Use of a "Living" Probiotic Medical Food in Eight Patients Confirms In Vitro Complete Inhibition of Clostridium difficile
Abstract
Objective : The objective of the use of a medical food containing 15 patented strains of “Living” probiotics was to evaluate whether it supports our earlier in vitro findings, which demonstrated that a juice-based living probiotic dietary supplement could inhibit Clostridium difficile.
Results : Among eight patients with confirmed active infection, toxin B was detectable in all participants, while toxin A was identified in only about half. Both toxins exhibited large standard deviations in pre- and post-trial measurements, reflecting considerable variability among participants.
Importantly, significant reductions in concentration were observed for both toxins following the intervention. Symptom severity scores also declined consistently across all categories. Bloating, the most pronounced symptom at baseline, dropped from an average score above 2 (moderate) to approximately 1 (mild) after the probiotic treatment.
These clinical findings align with our in vitro results, adding to the evidence that the juice-based living probiotic medical food inhibits C. difficile. A key reason for the efficacy of this living probiotic medical food seems to be its ability to survive gastric transit and reach the colon. These promising outcomes provide a strong foundation for conducting a double-blind, randomized clinical trial under Good Clinical Practice guidelines at a reputable Contract Research Organization.
Keywords
Clostridium difficile, C. diff, Clostridium difficile infection (CDI), Open-Label clinical trial, Probiotics, Juice-Based probiotic medical food
Abbreviations
CDI = C. diff Infection
EIA = Enzyme Immunoassay
HIPAA = Health Insurance Portability and Accountability Act
NAAT = Nucleic Acid Amplification Test
PCR = Polymerase Chain Reaction
Introduction
In our previous in vitro study, we demonstrated that our juice-based living probiotic dietary supplement could completely inhibit the growth of Clostridium difficile (C. diff) [1]. As a logical next step, we looked for volunteers with confirmed C. diff. infection to assess whether these in vitro findings could be confirmed in a real-world clinical setting.
The objective of this was to endeavor to confirm our earlier in vitro findings, which demonstrated inhibition of Clostridium difficile by a juice-based living probiotic dietary supplement.
Methods
Investigational product
We enhanced the juice-based living probiotic dietary supplement and formulated it as a juice-based probiotic medical food specifically designed for the dietary management of Clostridium difficile infection (MF-CDI). The supplement in question was Doctor’s Biome Signature Probiotic Blend, which contains 342 mg (equivalent to 60 billion colony-forming units) of Bifidobacterium bifidum strain SP 9, Bifidobacterium breve BBR8, Bifidobacterium infantis SP 37, Bifidobacterium longum SP 54, Bifidobacterium animalis subsp. lactis BLC 1, Lactobacillus acidophilus LA1, Lactobacillus brevis SP 48, Lactobacillus bulgaricus LB2, Lactobacillus casei BGP 93, Lactobacillus gasseri LG050, Lactobacillus paracasei 101/37, Lactobacillus plantarum 14D, Lactobacillus reuteri LR92, Lactobacillus rhamnosus SP 1, and Lactobacillus salivarius SP 2. The probiotic blend was added to a two-fluid-ounce serving (61 g) of Doctor’s Biome’s proprietary organic green juice blend, which consists of diluted organic mint juice, organic cucumber juice, organic apple juice, organic kale juice, organic lettuce juice, organic celery juice, and organic lemon juice (Figure 1).
Patient testing
We asked for volunteers to participate in an initial evaluation of the formulation's effectiveness. If the outcomes of this proved promising, we planned that a double-blind, randomized clinical trial would subsequently be conducted at a reputable Contract Research Organization.
Ethical Approval and Consent
All participants provided written informed consent and received a Health Insurance Portability and Accountability Act (HIPAA) Notice of Privacy Practices prior to enrollment.
Inclusion and exclusion criteria
Participants were eligible for inclusion if they had a confirmed active Clostridium difficile infection (CDI). The sole exclusion criterion was the concurrent use of other probiotic dietary supplements or fermented foods.
Diagnostic criteria for CDI
Diagnosis of CDI was confirmed using quantitative polymerase chain reaction (PCR) stool analysis (GI-MAP, Diagnostic Solutions Laboratory) [2], which detects genes encoding toxins A and/or B, in conjunction with clinical symptoms consistent with CDI. One participant provided post-intervention toxin enzyme immunoassay (EIA) and nucleic acid amplification test (NAAT) results instead of PCR stool analysis.
Questionnaire and clinical assessment
A standardized symptom questionnaire was administered at baseline and weekly throughout the four-week study period. The questionnaire assessed the frequency and severity of gastrointestinal and systemic symptoms, history of previous CDI episodes, prior treatments, and the overall impact on quality of life. Symptom severity, including abdominal pain, cramping, bloating, and fatigue was rated using a numerical scale from 1 (mild) to 5 (severe). Stool consistency was evaluated using the Bristol Stool Chart.
Questionnaire administration and data handling
Informed consent forms, HIPAA privacy notices, and symptom questionnaires were distributed electronically using Adobe secure web forms. Completed forms were encrypted, password-protected, and securely stored. PCR stool test results were securely transmitted by participants via email to the Principal Investigator.
Intervention
Participants consumed the investigational product (MF-CDI) once daily (2 fl. oz) on an empty stomach for a duration of four weeks.
Design and Follow-Up
This was designed to evaluate the safety and efficacy of MF-CDI for the dietary management of patients with CDI. The primary endpoints were (1) PCR-confirmed eradication of C. diff toxin genes and (2) symptomatic improvement as measured by weekly participant-reported symptom questionnaires. At enrollment, participants completed a baseline symptom questionnaire and underwent stool PCR testing. Follow-up questionnaires were submitted electronically on a weekly basis throughout the four-week intervention period. At the end of the testing period all participants underwent a repeat stool PCR test to assess the persistence or resolution of CDI.
Study population
Eight (8) participants were enrolled (75% female; age range 4-79 years). Five participants were experiencing their first episode of CDI, while three had a history of recurrent infections.
Results
The results are presented in Table 1 (provided as Additional file 1 due to width constraints). Pre-trial stool PCR analysis revealed that toxin B was detectable in all participants, while toxin A was present in approximately half. In post-treatment stool PCR and/or EIA results, both toxin A and toxin B were undetectable in all participants except one (participant 4), whose post-treatment results showed approximate 50% reductions in both. Participant 4 also received a diagnosis of gallstones (cholelithiasis) during the treatment period, which is consistent with their abdominal symptom reporting and with pre-treatment PCR stool results showing increased levels of colonization by biofilm-producing microbes (Pseudomonas aeruginosa, Morganella spp., and Enterobacteriaceae). It is possible that gallstone-induced alterations to bile acid metabolism and microbiome composition limited the effectiveness of probiotic medical food intervention. Participant 2 continued using the MF-CDI for an additional four weeks, reportedly due to experiencing no change in symptoms; after the extended treatment, both toxins were below detectable limits in stool PCR and average symptom severity was reduced from 4.375 to 2.25. For participant 8, although toxin EIA results were negative, their NAAT results for toxigenic C. diff were positive, potentially indicating colonization without active infection. Notably, wide standard deviations were observed in pre-treatment and post-treatment concentration measurements for toxin A and B alike, indicating inter-individual variability.
Symptom assessment focused on four key indicators: diarrhea, bloating, abdominal pain/cramping, and nausea. None of the participants reported a fever throughout the duration of the trial. Five of the eight participants did not report experiencing diarrhea pre-trial, potentially indicating toxigenic C. diff colonization without active infection or mild or atypical clinical presentations. Not all symptoms were present in every participant, with bloating being the most prevalent and severe. Across all participants, symptom severity scores decreased following the intervention period, suggesting overall symptomatic improvement.
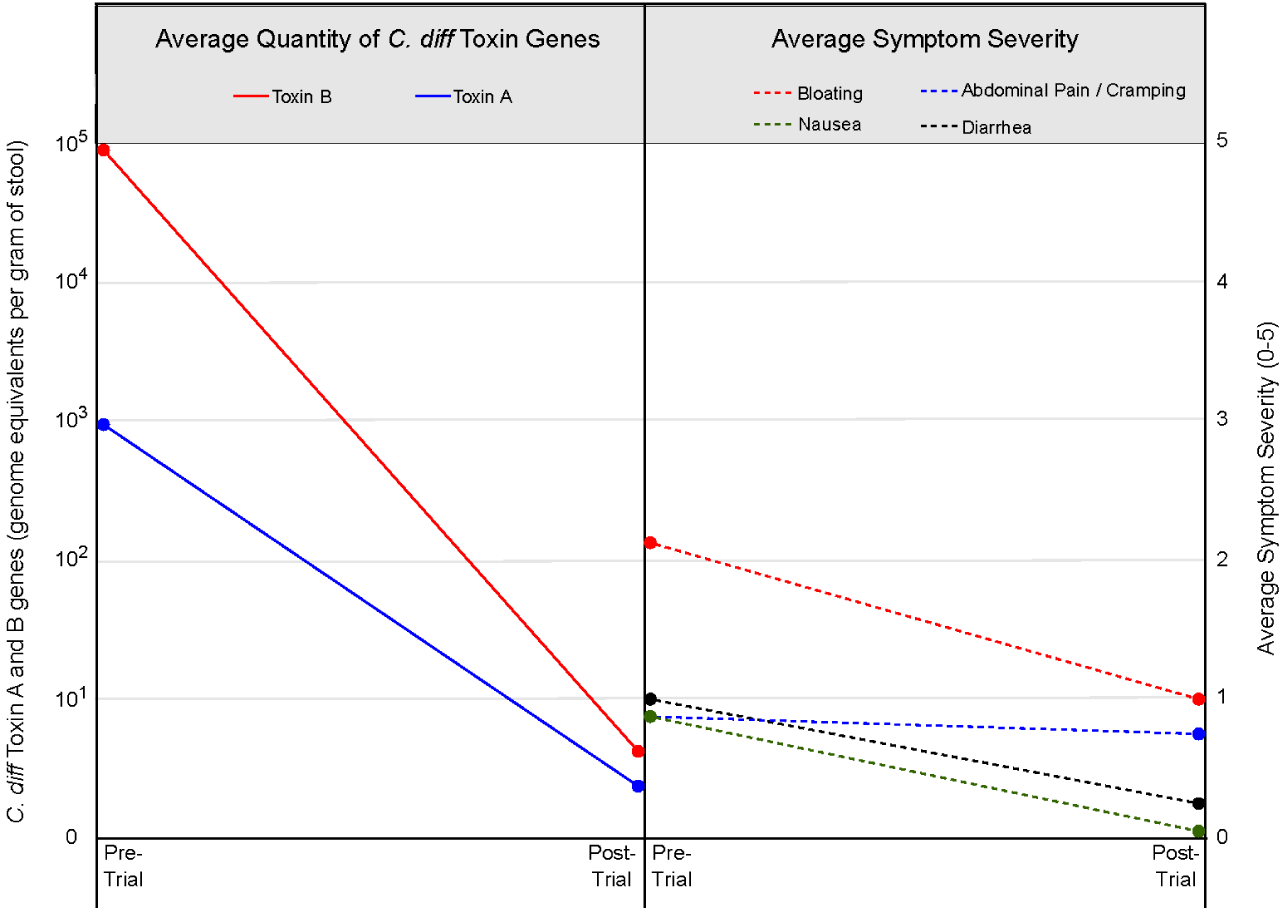
Figure 2 illustrates the average quantity of C. difficile toxin genes detected (left) and the average symptom severity scores among participants (right). A marked post-intervention reduction in the gene quantity was clearly observed for both toxins A and B. Likewise, average symptom severity scores demonstrated a consistent post-intervention decrease across all evaluated symptoms. Bloating, the most pronounced symptom, declined from a severity score above 2 to approximately 1 (mild). Nausea and abdominal pain/cramping were reduced to near-zero levels, while diarrhea showed only a modest reduction.
Discussion
We believe the results clearly confirm our earlier in vitro findings demonstrating inhibition of Clostridium difficile by a juice-based probiotic dietary supplement.
A critical factor contributing to the observed beneficial effects of MF-CDI is its ability to reach the colon effectively. In a recent study [3], we demonstrated that probiotics delivered in a juice matrix exhibit significantly greater resistance to stomach acid compared to their dry counterparts in capsules. This enhanced survivability of hydrated probiotics is likely due to a combination of factors, including cellular hydration, the buffering capacity of the juice, and the presence of glucose. In essence, incorporating probiotics into a fruit and vegetable juice carrier improves their resistance to gastric acidity, thereby serving as a more effective delivery system for probiotic dietary supplements and medical foods.
In explaining the observed inhibition of Clostridium difficile by the juice-based living probiotic medical food, both quantitative and qualitative perspectives should be considered:
From a quantitative perspective, one general mechanism of microbial inhibition is the competitive exclusion principle. This principle states that when two species compete for limited resources within a constrained environment-such as the colon-the species with a competitive advantage (e.g., greater population number or faster growth rate) will dominate and ultimately exclude the weaker competitor.
From a qualitative perspective, the selected blend of Bifidobacteria and Lactobacilli appears to have functioned in a complementary, additive, and possibly synergistic manner to completely inhibit the growth of Clostridium difficile and suppress production of its toxins. It is likely that the probiotic strains released bioactive compounds into the colon that interfered with C. difficile growth and toxin expression. One such compound may be lactic acid (along with other organic acids), which can lower the pH of the local environment to a range that is unfavorable for C. difficile spore germination and/or vegetative growth.
Additional information related to Clostridium difficile can be found in references [4-10].
Limitations
The study we conducted was a preliminary study. While we remain appropriately cautious regarding the interpretive limitations of this study, the promising results provide a compelling rationale to advance to a double-blind, randomized clinical trial. This next phase will be conducted in compliance with Good Clinical Practice guidelines at a reputable Contract Research Organization.
Declarations
Ethics approval and consent to participate
Written informed consent was obtained from all participants prior to their participation. This research was conducted in accordance with the principles set forth in the Declaration of Helsinki.
Consent for publication
Not applicable.
Availability of data and materials
All data generated or analyzed are included in this published article and its supplementary information files. The original raw data documents contain identifiable patient information and thus cannot be publicly shared. De-identified data supporting the findings are available from the corresponding author upon reasonable request and following ethical approval.
Competing interests
Funding
This was fully sponsored and funded by Doctor’s Biome. The sponsor had a role in design, data collection, analysis, and manuscript preparation.
Authors’ contributions
Acknowledgements: Not applicable.
References
- Robins Howard F, Reza KA (2020) Complete inhibition of clostridium difficile with a probiotic juice beverage. J gastroenterol res 4: 104-111.
- Quantitative PCR Stool Technology for the Integrative and Functional Medicine Practitioner. (2024).
- Kamarei A, Robins H, Finkelstein E (2024) Juice-Based living probiotics survive stomach acid significantly better than dry powder live probiotics. J Biochem Physiol 7.
- Cai J, Zhao C, Du Y, et al. (2017) Comparative efficacy and tolerability of probiotics for antibiotic-associated diarrhea: Systematic review with network meta-analysis. United European Gastroenterol J 6: 169-180.
- Zilberberg MD, Shorr AF, Jesdale WM, et al. (2017) Recurrent Clostridium difficile infection among Medicare patients in nursing homes. Medicine 96: e6231.
- Rondanelli M, Faliva MA, Perna S, et al. (2017) Using probiotics in clinical practice: Where are we now? A review of existing meta-analyses. Gut Microbes 8: 521-543.
- Hudson SL, Arnoczy G, Gibson H, et al. (2019) Probiotic use as prophylaxis for Clostridium difficile-associated diarrhea in a community hospital. Am J Infect Control 47: 1028-1029.
- Bubnov RV, Babenko LP, Lazarenko LM, et al. (2018) Specific properties of probiotic strains: Relevance and benefits for the host. EPMA J 9: 205-223.
- McFarland LV (2017) Primary prevention of Clostridium difficile infections - how difficult can it be? Expert Rev Gastroenterol Hepatol 11: 507-521.
- Wilkins T, Sequoia J (2017) Probiotics for gastrointestinal conditions: A summary of the evidence. Am Fam Physician 96: 170-178.
Corresponding Author
Howard F Robins, Department of Health Education & Communication, Doctor's Biome, 106 Lake Avenue S, Unit 4, Nesconset, NY, USA, Tel +1-516-967-1009.
Copyright
© 2025 Robins HF. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.