massardier, et al. found that prematurity is important and the rate of live births varies between 16 and 56% [1]. Thus, a twin pregnancy with a hydatidiform mole and a coexisting live foetus requires a thorough evaluation, and pregnancy may be continued under close surveillance for an optimal outcome especially in the presence of normal karyotype and in the absence of ultrasound anomalies. In our observation, the therapeutic interruption of pregnancy was justified b the severity of the clinical situation with severe preeclampsia and premonitory signs of convulsion.
As concerns the risk of persistent trophoblastic disease, it was estimated as 15% to 20% [10]. Steller, et al. suggest that it is higher in cases of CHMF compared with single molar pregnancies and that, when present, it more commonly progresses to metastatic disease. Sebire, et al. [8], find out that the risk of PTD after CHMF is not significantly higher than in single molar pregnancies (16% vs. 21%). Niemann, et al., confirmed this results in a more recent study [11].
This risk involves a rigorous biological monitoring of the HCG level with serial quantitative serum hCG values every week until hCG levels normalize then monthly for six to 12 months [12]. Indeed, the evolution toward a persistent gestational disease is defined as an abnormal evolution of HCG after evacuation of HM:
• A reascension of 10% or more of the β-hCG on 3 consecutive dosages at one week intervals (days 1, 7 and 14), such our case,
• Stagnation of β-hCG (less than 10% variation) on at least 4 consecutive dosages at one week intervals (days 1, 7, 14 and 21),
• Persistence of β-hCG 24 weeks after the evacuation of the mole.
Histological examination is not essential for the diagnosis and for chemotherapy.
The diagnosis can also suspected on clinical features:
• Persistent metrorrhagia in the postpartum or became abundant after a normal decrease.
• The appearance of signs of hyperthyroidism without apparent cause in a young woman,
• Respiratory disorders related to pulmonary matastases or neurological disorders related to brain metastases without primitive known in women during periods of genital activity.
• Acute hemorragia syndrome in a metastatic site: Hemoperitoneum, hemothorax.
In addition, a TTG can be diagnosed by histological examination of product of conception after a suction curtage.
Conclusion
Twin pregnancy involving CHM and a coexisting live fetus is an exceptional entity that can be suspected early with obstetrical ultrasound and HCG levels. But only histological examination will confirm the diagnosis. There is no consensus for management of such pregnancies. However, close surveillance to detect potential early signs of maternal and fetal complications is compulsory and a detailed discussion (and informed consent) with the couple is necessary. Finally, we will insist on weekly monitoring of HCG levels to eliminate a progression towards gestational trophoblastic disease after a pregnancy evacuation to exclude PTD.
References
- Massardier J, Golfier F, Journet D, et al. (2009) Twin pregnancy with complete hydatiformmole and coexistent fetus: Obstetrical and oncological outcomes in a series of 14 cases. Eur J Obstet Gynecol Reprod Biol 143: 84-87.
- Sharma D, Usha MG, Gaikwad R (2013) Twin pregnancy with complete hydatidiform mole and coexisting live fetus complicated with HELLP syndrome. Int J Reprod Contracept Obstet Gynecol 2: 92-94.
- Piura B, Rabinovich A, Hershkovitz R, et al. (2008) Twin pregnancy with a complete hydatiform mole and surviving coexistent fetus. Arch Gynecol Obstet 278: 377-378.
- Shazly SA, Ali MK, Abdel Badee AY, et al. (2012) Twin pregnancy with complete hydatidiform mole and coexisting fetus following ovulation induction with a non-prescribed clomiphene citrate regimen: A case report. J Med Case Reports 6: 95.
- Niemann I, Bolund L, Sunde L (2008) Twin pregnancies with diploid hydatiform mole and co-existing normal fetus may originate from one oocyte. Hum Reprod 23: 2031-2035.
- Sophie Patrier (2010) Pathologie du placenta. Cas n°5. Môle Hydatiforme partielle. Annales de Pathologie 30: 296-300.
- Allias F, Lebreton F, Collardeau-Frachon S, et al. (2008) Placental mesenchymal dysplasia. Ann Pathol 28: 85-94.
- Sebire NJ, Foskett M, Paradinas FJ, et al. (2002) Outcome of twin pregnancies with complete hydatidiform mole and healthy co-twin. Lancet 359: 2165-2166.
- Vaisbuch E, Ben-Arie A, Dgani R, et al. (2005) Twin pregnancy consisting of a complete hydatidiform mole and co-existent fetus: Report of two cases and review of literature. Gynecol Oncol 98: 19-23.
- Sophie Patrier (2010) Pathologie du placenta. Cas n°1. Môle hydatiforme complète vue précocement. Annales de Pathologie 30: 275-279.
- Niemann I, Sunde L, Petersen LK (2007) Evaluation of the risk of persistent trophoblastic disease after twin pregnancy with diploid hydatidiform mole and coexisting normal fetus. Am J Obstet Gynecol 97: 45.e1-45.e5.
- Boufettal H, Coullin P, Mahdaoui S, et al. (2011) Les môles hydatiformes complètes au Maroc: étude épidémiologique et clinique. Journal de Gynecologie Obstetrique et Biologie da la Reproduction 40: 419-429.
Corresponding Author
Fatnassi Ridha, Department of Gynecology and Obstetrics, Ibn Al Jazzar Hospital 3140 Kairouan, Tunisia.
Copyright
© 2018 Fatnassi MR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.