Epidemiology of Intussusception among Children Under-Two Years of Age from 2010-2017 in Odisha, India
Abstract
Background
Intussusception is an adverse event associated with rotavirus vaccines (RVV). RVV was introduced phase-wise in India since April 2016. Background intussusception rates are needed to document changes with RVV introduction. We describe the epidemiology of intussusception among children aged under-two years in Odisha, India.
Methods
This bidirectional surveillance (retrospective from July 2010 to March 2016 and prospective from April 2016 to September 2017) at three hospitals in Odisha recruited children aged 2-23 months with intussusception. Data on sociodemography, immunization, clinical, treatment and outcome were collected. Incidences of intussusception among infants and children > 1 year were estimated.
Results
371 children with intussusception (retrospective, n = 266; prospective, n = 105) were recruited. Among them, 78.7% were infants with median age 8 (IQR 6-12) months and 70.6% were males. Abdominal pain (60.9%), vomiting (55.5%), and bloody stools (53.4%) were the leading symptoms and triad was observed in 51.8% cases. 57.4% cases underwent surgery and 16.0% were managed by reduction. Nine (2.4%) children died. 71.4% cases met Brighton criteria Level-1. Intussusception cases increased 2014 onwards and the pooled incidence was estimated to be 5 (3.9-7.9) cases per 100000 infants per year.
Conclusions
Intussusception in children was observed prior to rotavirus vaccination in Odisha, India. The risk factors for rising intussusception in children need further evaluation.
Keywords
Intussusception, Prospective surveillance, Retrospective surveillance, Children, Epidemiology, Rotavirus vaccine, Odisha, India
Introduction
Intussusception is one of the common intestinal obstruction in young children, characterised by invagination of an intestinal segment into another segment. It usually occurs in infants and commonly between 4-10 months of age [1,2]. If not intervened and relieved early, it may progress to ischemia and perforation of intestine and even may be fatal [2]. The ileocecal is the most commonly reported intussusception site. While imbalance in the peristalsis and neuromuscular coordination have been hypothesized as the cause(s), in 10-15% of cases, some pathological lead points (PLPs) may be found [2-4]. Several enteric and non-enteric viral infections and bacterial enteritis have also been reported as triggers for intussusceptions [5-7]. Intussusception gained importance with its association as an adverse event following introduction of the rotavirus vaccine (RVV), Rotashield™ (Wyeth Lederle, Marietta, PA, USA) in 1999, which forced withdrawal of the vaccine [8-10]. Since then, intussusception has been documented in the clinical trials for subsequent candidate RVVs [11,12]. Variable risks of intussusception with the RVVs including Rotarix™ (GlaxoSmithKline Biologicals, Rixensart, Belgium) and Rotateq™ (Merck & Co. Inc., Kenilworth, USA) have been reported; from no increased risk in USA and Brazil to 1-2 additional cases per 100,000 infants vaccinated in Mexico and Australia [13-15]. Considering the benefit of child mortality reduction with RVV, World Health Organization (WHO) has recommended universal introduction in national immunization programmes (NIPs). WHO also recommends surveillance to document the potential changes and attributable risks of intussusception with vaccine introduction [16].
RVV was introduced in India's NIP in April 2016 and expanded countrywide in a phased manner. Both Rotavac™ (Bharat Biotech, Hyderabad, India) and Rotasiil™ (Serum Institute of India, Pune, India) RVVs are in use with 6, 10, 14 weeks schedule. The available intussusception incidence among Indian children vary from 17.7 (95% CI: 5.9, 41.4) in north India to 254 (95% CI: 5.9, 41.4) cases per 100,000 child-years in south India [17,18]. The incidence information from other parts of the country are not available. There is wide variations in intussusception incidence and case load globally [19]. A regional variation in the intussusception case load have been documented in India, although the reasons for the variation are yet to be explored [4,20]. In view of the recent introduction of RVV in India, limited information on intussusception rates across the regions and states, there is need to contribute information that allows monitoring the trend and identifying the associated risk factors [21,22]. We describe the epidemiology, incidence, clinical features of intussusception in children aged under-two years through a hospital based surveillance network in eastern India.
Methods
Study area and participating hospitals
This hospital-based sentinel surveillance involved three medical college hospitals in Odisha state in eastern India. Out of these hospitals located in three cities from different regions of the state (Bhubaneswar, Cuttack and Berhampur), two were government funded and one was private. These were the leading hospitals with functional paediatric surgery facility. Most of the patients from Odisha state attended one of these hospitals.
Study design
At these hospitals, a bidirectional surveillance was set up including a retrospective (July 2010 to March 2016) and prospective (April 2016 to September 2017).
Case definition, case selection and data collection
For the retrospective surveillance, the list of children aged 2-23 months with compatible diagnoses were retrieved from the medical records section. For retrieval of the cases all possible sources were considered. (a) At the institutions using international classification of diseases (ICD), the cases with any of the compatible ICD-10 codes (K56.1, K56.2, K56.3, K56.4, K56.5, K56.6, K56.7 and K56.0) or ICD 9 codes (560.0, 560.2, 560.31, 560.30, 560.81, 560.9 and 560.1) were identified. (b) At the institutes without ICD classification, cases with compatible diagnoses (intussusception, acute intestinal obstruction, subacute intestinal obstruction, acute abdomen, and blood in stool with vomiting) were identified. (c) These lists were supplemented with review of the registers from clinical wards (pediatrics, pediatric surgery and emergency), operation theatres, radiology (ultrasound, barium studies and CT scan) and pathology departments to identify any missed case. The records of these potential cases were reviewed to identify the confirmed intussusception cases.
For the prospective surveillance, all the children aged 2-23 months admitted to these hospitals were screened and actively tracked till final diagnosis. All confirmed intussusception cases were recruited after written informed consent from their parent or legally authorised representative. For the confirmed intussusception cases identified through retrospective and prospective surveillance, the data on demography, clinical features, management, and final outcome were abstracted. For the prospective surveillance, the socioeconomic status (SES), dietary practices and immunization exposure (from definite sources: Immunization card or register) were documented. Diagnostic certainty levels were assigned according to the Brighton Collaboration case definition (BCCD) by an independent expert group (paediatrician, paediatric surgeon, and radiologist) [1].
Quality assurance
The research staffs were trained on the protocol and data collection. The data received from sites were reviewed and queries were resolved in reference to the source documents. Site visits were made to assess the protocol adherence and data quality and completeness. Additionally, the data team visited sites and checked the data retrieval from the medical records using the diagnoses and ICD-9/10 codes for the study period to identify any missed case.
Data management and analysis
Double data entry was done followed by matching between the entries and with the filled forms. The verified data were stored in the server with authorised access and daily backup. The SES data was represented as standard of living index (SLI), which was estimated using the scores for household assets ownership and categorised into high, medium and low categories, with reference to the National Family and Health Survey for India [23]. Using the BCCD criteria the cases were categorised into levels 1 to 3 [1]. Descriptive analysis was done and findings were expressed as proportions, means (with standard deviations), or median (with interquartile range, IQR), as appropriate. For statistical significance, the values between groups were compared using Chi-Square or Fisher's exact tests for the proportions and Mann-Whitney-U or Kruskal-Wallis tests for the medians depending on the skewness, sample and number of groups. The missing data were excluded from analysis. The statistical significance was considered if p < 0.05. It was assumed that the children with intussusception in the state would attend these hospitals. The census (2011) population data for Odisha (n = 41,947,358) was used as the base population and growth rate (1.4%) as per the Sample Registration System (SRS) data was applied to estimate the population for 2016-2017 [24]. The live birth rate and infant mortality rate data from SRS were used for estimating the number of children in the corresponding years (Appendix A) [24]. The intussusception incidence rate was estimated as number of intussusception cases among infants or under-two-children per 100000 infants or under-two-children/year in Odisha. The statistical analysis was performed using STATA version 15.0 (StataCorp LLC, Texas, USA).
Ethical issues
The study protocol approved by the ethics committees of all participating institutes. Informed written consent was obtained for all the eligible cases before recruitment and data collection. Confidentiality in data handling was maintained.
Results
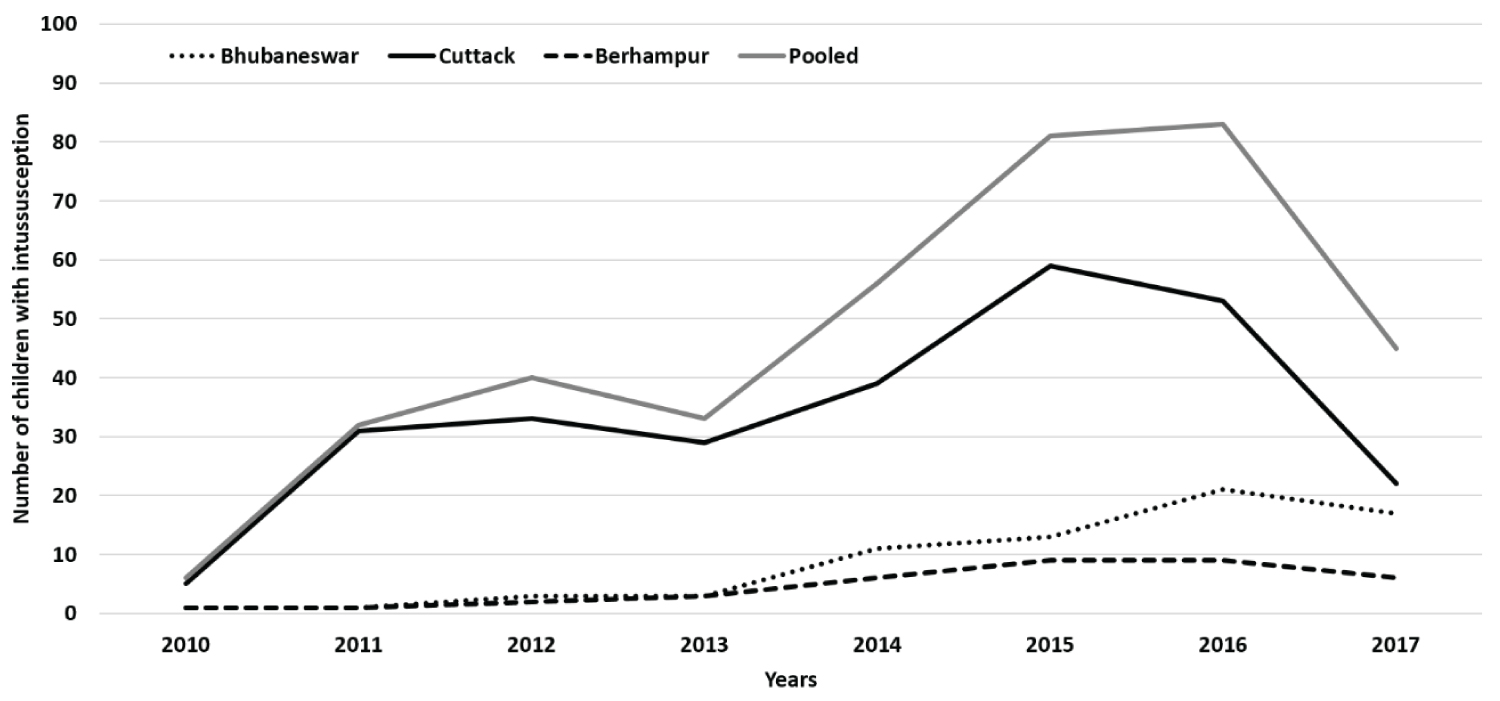
Between July 2010 and September 2017, out of the 160,009 children (142317 paediatrics and 17692 paediatric surgery admissions) admitted to these hospitals, 568 suspected intussusception cases were identified and 371 children with confirmed intussusception were recruited. The number of intussusception cases increased over the years, especially after 2013 (Figure 1).
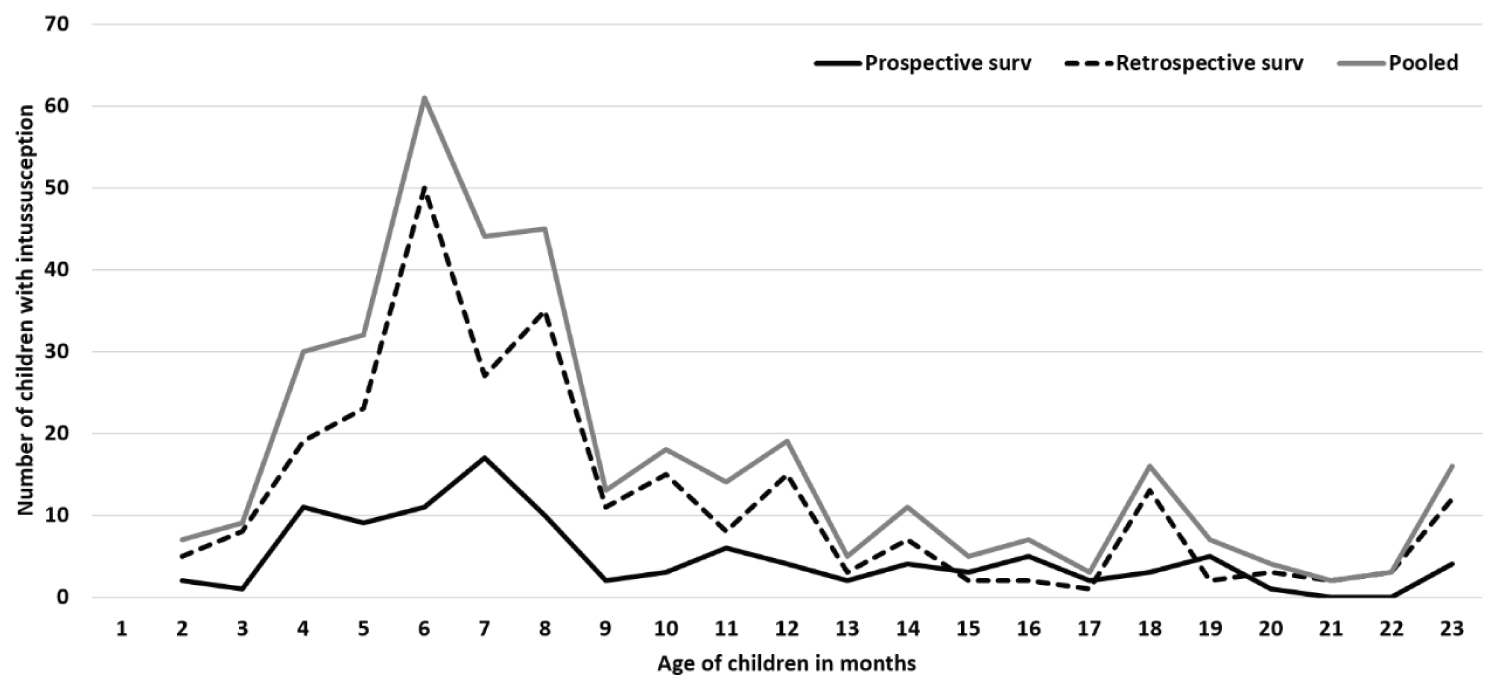
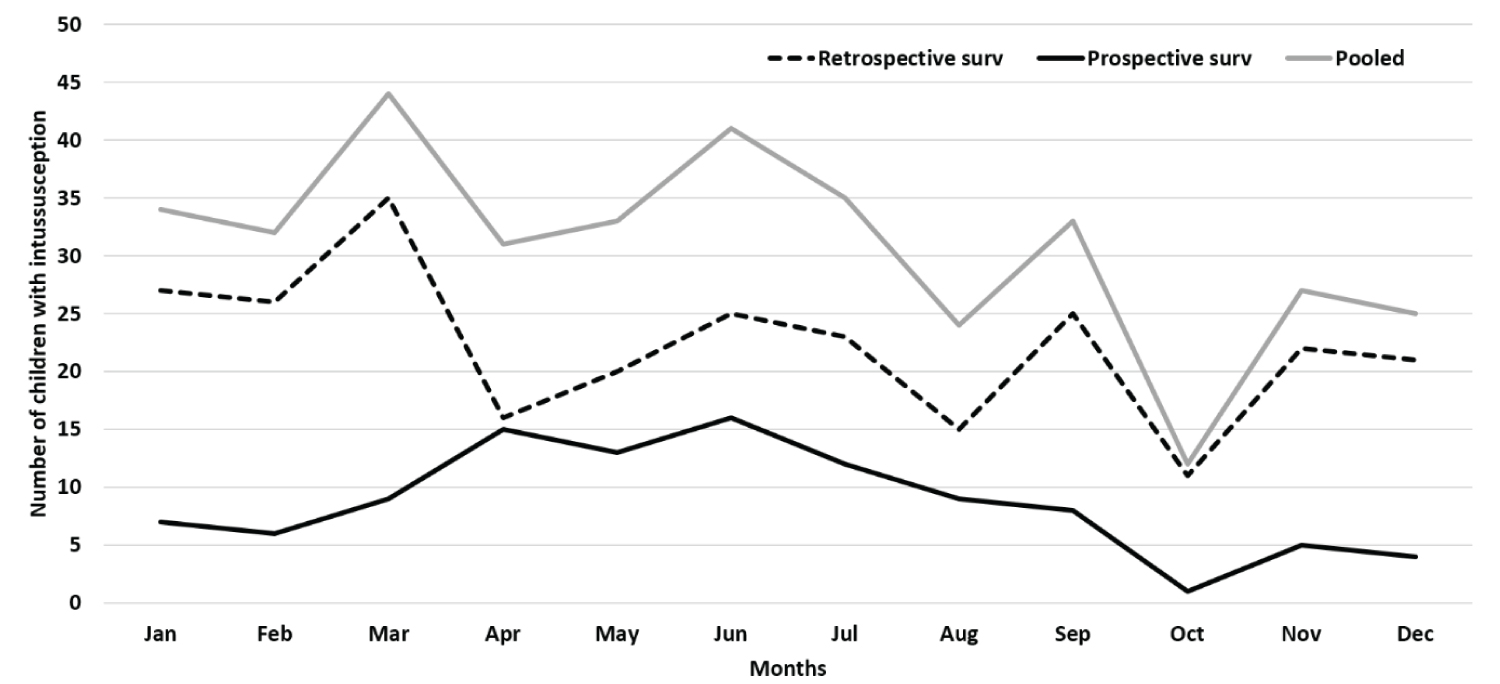
Table 1 shows the sociodemographic and clinical characteristics of children with intussusception for the retrospective and prospective surveillance periods. Majority (78.7%) of the children were infants with median age of 8 months (IQR, 6-12 months). The children aged 4-8 months contributed to 57.1% of pooled cases (57.9% in retrospective and 55.2% in prospective surveillance) (Figure 2). Males contributed more than females (male-female ratio = 2.4:1). More (53%) cases occurred during April to July months (Figure 3). While 22.4% of the patients came from the same district where hospital was based, 77.6% came from other districts of the state (Appendix B).
The sociodemographic and dietary practices were documented for children in the prospective surveillance. Overall 82.8% (87/105) children were ever breastfed. Among the children aged ≥ 6 months, 61% (42/69) were exclusive breastfed for minimum 6 months and median period of breastfeeding was 5 months (IQR 4-6 months). Mixed feeding was initiated in 41.2% infants before six months of age. Weaning food was given to 76.8% (53/69) children aged > 6 months and 19.3% (6/31) children aged < 6 months. The median age of weaning was 5 months (IQR 3-9 months). Rice was the major weaning food (74.6%). The intervals between onset of weaning and intussusception were similar for the different types of weaning food (Appendix B).
Abdominal pain (including excessive crying) was the most common symptom (retrospective: 54.1% and prospective: 78.1%) followed by vomiting (retrospective: 49.6% and prospective: 70.3%) and blood in stool (retrospective: 47.0% and prospective: 69.5%). The intussusception symptom triad (abdominal pain, vomiting and blood in stool) was observed in 44.7% and 69.5% of the children in retrospective and prospective surveillance, respectively. Abnormal/absent bowel sounds were documented in 83.1% (49/59) and 81.7% (67/82) of the children in retrospective and prospective surveillance, respectively. Blood on per-rectal examination was found in 32.2% prospective cases. Overall 97.3% of the cases (retrospective: 98.1% and prospective: 95.2%) were diagnosed by ultrasound and 93.8% were at ileocolic region (retrospective: 94.7% and prospective: 91.5%) was the most common site. A PLP was documented in 3.5% cases. Overall, 57.4% children were treated by surgery, 16.0% by reduction and 24.8% were managed conservatively. The proportion of children managed by reduction increased in parallel with decline in share of surgery. At two hospitals, reduction was not practiced and all cases were surgically managed. At the hospital with both facilities (reduction and surgery), one child (1/26, 3.8%) required surgery for failed reduction. Seven (2.6%) children in the retrospective and two (1.4%) in the prospective periods died. Out of the nine (2.4%) children died, four underwent surgery and no definite intervention could not be done for five children due to unstable clinical status. Eight of these deceased children were referred from other districts. The causes of death were sepsis and shock.
The pooled median illness onset-hospitalisation interval was 2 days (IQR 1, 3) and were similar for both the prospective and retrospective surveillance. In the retrospective component, the median onset-intervention intervals were 1 (IQR 1, 3), 2 (IQR 1, 3) and 3 (IQR 1, 3) days for the children managed by conservative, reduction and surgical mode, respectively. In the prospective component, the median onset-intervention intervals were similar (median 2 days; IQR 1-3 days), irrespective of the mode of management. The children underwent surgery (6-7 days) required longer hospitalisation compared to those treated by reduction or conservatively (2-3 days). History of diarrhoea and respiratory illnesses within four weeks prior to intussusception was reported in 1.9% and 3.8% children, respectively. Using BCCD criteria 70.6% of retrospective and 73.6% of prospective cases were classified as Level-1.
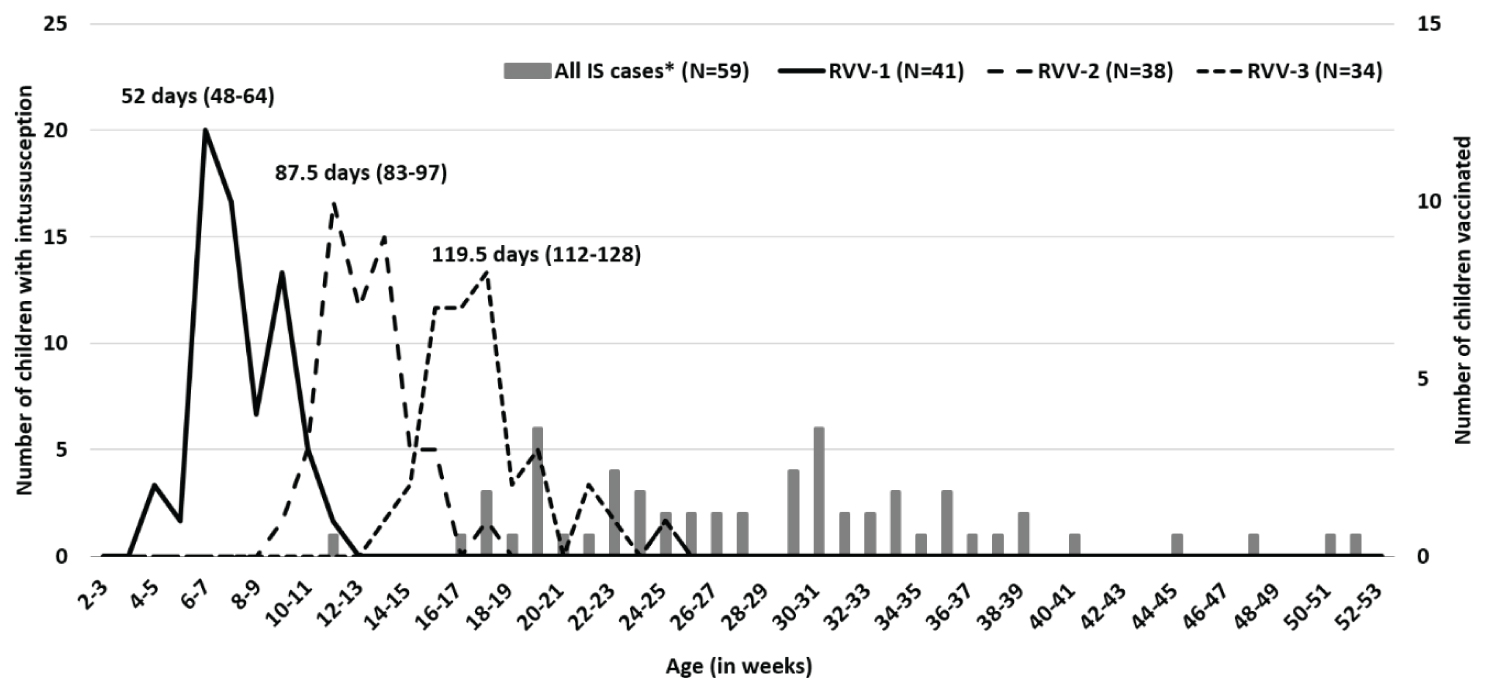
Immunization information was available for 75.2% (79/105) of the children. Among the children with immunization information available, and 51.9% (41/79), 48.1% (38/79) and 43.0% (34/79) received first, second and third RVV doses, respectively. All children received the Rotavac™ vaccine. The median ages of RVV doses administration were 52 (IQR 48, 64 days), 87.5 (IQR 83, 97 days) and 119.5 (IQR 112, 128 days) for first, second and third doses respectively (Figure 4). Five children received RVV (RVV-2, n = 1 and RVV-3, n = 4) within 8-21 days of last RVV dose and none received within the 1-7 days window (Appendix C).
The pooled incidence of intussusception in Odisha was estimated to be 5 cases (range 3.9-7.9) and 1.4 cases (range 1.1-2.8) per 100000 children per year during first and second year (Appendix A). The incidence for recent years (2015-2016) were 7.6-7.9cases per 100000 infant years.
Discussion
This study documented the intussusception case load in children under-two years in Odisha and the trend during 2010-2017. Predominance of intussusception among males (70.6%) and infants (78.7%) with median age (8 months, IQR 6-12 months) and high case load during 4-8 months of age were similar to the reports from India and globally [1,4,18-20,25-30]. Children from all social classes and religion were affected. The breastfeeding pattern, 61% children were exclusive breastfed was comparable to State average (2015-16) [31]. Abdominal pain, vomiting and blood in stool were the top three symptoms, which were similar to the other reports from India and globally [1,4,18-20,25-30]. The classical triad was observed in 51.8 (44.9%-69.5%), higher than reports from some reports from India (19%-34.8%) [4,20,26], but similar (50%) to another report from Odisha (same state) [32]. Most (97.3%) cases were diagnosed by ultrasound. Intussusception mostly occurred at ileocolic site, similar (68-85.3%) to reports from India and globally [1,4,18-20,25-30].
In 3.5% cases any PLPs was reported, was lower than other reports from India (14.6%-41%) [4,20,32,33] and comparable to studies from North India (2.1%-3.7%) [27,28]. The proportion of cases meeting Level-1 (71.4%) were lower than other reports from India (89.1%-96.7%) [4,18,20]. Non-documentation of intussusception reduction as routinely prevented the cases meeting BCCD Level- 1 certainty. Transient intussusception has been observed in children that resolves spontaneously, without any intervention [18,34]. Such transient intussusception cases wouldn't meet Level-1 criteria.
Over half (57.4%) of the cases were managed surgically and 15.2% of them required bowel resection, which were comparable to other reports from India [4,20,28]. Two of the three hospitals practiced only surgical intervention, like some of the other reports from India. At one hospital where hydrostatic reduction was practiced, 74.3% cases were managed by reduction and 10% underwent surgery. The death rate (2.4%) was higher than other parts of India (0%-1.7%) [4,18,20,25-29]. Most of the children died were from far-off places with 24-48 hours interval from onset and five of them were clinical unstable for any intervention. These highlight the importance of early identification, faster referral and pre-referral management of the infants for preventing deaths. There are differences in the proportions of children with clinical symptoms and signs between retrospective and prospective periods, which reflects the quality of case record documentation. The proportion of children with triad, surgical intervention and mortality reflected the case detection, referral, time taken to reach the facilities and response status.
No intussusception case occurred within 1-7 days window after any dose and five cases occurred within 8-21 days window, four after the third dose of RVV. The RVV doses are administered at 6, 10 and 14 weeks of age. The overlap in the ages at intussusception following RVV with natural occurrence may make it difficult to assign causality. Although no definite increased risk of intussusception among Indian children following RVV has been observed from the preliminary findings, longer period of surveillance may be needed to confirm [35,36]. This highlights the importance of documenting vaccine exposure for intussusception patients in routine clinical practice.
A progressive rise in the intussusception cases was observed at all sites, especially 2014 onwards. Although the exact cause of rise remains unknown, the possibilities include increased awareness among the clinicians, availability of diagnostic facilities at peripheral levels, better referral, and availability of management facilities, apart from possible rise in the case load. A rise in the number of cases across years have been also documented across other parts of India [20,28] and other countries [37,38]. More cases were observed during April-July months (summer to early monsoon), which was similar to the other reports from India [4,20,26,28]. But no seasonal variation was observed in South India [18,25].
The intussusception incidence for the years 2015 and 2016 were 7.9 and 7.6 per 100,000 infants, which were lower than those reported from North India (17.7-20) [17,28] and South India (62-254) per 100,000 infants [18,39]. The observed incidence was comparable to the reports from some countries (Bangladesh- 9, Brazil- 3.8, and Italy-7.3 per 100,000 infants) [40-42].
Documentation of changes in intussusception rate with RVV introduction is recommended as part of the vaccine safety surveillance effort [16,43]. Considering the geographic, dietary practices and other possible risk factors for intussusception, there is need for documentation of the intussusception epidemiology from different parts of India. Additionally, there is also need for documenting the clinical and risk factors epidemiology and management for improving case detection, referral and enabling non-surgical management to minimise morbidity and mortality. Despite the risk of intussusception and other possible adverse events, role of RVV and other routine vaccines for preventing child deaths are critical and must be reaffirmed.
Inclusion of multiple hospitals from regions of the state, adoption of both retrospective and prospective surveillance, use of common protocol and standard case definition, reliable documentation and quality assurance measures are the strengths of our study. The site institution level investigators (paediatrician, paediatric surgeon and radiologist) involvement, dedicated research staffs and review of all source documents minimised retrieval, classification and data abstraction errors. A definite source document was used to record immunization exposure. There are several limitations in the study. This was a hospital-based surveillance. In absence of the definite catchment area and referral pattern, the estimated incidence of intussusception might be an underestimation, as some cases might have been treated at other private facilities in the state or outside the state. The aetiology and risk factors for intussusception were not explored.
Conclusion
This study documented that the intussusception occurred mostly in infants and males. The high case load age overlapped with the third dose routine vaccination. Transient intussusception occurred in children frequently. For minimising surgical intervention, morbidity and mortality, high suspicion, quick diagnosis, faster referral and availability of non-surgical methods of management are needed. Immunization exposures should be routinely documented to identify vaccine associated risk and reporting. The estimated intussusception incidence was lower that other parts of India, but future community-cum-hospital based studies are needed for reliable estimates including documentation of aetiology and risk factors.
Declarations
Ethics approval and consent to participate
The study protocol was reviewed and approved by all participating study site institute ethics committees: INCLEN Ethics Committee, New Delhi, India; Institutional Ethics Committee, SCB Medical College, Cuttack, Odisha, India; Institutional Ethics Committee, IMS & SUM Medical College & Hospital, Bhubaneswar, Odisha, India; and Institutional Ethics Committee, MKCG Medical College, Berhampur, Odisha, India. The information about the children were collected after obtaining written informed consent.
Consent for publication
Not applicable.
Availability of data and materials
All data are available with the investigators and can be provided by the corresponding author upon reasonable request.
Competing interests and conflict of interest
The authors declare that there is no competing interests and conflict of interest.
Disclosure statement
None. There is no financial interest or benefit for the authors arisen from this project or its direct application.
Funding
Part of this project (two study sites) was supported by the Bill and Melinda Gates Foundation, USA to The INCLEN Trust International (grant number OPP1116433). The funder or its representative had no role in the design of the study and collection, analysis, and interpretation of data and writing the manuscript.
Authors' contributions
MKD and NKA conceptualised the framework for the study protocol, training, data analysis and interpretation. All site investigators (PKJ, RR, NB, SKS, AKD, BBT, and SSGM) supervised the data collection at respective study site institutes. MKD and BG coordinated the data collection and collation. MKD and BG analysed the data. MKD wrote the first draft of the manuscript. All authors reviewed, provided critical input and approved the final version.
Disclaimer
The content represents the views of the authors alone and do not necessarily represent the official positions of their organizations.
Acknowledgments
We acknowledge the support from Ministry of Health and Health Welfare, Government of India for undertaking the study. We are thankful to the institute and hospital administration and the clinicians who supported and facilitate undertaking the study.
We acknowledge the contribution of the research staffs at The INCLEN Trust International: Apoorva Sharan, Harshpreet Kaur, Janvi Chaubey, Mrimmaya Das, Shweta Sharma and Vaibhav Jain.
We highly appreciate the efforts made by the research staffs at the study sites: Goutam Benia, IMS & SUM Medical College & Hospital, Bhubaneshwar, Odisha, India; Prasntajyoti Mohanty, SVP Post Graduate Institute of Paediatrics and SCB Medical College, Cuttack, Odisha, India; and Asit Pradhan, MKCG Medical College, Berhampur, Odisha, India.
References
- Bines JE, Kohl KS, Forster J, et al. (2004) Acute intussusception in infants and children as an adverse event following immunization: Case definition and guidelines of data collection, analysis, and presentation. Vaccine 22: 569-574.
- Bines JE, Ivanoff B (2020) Acute intussusception in infants and children: Incidence, clinical presentation and management: A global perspective. World Health Organisation.
- Perrin WS, Lindsay EC (1921) Intussusception: A monograph based on 400 cases. Br J Surg 9: 46-71.
- The INCLEN Intussusception Surveillance Network Study Group (2020) Prospective surveillance for intussusception in Indian children aged under two years at nineteen tertiary care hospitals. BMC Pediatrics 20: 413.
- Guarner J, de Leon-Bojorge B, Lopez-Corella E, et al. (2003) Intestinal intussusception associated with adenovirus infection in Mexican children. Am J Clin Pathol 120: 845-850.
- Bines JE, Liem NT, Justice FA, et al. (2006) Risk factors for intussusception in infants in Vietnam and Australia: Adenovirus implicated, but not rotavirus. J Pediatr 149: 452-460.
- Nylund CM, Denson LA, Noel JM (2010) Bacterial enteritis as a risk factor for childhood intussusception: A retrospective cohort study. J Pediatr 156: 761-765.
- Murphy TV, Gargiullo PM, Massoudi MS, et al. (2001) Intussusception among infants given an oral rotavirus vaccine. N Engl J Med 344: 564-572.
- Patel MM, Haber P, Baggs J, et al. (2009) Intussusception and rotavirus vaccination: A review of the available evidence. Expert Rev Vaccines 8: 1555-1564.
- World Health Organization (2007) Rotavirus vaccines. Wkly Epidemiol Rec 82: 285-295.
- Vesikari T, Matson DO, Dennehy P, et al. (2006) Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 354: 23-33.
- Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, et al. (2006) Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 354: 11-22.
- Yen C, Tate JE, Steiner CA, et al. (2012) Trends in intussusception hospitalizations among US infants before and after implementation of the rotavirus vaccination program, 2000-2009. J Infect Dis 206: 41-48.
- Patel MM, López-Collada VR, Bulhões MM, et al. (2011) Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med 364: 2283-2292.
- Buttery JP, Danchin MH, Lee KJ, et al. (2011) Intussusception following rotavirus vaccine administration: Post-marketing surveillance in the National Immunization Program in Australia. Vaccine 29: 3061-3066.
- WHO (2009) Post-marketing surveillance of rotavirus vaccine safety. World Health Organization, Geneva.
- Bahl R, Saxena M, Bhandari N, et al. (2009) Population‐based incidence of intussusception and a case‐control study to examine the association of intussusception with natural rotavirus infection among Indian children. J Infect Dis, S277-S281.
- Jehangir S, John J, Rajkumar S, et al. (2014) Intussusception in southern India: Comparison of retrospective analysis and active surveillance. Vaccine 32: 99-103.
- Jiang J, Jiang B, Parashar U, et al. (2013) Childhood intussusception: A literature review. PLoS One 8: e68482.
- Das MK, Arora NK, Gupta B, et al. (2020) Intussusception in children aged under two years in India: Retrospective surveillance at nineteen tertiary care hospitals. Vaccine 38: 6849-6857.
- (2013) Rotavirus vaccines WHO position paper: January 2013-recommendations. Vaccine 31: 6170-6171.
- Tate JE, Steele AD, Bines JE, et al. (2012) Research priorities regarding rotavirus vaccine and intussusception: A meeting summary. Vaccine 30: 179-184.
- International Institute for Population Sciences (2017) National family health survey, India.
- Office of the Registrar General & Census Commissioner, India (2020) Sample registration system.
- Bhowmick K, Kang G, Bose A, et al. (2009) Retrospective surveillance for intussusception in children aged less than five years in a South Indian tertiary-care hospital. J Health Popul Nutr 27: 660-665.
- Singh JV, Kamath V, Shetty R, et al. (2014) Retrospective surveillance for intussusception in children aged less than five years at two tertiary care centers in India. Vaccine 32: 95-98.
- Fahiem-Ul-Hassan M, Mufti G, Bhat N, et al. (2020) Management of intussusception in the era of ultrasound-guided hydrostatic reduction: A 3-year experience from a tertiary care center. J Indian Assoc Pediatr Surg 25: 71-75.
- Gupta M, Kanojia R, Singha R, et al. (2018) Intussusception rate among under-five-children before introduction of rotavirus vaccine in North India. J Trop Pediatr 64: 326-335.
- Srinivasan R, Girish Kumar CP, Naaraayan SA, et al. (2018) Intussusception hospitalizations before rotavirus vaccine introduction: Retrospective data from two referral hospitals in Tamil Nadu, India. Vaccine 36: 7820-7825.
- Patel MM, Clark AD, Sanderson CFB, et al. (2012) Removing the age restrictions for rotavirus vaccination: A benefit-risk modeling analysis. PLoS Med 9: e1001330.
- International Institute for Population Sciences (2017) State fact sheet- Odisha, National family health survey-4 (2015-16).
- Nayak MK, Kumar M, Nayak RK, et al. (2019) Prevalence of intussusception after rotavirus vaccination: A hospital based study from Odisha, India. J Clin Diagn Res 13: 9-12.
- Raman T, Mukhopadhyaya A, Eapen CE, et al. (2003) Intussusception in southern Indian children: Lack of association with diarrheal disease and oral polio vaccine immunization. Indian J Gastroenterol 22: 82-84.
- Strouse PJ, DiPietro MA, Saez F (2003) Transient small-bowel intussusception in children on CT. Pediatr Radiol 33: 316-320.
- Bhandari N, Antony K, Balraj V, et al. (2020) Assessment of risk of intussusception after pilot rollout of rotavirus vaccine in the Indian public health system. Vaccine 38: 5241-5248.
- Das MK, Das MK, Arora NK, et al. (2020) Risk of intussusception after monovalent rotavirus vaccine (Rotavac) in Indian infants: A self-controlled case series analysis. Vaccine.
- Restivo V, Costantino C, Tramuto F, et al. (2017) Hospitalization rates for intussusception in children aged 0-59 months from 2009 to 2014 in Italy. Hum Vaccin Immunother 13: 445-449.
- Noel G, Minodier P, Merrot T (2014) Intussusception risk after rotavirus vaccination in U.S. Infants. N Engl J Med 370: 1766-1766.
- Mathew MA, Venugopal S, Arora R, et al. (2016) Leveraging the national rotavirus surveillance network for monitoring intussusception. Indian Pediatrics 53: 635-638.
- Zaman K, Breiman RF, Yunus Md, et al. (2009) Intussusception surveillance in a rural demographic surveillance area in Bangladesh. J Infect Dis 200: S271-S276.
- Sáez-Llorens X, Velázquez FR, Lopez P, et al. (2013) A multi-country study of intussusception in children under 2 years of age in Latin America: Analysis of prospective surveillance data. BMC Gastroenterol 13: 95.
- Costantino C, Restivo V, Cuccia M, et al. (2015) Analysis of hospitalizations due to intussusception in Sicily in the pre-rotavirus vaccination era (2003-2012). Ital J Pediatr 41: 52.
- Mwenda JM, Tate JE, Steele AD, et al. (2014) Preparing for the scale-up of rotavirus vaccine introduction in Africa: Establishing surveillance platforms to monitor disease burden and vaccine impact. Pediatr Infect Dis J 33: S1-S5.
Corresponding Author
Manoja Kumar Das, Director Projects, The INCLEN Trust International, F1/5, Okhla Industrial Area, Phase 1, New Delhi -110020, India, Tel: Off: +91-11-47730000; Mob: +91-9810203768; Fax: +91-11-47730001, E-mail: manoj@inclentrust.org
Copyright
© 2020 Jena PK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.