Complete Inhibition of Clostridium difficile with a Probiotic Juice Beverage
Abstract
Background & Aims
Clostridium difficile ("C. difficile" or "C. diff") is a Gram-positive, spore-forming, anaerobic, motile bacterium which produces two types of toxins, enterotoxin A and cytotoxin B. Clostridium difficile Infection (CDI) causes life-threatening diarrhea which is usually a side-effect of taking antibiotics. C. diff can easily spread from person to person and is considered a major health threat. In 2017, there were an estimated 223,900 cases in hospitalized patients and 12,800 deaths in the United States. There has been considerable scientific research in using various probiotic strains against C. diff with diverse observations and inconsistent results. Our first goal was to develop an "optimum probiotic product" to inhibit growth and pathogenicity of C. diff. Our second goal was to prove an inhibitory effect of bioactive compounds (secreted by the 15 strains of probiotic bacteria into the juice) on C. diff.
Methods
For optimum profile of probiotics, we considered positive results from specific strains in the published literature and commercially-available probiotic bacteria from credible global suppliers. As a result, we chose a blend of five strains of Bifidobacteria and ten strains of Lactobacilli. For an optimum carrier, we chose a proprietary blend of organic green vegetable and fruit juices. Upon culture propagation and strain verification of Clostridium difficile ATCC #9689, inoculum was prepared using reinforced clostridial broth and a sample was prepared using centrifuged, microfiltered supernatant of Doctor's Biome Colon Health (DBCH). The inoculated control was serially diluted with sterile 0.1% Peptone Buffer. The inoculated sample was serially diluted with the centrifuged, filtered supernatant of DBCH. The serially diluted control and diluted sample were transferred to agar plates and incubated anaerobically at 35-37 ℃ for 18-24 hours.
Results
Serially diluted agar plates of the control showed presence of C. diff colony forming units, which were biochemically identified via VITEK ANC. Serially diluted agar plates of the sample did not show any C. diff colony forming units due to the inhibitory effect of the DBCH bioactive compounds.
Conclusions
We believe inhibition of C. diff by DBCH probiotics should be considered from both a quantitative and qualitative points of view. From a quantitative point of view, a general mechanism of microbial inhibition is through the "competitive exclusion principle". This means that when two species compete in a limited environment (e.g., colon) and compete for the same limited amount of nutrients, the species that has advantage over the others (e.g., larger numbers) will dominate the environment and lead to the exclusion of the weaker competitor. From a qualitative point of view, it seems that the chosen blend of Bifidobacteria and Lactobacilli have released some bioactive compounds into the juice that has completely inhibited growth of C. diff. We conclude that the bioactive compounds secreted from the 15 probiotic strains into the organic vegetable and fruit juice has played a decisive inhibitory role against growth of C. diff.
Keywords
Clostridium difficile, C. difficile, C. diff., Clostridium difficile infection (CDI), Probiotics, Multi-strain probiotics, Liquid probiotics
Introduction
Clostridium difficile, also known as "C. difficile" or "C. diff" is a Gram-positive, spore-forming, anaerobic, motile bacterium (Figure 1). Clostridium difficile cells show optimum growth on blood agar at human body temperatures in the absence of oxygen. Clostridium difficile produces two types of toxins, enterotoxin A and cytotoxin B.
C. difficile is a bacterium that causes life-threatening diarrhea. It is usually caused as an adverse effect of taking antibiotics. It is considered a major health threat and listed as an "Urgent Threat" by the Center for Disease Control and Prevention (CDC) [1]. According to the CDC symptoms may begin within a few days or several weeks after beginning or following the taking of antibiotics or when people touch surfaces that are contaminated with feces from an infected person. Symptoms of Clostridium difficile Infection (CDI) include diarrhea, loose or watery stools lasting for several days, fever, stomach tenderness, loss of appetite and nausea. Frequent recurrent and duplicate episodes are common with one in six patients with CDI occurring again within two to eight weeks [1].
These infections are common in people 65 and older (80+%) and in children who take antibiotics and receive outpatient medical care or people who stay in hospitals and nursing homes for a long period of time [1,2]. Also, it is common in patients with weakened immune systems on antibiotics or with previous infection with C. diff. One out of eleven patients diagnosed with CDI will die within a month [1].
The estimated national burden of C. difficile infection (CDI) was 476,400 cases in 2011 and 462,100 in 2017 with an average number of deaths around 28,000 per year [3].
CDI puts a tremendous strain on the healthcare system. The total financial burden of CDI inpatient management was estimated to be $5.4-6.3 billion USD per year. This includes 2.4 million days of hospital stays and growing in number yearly [3-6].
With the increased use of appropriate and inappropriate prescriptions of antibiotics both in hospital and outpatient care the financial burden from CDI has risen over the past decade [4,7-9].
The scientific definition of the term ‘probiotic' was proposed in a 2001 joint report of the Food and Agriculture Organization of the United Nations and the World Health Organization (FAO/WHO), and confirmed by an expert panel convened by International Scientific Association for Probiotics and Prebiotics (ISAPP) held in 2013. The currently accepted definition of probiotics is: "Live microorganisms that, when administered in adequate amounts, confer a health benefit on the host". This means if the microorganisms are not "live", they cannot be considered probiotic and if their amount is not adequate enough to confer a health benefit, they cannot be considered probiotic either.
A review of the literature shows it is replete with hundreds of published papers showing various positive and some mixed results on the use of specific probiotic strains to help prevent and help treat CDI [10-15].
The probiotics used in most of the consumer available preparations are usually taken from a limited range of organisms. As this area of study has become more important and prevalent, new strains have come under investigation.
"Next Generation Probiotics" (NGP) are bacteria that due to their development act as live biotherapeutic products and lend themselves to act more like pharmaceuticals than traditional food supplements [16,17].
Despite the above findings reported in the literature, McDonald, et al., in 2017 update on Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children, using earlier publications up to 2013 and referring to issues such as differences in probiotic formulations studied, duration of probiotic administration, definitions of CDI, duration of study follow-up, inclusion of patients not typically considered at high risk for CDI, and the potential for organisms in probiotic formulations which can cause infections in hospitalized patients, concluded that there is insufficient data at this time to recommend administration of probiotics for primary prevention of CDI outside of clinical trials of probiotics for primary prevention of CDI. Yet the literature is replete with numerous studies showing that specific strains of patented bacteria have had beneficial results in the prevention and treatment of CDI.
The Harvard School of Public Health says "Because probiotics fall under the category of supplements and not food, they are not regulated by the Food and Drug Administration in the U.S. This means that unless the supplement company voluntarily discloses information on quality, such as carrying the USP seal that provides standards for quality and purity, a probiotic pill may not contain the amounts listed on the label or even guarantee that the bacteria are alive and active at the time of use" [18].
Several studies have been performed in an attempt to find a way to improve survival of probiotics before reaching the large intestine with limited and minimal success. This is one of the main concerns over capsule, powder, tablet and spore probiotics. Less than 59% survival using unique and somewhat expensive measures have been employed, though rarely found and used in most probiotic preparations currently available [19-24].
The FDA states "Many dietary supplements, often marketed as "probiotics," include claims that the live microbial ingredient, formulated in adequate amounts, will confer a health benefit on consumers. The weight of microbial dietary ingredient in a product represents the product's total cellular mass, consisting of both live and dead microorganisms, and therefore does not necessarily correlate with the number of viable microorganisms in that product" [25].
Aims of the Study
It became evident that certain strains of NGP "Smart Bacteria" (SB) achieved consistently the best results in helping to prevent and assist in CDI amelioration along with the usual treatment regimens. SB can sense they're attached to our intestinal cells, and then remodel their expression of specific genes, including those involved in metabolism, to beneficially exploit our cells and colonize our gut [26,27].
Bacteria have evolved to develop remarkable systems that can sense neighboring cells called "target" cells, and can initiate upon contact a series of changes that enhance their ability to survive and grow at the expense of these "target" cells. Smart Bacteria do this by employing a mechanism called "Horizontal Gene Transfer" (HGT). This is a very interesting and complex mechanism. Simply put, HGT shares genetic material between organisms that are not in a parent-offspring relationship and is a widely recognized mechanism for adaptation in bacteria [28-33].
With this concern in mind, NGP SB's were created to survive the stomach acids and digestive enzymes 80% or more [34]. Also, to ensure that they are "living" and thriving at the time of ingestion an appropriate environment and media, a 100% organic vegetable-fruit juice needed to be found meeting all these needs including the range of acidity needed for survival and growth. The NGP SB's that were chosen needed to be compatible with each other so as not to overwhelm each other's growth and benefits. They also needed to be in a sufficient number of Colony Forming Units (CFU) to repopulate the colon and attack the CDI effectively.
Considering diverse observations and inconsistent results of various probiotic strains on Clostridium difficile, it was not possible to choose one single, most effective strain for inhibition of C. difficile. Therefore, we decided to develop an "optimum probiotic product" to inhibit growth and pathogenicity of C. difficile. We named it Doctor's Biome Colon Health™ or DBCH (Figure 2). By definition, DBCH should have the optimum profile of probiotic bacteria and be an optimum carrier for the chosen probiotic bacteria. The immediate goal of our study was to prove the inhibitory effect of DBCH bioactive compounds (secreted by the 15 strains of NG Smart probiotic bacteria in the juice) on C. difficile.
Materials and Methods
Optimum profile of probiotics
Bifidobacteria and Lactobacilli are broadly recognized for their key roles in the human intestinal microflora throughout life. A high proportion of bifidobacteria and lactobacilli in the intestinal tract is considered beneficial to health. Considering the results of published literature mentioned above and reviewing commercially-available probiotic bacteria from credible global suppliers, we chose a blend of five strains of Bifidobacteria (Table 1a) and ten strains of Lactobacilli (Table 1b) from DuPont Nutrition & Biosciences.
Optimum carrier
In search of an optimum carrier (also known as excipients or medium), we chose a proprietary blend of organic green vegetable and fruit juices consisting of 100% organic diluted mint juice, cucumber juice, apple juice, lettuce juice, kale juice, celery juice and lemon juice. Sensory evaluation of DBCH (blend of probiotics in blend of organic juices) showed a pleasant taste of this product to consumers across the board. Furthermore, every batch of Doctor's Biome Colon Health is tested by an independent, FDA-registered accredited microbiology lab to confirm absence of pathogenic microorganisms.
Design of the Study
Culture propagation and strain verification
A sterile inoculating loop of Clostridium difficile ATCC #9689 was streaked onto a Reinforced Clostridial agar slant, broth tubes, and two Sheep Blood agar plates. The slant and broth tubes and one Sheep Blood agar plate were incubated anaerobically at 36-38 ℃ for 48 ± 2 hours. The second Sheep Blood agar plate was incubated aerobically at 36-38 ℃ for 48 ± 2 hours and served as the contamination check. Following incubation, the agar plates and tube were observed for colony growth and turbidity. The Sheep Blood agar plate incubated anaerobically was biochemically identified using the VITEK ANC card to verify purity.
Inoculum preparation
A well isolated colony from the Sheep Blood agar plate was used to inoculate a Reinforced Clostridial broth, which was then incubated anaerobically at 36-38 ℃ for 18 to 20 hours. Following incubation, the culture was stored overnight at 3 ± 1 ℃.
Sample preparation
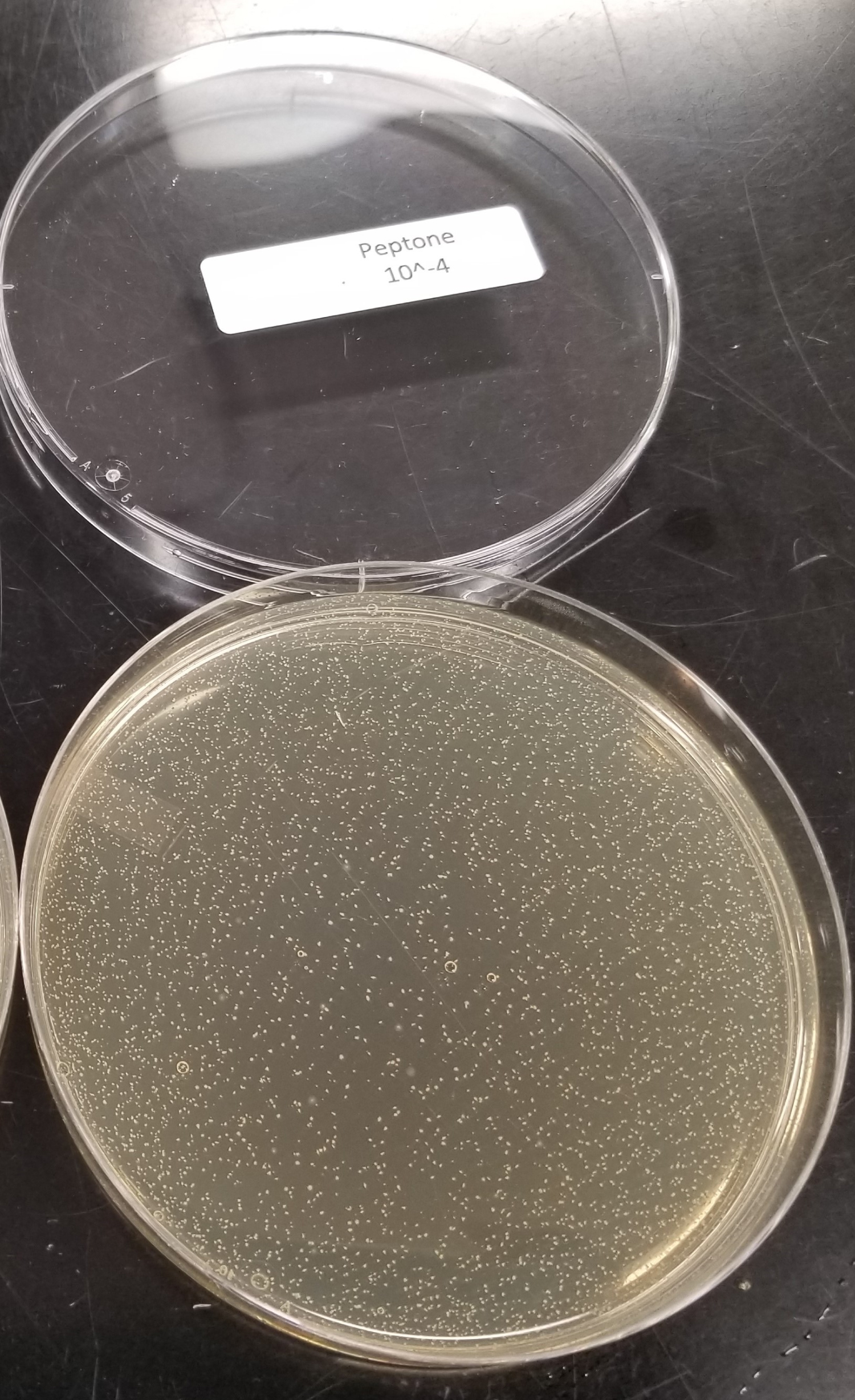
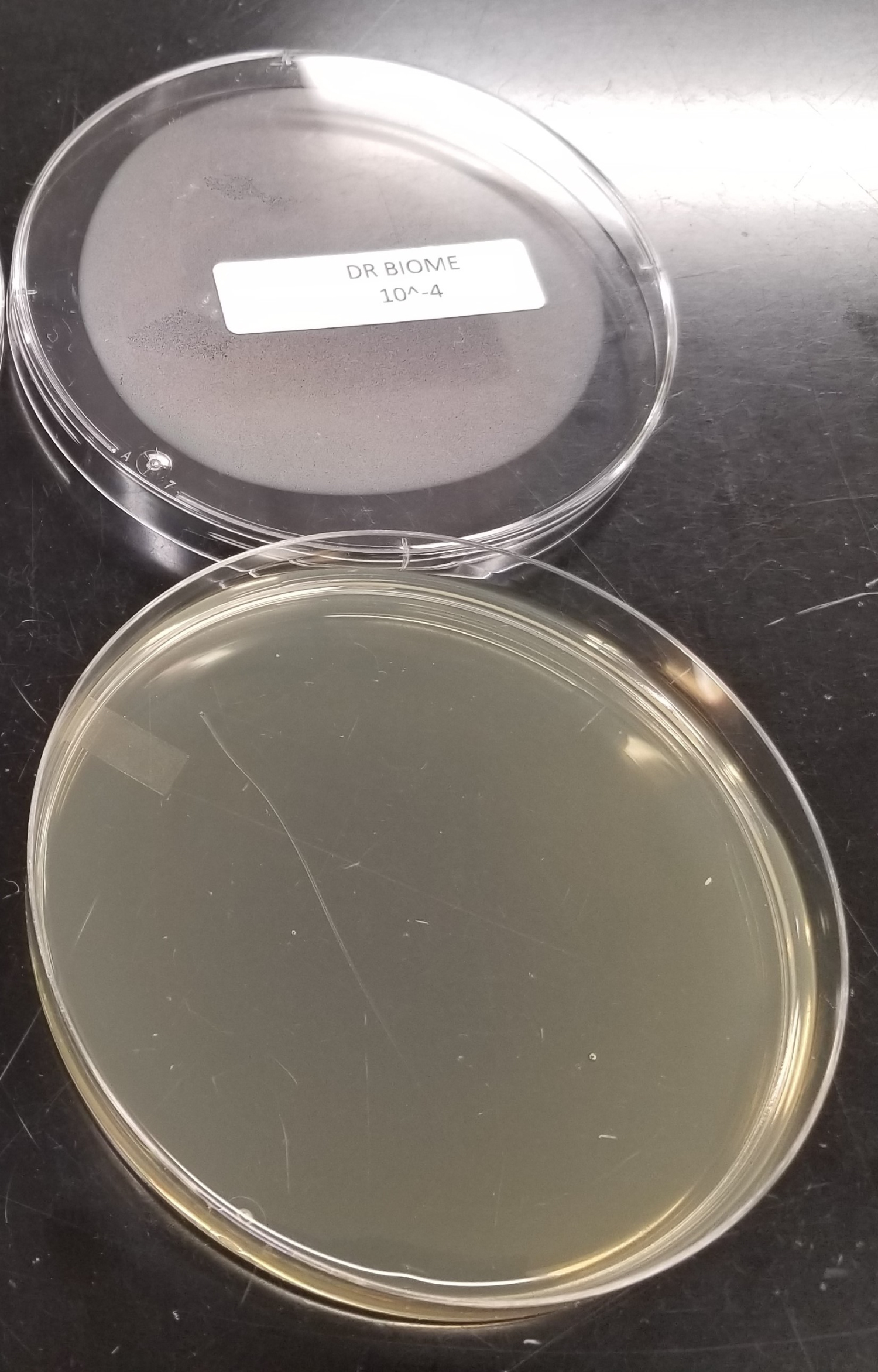
Six, individually packaged, intact 2-ounce DBCH glasses were composited into a sterile 500 mL glass bottle under a laminar flow hood. This bottle was mixed by gentle swirling and stored at ambient temperature (20-25 ℃) until testing. At that time, the composited product was homogenized. Then 10 mL was pipetted into a test tube for a before (Figure 3) and after comparison photo with the centrifuged product. Following centrifugation at 4500 RPM for 15 minutes, the supernatant liquid was filtered through a 0.22 µm filter to ensure that the supernatant liquid was free of cells. A 10 mL portion was pipetted into a test tube and photographed (Figure 4). The filtered sample was used as the test product sample and the 9 mL diluent tubes for its ten-fold serial dilution. The inoculated control was analyzed with sterile 0.1% Peptone Buffer.
Test procedure
Test procedure was performed according to the Compendium of Methods for the Microbiological Examination of Foods, 5th Edition, Chapter 33, Section 33.7 (Editors: Yvonne Salfinger and Mary Lou Tortorello, American Public Health Association).
Control test procedure
The inoculum used for the sample test procedure was vortexed for 5 seconds. 1 mL of the inoculum was transferred to a 9 mL tube of 0.1% Peptone Buffer and vortexed for 5 seconds. This served as the 10-1 dilution tube. 1 mL from the 10-1 dilution tube was transferred in duplicate into sterile Petri Dishes. With the same pipet, 1 mL was transferred into a second 9 mL tube containing the 0.1% Peptone Buffer for the 10-2 dilution tube and vortexed for 5 seconds. 1 mL from the 10-2 dilution tube was transferred in duplicate into sterile Petri Dishes. With the same pipet, 1 mL was transferred into a third 9 mL tube containing the 0.1% Peptone Buffer for the 10-3 dilution tube and vortexed for 5 seconds. Serial dilution was repeated until a 10-8 dilution plate was reached. Approximately 20 mL of tempered (44-46 ℃) Reinforced Clostridial agar was added to the Petri Dishes and gently swirled to evenly disperse inoculum throughout the agar. After solidification, 15 mL of Reinforced Clostridial agar was overlaid onto each plate. The plates were incubated anaerobically in an upright position at 35-37 ℃ for 18-24 hours.
Sample test procedure
The refrigerated anaerobic chamber containing the inoculum was equilibrated to ambient temperature prior to inoculation. The inoculum was vortexed for 5 seconds to homogenize. 1 mL of the inoculum was transferred to a 9 mL tube of the supernatant test sample and vortexed for 5 seconds. This served as the 10-1 dilution tube. 1 mL from the 10-1 dilution tube was transferred in duplicate into sterile Petri Dishes. With the same pipet, 1 mL was transferred into a second 9 mL tube containing the supernatant test sample for the 10-2 dilution tube and vortexed for 5 seconds. 1 mL from the 10-2 dilution tube was transferred in duplicate into sterile Petri Dishes. With the same pipet, 1 mL will be transferred into a third 9 mL tube containing the supernatant test sample for the 10-3 dilution tube and vortexed for 5 seconds. Serial dilution was repeated until a 10-8 dilution plate was reached. Approximately 20 mL of tempered (44-46 ℃) Reinforced Clostridial agar was added to the Petri Dishes and gently swirled to evenly disperse inoculums throughout the agar. After solidification, 15 mL of Reinforced Clostridial agar was overlaid onto each plate. The plates were incubated anaerobically in an upright position at 35-37 ℃ for 18-24 hours.
Results
Following incubation, photographs were taken of the serially diluted agar plates of the control to visually show presence of Clostridium difficile colony forming units (Figure 5). The plates which exhibited less than 300 colonies were enumerated and representative colonies for Clostridium difficile were biochemically identified via VITEK ANC (Table 2).
Similarly, following incubation, photographs were taken of the serially diluted agar plates of the sample to visually show absence of Clostridium difficile colony forming units due to the inhibitory effect of the DBCH bioactive compounds (Figure 6 and Table 2).
Discussion and Conclusion
The obtained results of our in-vitro study are consistent with several published investigations in PubMed. We believe inhibition of C. difficile by DBCH probiotics should be considered from both quantitative and qualitative points of view:
From a quantitative point of view, a general mechanism of microbial inhibition is through the "competitive exclusion principle". This means that when two species compete in a limited environment (e.g., colon) and compete for the same limited amount of nutrients, the species that has advantage over the others (e.g., larger numbers) will dominate the environment and lead to the exclusion of the weaker competitor. For example, in our study the probiotic dose of 27 billion live and active Colony Forming Units (CFU) for a daily serving of DBCH is considered among the higher quantities commercially available.
From a qualitative point of view, it seems that the chosen blend of Bifidobacteria and Lactobacilli have functioned in a complementary, additive and possibly synergistic way to completely inhibit growth of C. difficile. In other words, the chosen probiotics have released some bioactive compounds into the juice that has inhibited growth of C. difficile. One such compound could possibly be lactic acid (and possibly other organic acids) which can reduce the pH of the environment to an acidic range unfavorable for spore germination and/or growth of C. difficile. We can conclude that the bioactive compounds secreted from the chosen 15 probiotic strains into the organic vegetable and fruit juice has played a decisive inhibitory role against growth C. difficile.
The combined quantitative and qualitative properties of juice-based DBCH is expected to positively and impressivlely impact prevention and/or treatment of CDI.
Based on these results, and in line with the recent guidelines of American Gastroenterological Association on the role of probiotics in the management of gastrointestinal disorders [35], a prospective, randomized, double-blind clinical trial (based on Good Clinical Practice) on the safety and efficacy of DBCH for CDI patients is warranted.
Acknowledgment
Authors acknowledge the valuable contribution of Mary Lee Kluger and Andrew Liles from the independent Certified Laboratories (Melville, New York) for microbiological tests.
Financial Disclosure
Dr. Kamarei was paid as a consultant. Certified Laboratories was monetarily compensated by Doctor's Biome microbiological tests.
Conflict of Interest
Dr. Robins and Dr. Kamarei are partners in Doctor's Biome Company (Newgen 27 LLC).
References
- Center for Disease Control: Healthcare associated diseases-community interface (HAIC).
- Li N, Zeng B, Cai HE, et al. (2018) Cost-effective analysis of oral antibiotics for the prevention of clostridium difficile-associated diarrhea in children and adolecents. J Hosp Infect 99: 469-474.
- Guh AY, Mu Y, Winston LG, et al. (2020) Trends in U.S. burden of clostridioides difficile infection and outcomes. N Engl J Med 382: 1320-1330.
- Dongmu Zhang, Vimalanand S Prabhu, Stephen W Marcella (2018) Attributable healthcare resource utilization and costs for patients with primary and recurrent clostridium difficile infection in the United States. Clinical Infectious Disease 66: 1326-1332.
- Bond SE, Boutlis CS, Yeo WW, et al. (2017) The burden of healthcare-associated clostridium difficile infection in a non-metropolitan setting. J Hosp Infect 95: 387-393.
- Heimann SM, Cruz Aguilar MR, Mellinghof S, et al. (2018) Economic burden and cost-effective management of Clostridium difficile infections. Med Mal Infect 48: 23-29.
- Mundkur ML, Franklin J, Huybrechts KF, et al. (2018) Changes in outpatient use of antibiotics by adults in the United States 2006-2015. Drug Saf 41: 1333-1342.
- Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. (2016) Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA 315: 1864-1873.
- Donskey CJ (2017) Clostridium difficile in older adults. Infect Dis Clin North Am 31: 724-756.
- Thad Wilkins, Jacqueline Sequoia (2017) Probiotics for gastrointestinal conditions: A summary of the evidence. Am Family Physician 96: 170-178.
- Joshua Z Goldenberg, Christina Yap, Lyubov Lytvyn, et al. (2017) Probiotics for the prevention of clostridium difficile-associated diarrhea in adults and children. Crochrane Database Sys Rev 19.
- John P Mills, Krishna Rao, Vincent B Young (2018) Probiotics for prevention of clostridium difficile infection. Curr Opin Gastroenterol 34: 3-10.
- Johnston BC, Ma SS, Goldenberg JZ, et al. (2012) Probiotics for the prevention of clostridium difficile-associated diarrhea: A systematic review and meta-analysis. Ann Intern Med 157: 878-888.
- Goldenberg JZ, Ma SS, Saxton JD, et al. (2013) Probiotics for the prevention of clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev 31.
- Goldstein EJC, Johnson SJ, Maziade PJ, et al. (2017) Probiotics and prevention of clostridium difficile infection. Anaerobe 45: 114-119.
- O'Toole PW, Marchesi JR, Hill C (2017) Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat Microbiol 2: 17057.
- Chang CJ, Lin TL, Tsai YL, et al. (2019) Next generation probiotics in disease amelioration. J Food Drug Anal 27: 615-622.
- (2020) The microbiome. The nutrition source, Harvard School of Public Health.
- Keller D, Verbruggen S, Cash H, et al. (2019) Spores of bacillus coagulans GBI-30, 6086 show high germination, survival and enzyme activity in a dynamic, computer-controlled in vitro model of the gastrointestinal tract. Benef Microbes 10: 77-87.
- Maathuis AJ, Keller D, Farmer S (2010) Survival and metabolic activity of the GanedenBC30 strain of bacillus coagulans in a dynamic in vitro model of the stomach and small intestine. Benef Microbes 1: 31-36.
- Varankovich N, Martinez MF, Nickerson MT, et al. (2017) Survival of probiotics in pea protein-alginate microcapsules with or without chitosan coating during storage and in a simulated gastrointestinal environment. Food Sci Biotechnol 26: 189-194.
- Rui Ji, Wu J, Zhang J, et al. (2019) Extending viability of bifidobacterium longum in chitosan-coated alginate microcapsules using emulsification and internal gelation encapsulation technology. Front Microbiol 10: 1389.
- Ding WK, Shah NP (2009) An improved method of microencapsulation of probiotic bacteria for their stability in acidic and bile conditions during storage. J Food Sci 74: 53-61.
- Ramos PE, Cerqueira MA, Teixeira JA, et al. (2018) Physiological protection of probiotic microcapsules by coatings. Crit Rev Food Sci Nutr 58: 1864-1877.
- FDA policy regarding quantitative labeling of dietary supplements containing live microbials: Guidance for industry 21 CFR 10.115(g)(5)).
- Landry BP, Tabor JJ (2017) Engineering diagnostic and therapeutic gut bacteria. Microbiol Spectr 5.
- El Hage R, Hernandez-Sanabria E, Tom Van de Wiele (2017) Emerging trends in "smart probiotics": Functional consideration for the development of novel health and industrial applications. Front Microbiol 8: 1889.
- Soucy SM, Huang J, Gogarten JP (2015) Horizontal gene transfer: Building the web of life. Nat Rev Genet 16: 472-482.
- Ochman H, Lawrence JG, Groisman EA (2000) Lateral gene transfer and the nature of bacterial innovation. Nature 405: 299-304.
- Olivera PH, Touchon M, Cury J, et al. (2017) The chromosomal organization of horizontal gene transfer in bacteria. Nat Comm 8: 841.
- Gilbert JA, Blaser MJ, Caporaso JG, et al. (2018) Current understanding of the human microbiome. Nat Med 24: 392-400.
- Hayes CS, Aoki SK, Low DA (2010) Bacterial contact-dependent delivery systems. Annu Rev Genet 44: 71-90.
- Manrique P, Dills M, Young MJ (2017) The human gut phage community and its implications for health and disease. Viruses 9: 141.
- Acid tolerance of bacteria used in Doctors Biome™ DCUSA. Inc. subsidiary of Dupont, Danisco, USA.
- Su GL, Ko CW, Bercik P, et al. (2020) AGA clinical practice guidelines on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology.
Corresponding Author
Dr. Howard F Robins, Chief Medical Officer, Doctor's Biome (Newgen 27 LLC), 80 Davids Dr., Unit 2, Hauppauge, NY 11788, USA, Tel: (631)-240-3066, Cell: (516)-967-1009.
Copyright
© 2020 Robins HF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.