Atopic Dermatitis in the Adult Population
Abstract
Atopic Dermatitis (AD) is a chronic inflammatory disease of the skin characterized by cutaneous findings of dry skin, erythematous scaly papules, or plaques, and lichenification. The hallmark of AD is pruritis, which drives most of the disease burden. While this disease is thought to primarily affect children, it commonly affects adults as well [1]. In the adult population, AD may be persistent from childhood, relapsing AD that was thought to be resolved, or adult-onset AD. Although AD is commonly seen in the adult population and is associated with a significant disease burden, many providers are inadequately educated about the recognition and treatment of AD in adults. This paper will review the clinical features, pathophysiology, diagnosis, treatment, and management of AD in the adult population. Working to better understand AD and its impact on patients may be beneficial in minimizing the burden of this disease on the adult population.
Introduction
Atopic Dermatitis (AD) is a chronic inflammatory disease of the skin characterized by cutaneous findings of dry skin, erythematous scaly papules or plaques, and lichenification. Pruritis is the hallmark of AD and drives most of the disease burden on patients. While this disease most commonly affects children, it can also present in or persist into adulthood [1]. The disease in adults often deviates from the pattern of flexural dermatitis that is often seen in children, with some forms of AD being specific to adults. Bannister and Freeman introduced the term adult-onset atopic dermatitis in 2000 [2]. However, this concept has received little attention in the literature compared with AD in children despite the significant burden of severe AD on adult patients [3]. Better understanding AD in adults will allow providers to minimize potentially significant disease burden for their patients.
Epidemiology
The prevalence of AD in the United States pediatric population is reported to be 16%. However, data is limited about the prevalence of AD in the United States adult population. Prevalence rates among adults have been reported to be 10-14% in studies from Scandinavian countries [4-6]. A cross-sectional study in the United States reported a prevalence of 7.3% in a population of nearly 1300 adults [7].
Risk factors
There are several genetic and environmental risk factors associated with the development of AD. The strongest risk factor for AD is a family history of atopic diseases such as AD, asthma, or allergic rhinitis. It is thought that 70% of AD patients have a family history of atopy [8]. There are also several genes associated with an increased risk for AD, many of which code for proteins involved in the skin barrier or immune regulation [9]. One major genetic alteration associated with an increased risk for AD is a loss-of-function mutation in the FLG gene. This gene codes for the filaggrin protein, which is a component of natural moisturizing factors and is key for the barrier function of the skin.
Environmental risk factors include living in an urban area, stress, increased domestic water hardness, exposure to irritants (e.g., tobacco smoke), increased adiposity, and a Western diet [10]. It is also believed that increased exposure to pathogens early in life can be protective against AD, termed the "hygiene hypothesis." This hypothesis theorizes that exposure to dirt and pathogens early in life can steer the immune system away from allergic inflammation [11]. This hypothesis may explain regional differences in the incidence of AD, as well as the increasing prevalence of AD over the past half-century [10].
Pathogenesis
The pathogenesis of AD is thought to involve both skin barrier dysfunction and an inflammatory response to environmental allergens and irritants. Contributing factors include genetics, immune dysregulation, the skin microbiome, and environmental triggers. Combinations of these mechanisms are responsible for the variable phenotypes of AD [12]. Several of the genes associated with increased risk for AD code for proteins that are either involved in immune regulation or the structure and function of the skin barrier, including the FLG gene discussed above.
Skin barrier dysfunction plays a major role in the pathogenesis of AD. Dysfunction of the skin barrier leads to increased epidermal permeability, resulting in trans-epidermal water loss and decreased water retention [13]. Factors contributing to skin barrier dysfunction include abnormalities related to filaggrin levels, tight junctions, proteases and antiproteases, cytokine release, and epidermal lipid composition. These abnormalities primarily affect the stratum corneum, the outermost layer of the epidermis. Skin barrier dysfunction ultimately plays a major role in the development of the dry, scaly, and pruritic skin that is commonly seen in AD. Itching of the pruritic skin can further potentiate the damage to the epidermis, leading to a self-propagating cycle.
The role of the immune system in chronic and acute AD is primarily due to Th2 inflammation. When a pathogen or marker of tissue damage is recognized by pattern recognition receptors of the innate immune system, antigen- presenting cells and keratinocytes release several products such as cytokines, proteases, antimicrobial peptides, and extracellular matrix proteins. These "alarmins" are proteins or peptides with chemotactic and activating effects on the immune system [14].
When the epithelial barrier is disrupted in the setting of AD, alarmin secretion activates Th2 cells and ultimately leads to the release of IL-4 and IL-13. These interleukins initiate an inflammatory response and B cell class-switching to IgE, resulting in the production of antigen-specific IgE molecules [15]. These IgE molecules will prime the immune system to have a more robust immune response following subsequent recognition of that same antigen. Cytokine release from Th2 cells is also believed to play a role in the promotion of epidermal hyperplasia, suppression of keratinocyte differentiation proteins, and inhibition of antimicrobial protein production. Further, Th2 cells and their interactions with peripheral nerve fibres and keratinocytes play a role in the development of chronic pruritis, which is a defining feature of AD [16].
Alterations of the dermal microbiome have also been shown to play a role in the pathogenesis of AD. More specifically, Staphylococcus aureus overgrowth and reduced bacterial diversity are associated with the development of AD [17]. Further, it has been shown that during flares, AD patients have decreased bacterial diversity and increased S. aureus density [18]. Many bacterial virulence factors, as well as bacterial superantigens, can also play a role in the pathogenesis of AD by resulting in an IgE-mediated immune response and mast cell degranulation.
Adult onset AD vs. persistence from childhood
This condition may present in adults as persistent AD from childhood, relapsing AD that was thought to be resolved, or adult-onset AD [3]. A vast majority of patients with childhood AD will be improved or even clear by late childhood, but some patients will have AD that persists into adulthood. While the clinical appearance of AD in the adult population is similar to the appearance in later childhood, some variation does exist. For example, AD in the adult population is generally more localized and lichenified. Also, involvement of the forehead, cheeks, and anterolateral neck is common in adults.
Among the population of adult patients affected by AD, about one-third are patients in which the disease first appeared during adulthood. In the adult-onset form of AD, the disease often deviates from the classic pattern of flexural dermatitis. Some forms of AD are specific to adults, such as head-and-neck dermatitis, chronic eczema of the hands, or prurigo lesions. Because AD is made by clinical diagnosis and adult-onset AD frequently does not fit traditional diagnostic criteria that were developed for children, the diagnosis of AD in the adult population can be difficult. As a result, particularly in adult-onset AD, this condition is often a diagnosis of exclusion [3].
Disease course
The disease course of AD has a chronic, relapsing and remitting pattern. For most infants and children who are diagnosed with this condition, AD resolves by late childhood. A pooled analysis including 110,651 subjects from 15 countries found that 20% of childhood AD cases persisted for 8 or more years, with only 5% of childhood AD cases persisting for 20 or more years after diagnosis. Factors associated with an increased risk of persistence into adulthood include the development of AD after the age of 2 years, having more severe baseline AD, and being female. In addition, the more time that the AD had already persisted, the more likely it was to continue to persist [19].
Disease burden
AD can impact the entire family unit of patients who are affected. Because AD has increasingly been recognized as a disease that may onset or persist in adulthood, the burden of this disease can have an impact on patients at any age. The detrimental effects of AD include social, academic, and occupational impacts. Further, quality of life and mental health are impacted by AD. The direct and indirect financial burden of AD impacts the patients, their families, and society [20]. Higher social and economic burden is associated with greater disease severity, lesions that are located on the hands and the face, and persistent AD. While adult AD is commonly mild to moderate, the condition is severe in about 25% of patients.
Even though AD is commonly seen in the adult population and is associated with significant disease burden, many providers are undereducated about the recognition and treatment of AD in adults. Better characterizing the diagnostic and treatment guidelines of AD in this population, as well as working to better educate providers about this disease and its impact on patients may be beneficial in minimizing the burden of this disease on the adult population.
Diagnosis
The diagnosis of AD is made clinically and is based on historical features, morphology, associated clinical signs, and the distribution of lesions (Table 1). There are no definitive laboratory tests for the diagnosis of AD [21]. In 2003, the American Academy of Dermatology suggested a revision to the Hanifin and Rajka criteria to be more applicable to the full range of ages affected [22].
If the patient is not responding to therapy, the diagnosis of AD should be re-reviewed, and other conditions should be considered. The diagnosis of AD is made clinically, and currently, there are no biomarkers available to distinguish it from other conditions. Skin biopsy is not routinely used or recommended in the evaluation of patients with suspected AD. Skin biopsies may be utilized, however, to rule out other diagnoses in the differential [22] (Table 2).
Clinical (by location) variants
Atopic hand eczema is inflammation of the skin of the hands. Clinical findings include redness, infiltration of the skin, scaling, edema, vesicles, hyperkeratosis, fissures, and erosions. The prevalence of atopic hand eczema is 4% in the general population of adults, with incidence varying based on occupation. Occupations that are associated with a higher risk of atopic hand eczema include farming, healthcare, metalworking, and construction [23]. Roughly one-third to one-half of patients with hand eczema have AD [24]. Contact dermatitis should be ruled out before the diagnosis of AD is made, as irritants are a common factor in the pathogenesis of these conditions.
Patients with AD can present with red, scaly, lichenified eyelids as they are subjected to increased susceptibility to most contact irritants or allergens [25]. Such presentations are classified as eyelid eczema. Patients presenting exclusively with periorbital eczema should have a complete history taken for AD.
Atopic cheilitis, or lip dermatitis, is a chronic eczematous reaction affecting the skin, vermilion border, and/or the mucosa of the lips. Because other inflammatory or allergic responses typically involve the mucosal surfaces of the lips, involvement of the skin and vermilion border alone should raise suspicion for atopic cheilitis [26].
Treatment
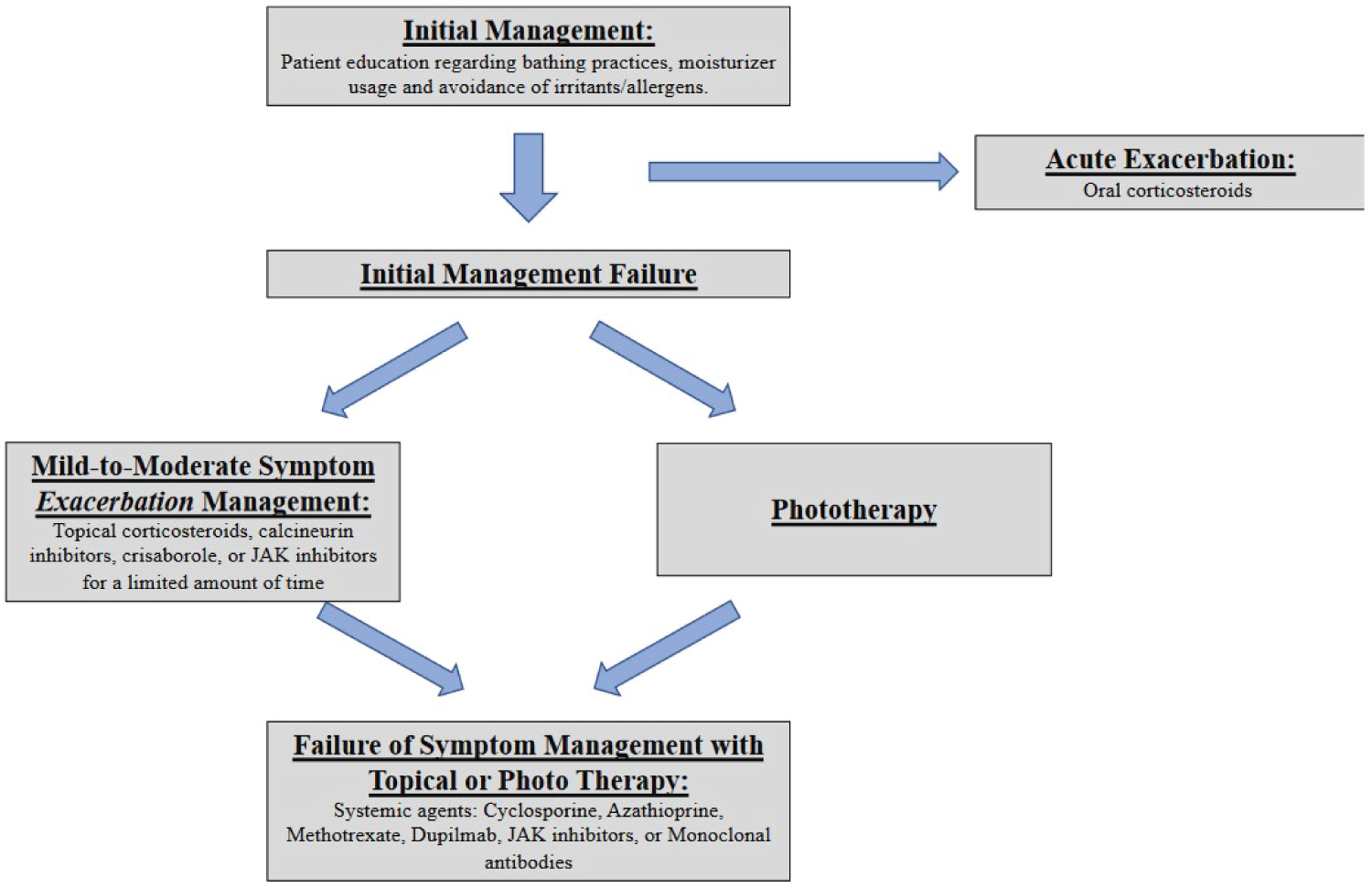
Treatment algorithm
Figure 1.
Initial management
Topical moisturizers have been proven to decrease the pruritis, erythema, fissuring, and lichenification associated with AD [34]. In 2020, Hebert, et al. demonstrated that daily moisturization increases skin hydration while decreasing transepidermal water loss through their analysis of multiple studies. Though the majority of the studies analyzed recommended twice-daily application of moisturizers, just once-daily application showed to improve patient outcomes as well [35]. Key ingredients for moisturizers for AD therapeutic relief include the following properties: Skin protection (colloidal oatmeal), antipruritic effects (hydrocortisone, menthol, pramoxine HCl, colloidal oatmeal), anti- inflammatory effects (hydrocortisone, licochalcone A, colloidal oatmeal), antioxidant effects (glycyrrhetinic acid, licochalcone A), barrier lipids (ceramides, plant oils containing linoleic acid), natural moisturizer factors (lactic acid, amino acids, PCA, urea), and pH buffering (citric acid) [35,36].
Patients with AD should be advised to bathe with water regularly as this can hydrate the skin and remove crust, irritants, scale, and allergens [37]. Bathing alone, without subsequent moisturizer application, can cause further drying of the patient’s skin. Thus, it is recommended that patients apply a moisturizer after bathing to maximize the hydration of their skin [38].
Local/topical therapy
Topical treatments are a cornerstone in the treatment of AD and are commonly used alongside other agents [34].
Topical corticosteroids are recommended as first-line therapy for acute control of moderate to severe AD [34]. They are used in patients who fail to respond to skincare techniques and moisturizers. While there are multiple strengths of topical corticosteroids available, current studies have not established an optimum strength and dosage recommendation. Typically, patients are advised to use a finger-tip amount on each surface area affected by the rule of 9’s. Therapy should be directed at acute disease flairs as these topical solutions are most effectively used in brief periods of 1 to 2 weeks. Side effects include skin striae, petechiae, telangiectasia, atrophy, and acne or rosacea at the site of application [39].
Topical calcineurin inhibitors such as tacrolimus ointment and pimecrolimus cream provide targeted anti- inflammatory effects by blocking cytokine expression [40]. Side effects include pruritus, stinging, and burning at the application site.
Crisaborole is a phosphodiesterase 4 (PDE4) inhibitor that reduces the circulating inflammatory cells in patients with AD. This topical treatment has shown to be efficacious with a positive safety profile in treating adults with AD [41]. Notable side effects include burning at the application site and redness of the skin.
Topical JAK inhibitors ruxolitinib, delgocitinib, and tofacitinib exert their effects through the inhibition of JAK signalling, thus reducing cytokine release. These topical JAK inhibitors have demonstrated their efficacy in treating AD [42]. Side effects include nasopharyngitis, pain at application site, headache, nausea, and neutropenia. At the time of this publication, topical ruxolitinib is FDA-approved with others listed under investigation.
Phototherapy
Phototherapy is a treatment option for AD that is recommended after the failure of non-pharmacological and pharmacological options. There are multiple forms of phototherapy available that are all associated with minimal adverse effects. While there is no definitive recommendation of the most effective form of phototherapy, narrowband UVB therapy is the most commonly recommended [43].
Systemic therapy
Systemic treatment for AD is typically recommended when topical regimens or phototherapy do not control the disease or when a patient’s quality of life is impacted substantially [43].
Cyclosporine inhibits T-cell activation, keratinocyte hyperproliferation, release of histamine from mast cells, and depletes lymphocytes and macrophages in the dermis and epidermis [44]. These mechanisms allow cyclosporine to be effective in the treatment of refractory AD. Short-term cyclosporine therapy effectively decreases AD severity, with higher initial doses leading to a more rapid response [45,46]. While long-term therapy is also effective in the treatment of AD, the patient’s renal function should be monitored during treatment [47,48].
Methotrexate is a folic acid analog that inhibits several folate-dependent enzymes in the DNA synthesis process. This medication leads to anti-inflammatory effects via inhibition of lymphocyte proliferation, though the exact mechanism in dermatologic disorders has not been elucidated [49]. The anti-inflammatory effects of methotrexate make it an effective treatment for refractory AD. In patients treated with this medication, it is important to follow folate levels and to monitor for complications of pulmonary or hepatic fibrosis [50].
Azathioprine is a pro-drug that is converted to 6-mercaptopurine and exerts its effects via purine anti-metabolism, thus decreasing T and B lymphocyte proliferation [51]. Studies have shown that azathioprine is a safe and effective treatment of refractory AD [52,53].
Dupilumab is an IL-4 receptor alpha antagonist that inhibits IL-4 and IL-13 signalling leading to reductions in Th2 response in AD. This treatment is administered subcutaneously, either weekly or biweekly. So far in its clinical use, dupilumab has demonstrated efficacy in moderate-severe AD and is generally well tolerated [54-56]. Common side effects include skin-related events (head and neck erythema, psoriasis, and alopecia) and mild to moderate ocular adverse effects (conjunctivitis, blepharitis, and keratitis) [57,58].
Mycophenolate mofetil and Interferon-gamma may be recommended in certain situations but are much less commonly used as systemic therapies for AD.
Emerging Therapies
The pathogenesis of AD is thought to involve an inflammatory response to environmental allergens and irritants. This skin inflammation relies on the interaction between cytokines as well as immune and tissue cells. Many cytokines rely on Janus kinases (JAKs) for signal transduction which subsequently leads to inflammation development. Newly developed oral JAK inhibitors inhibit these kinases and lead to a decreased inflammatory response [59]. These drugs have been proven to be safe and efficacious in the treatment of AD, with topical administration demonstrating more efficacy in comparison to oral treatment [60]. Examples of oral JAK inhibitors include abrocitinib and upadacitinib, which were both FDA approved in January 2022. Tofacitinib is another oral JAK inhibitor that is FDA approved for conditions such as rheumatoid arthritis but is not yet FDA-approved for AD.
In contrast to JAK inhibitors, monoclonal antibodies target specific cytokines to modulate the inflammatory response in AD. There are multiple monoclonal antibodies available for the treatment of AD, with some of the more commonly used medications discussed below:
IL-13 has an assumed role in the development and progression of AD and pharmacological therapies to inhibit its biological activity are in development [61]. Two biological treatments, lebrikizumab and tralokinumab, are being developed to specifically bind IL-13 and inhibit its activity. In a randomized controlled trial (RCT) of patients with AD refractory to topical corticosteroids, lebrikizumab demonstrated clinical efficacy with a tolerable safety Profile [62]. In its own RCT trial, tralokinumab demonstrated early and sustained improvements in AD symptoms with an acceptable safety profile [63]. The future of AD treatment with IL-13 monoclonal antibodies seems promising through the early results of their clinical trials.
IL-31 is a T-cell cytokine that correlates with severe pruritus and dermatitis found in patients with AD [64]. Nemolizumab is a monoclonal antibody that is targeted to the IL-31 receptor A and has demonstrated the ability to alleviate the pruritis associated with AD in patients while exhibiting an acceptable safety profile [65,66].
Summary
Atopic dermatitis is a chronic inflammatory disease of the skin characterized by pruritus, erythema, dry skin, scale, and lichenification. While this disease is thought to primarily affect children, it commonly affects adults as well. While data is limited about the prevalence of AD in the United States adult population, one cross-sectional study suggested a prevalence of 7.3%. There are several genetic and environmental risk factors associated with the development of AD, with a family history of other atopic diseased being the strongest risk factor. The pathogenesis of AD is thought to involve both skin barrier dysfunction and skin inflammation. Contributing factors include genetics, immune dysregulation, the skin microbiome, and environmental triggers.
This condition may present in adults as persistent AD from childhood, relapsing AD that was thought to be resolved, or adult-onset AD [3]. The disease in adults often deviates from the pattern seen in children. AD in the adult population is generally more localized and lichenified. Also, involvement of the forehead, cheeks, and anterolateral neck is common in adults. The diagnosis of AD is made clinically and is often a diagnosis of exclusion. Features that are essential for the diagnosis of atopic dermatitis include pruritis, eczema in a typical morphology and age-specific pattern, and a chronic or relapsing history.
Treatment of AD involves educating patients about the use of moisturizers in conjugation with regular bathing. Should patients not respond to this initial therapy, topical therapies should be utilized as first-line options. Patients can also be educated about the use of phototherapy as a treatment option. In patients with refractory AD, systemic medications such as dupilumab, cyclosporine, azathioprine, methotrexate, and FDA-approved JAK inhibitors (abrocitinib and upadacitinib) are effective and safe options.
Despite the significant prevalence and burden of atopic dermatitis in the adult population, many providers are inadequately educated about the diagnosis and treatment of AD in adults. Working to better educate providers about this disease and its impact on patients may help decrease the burden of this disease on the adult population.
Authors Declarations
The authors declare no financial support or conflicts of interest.
References
- Weidinger S, Novak N (2016) Atopic dermatitis. Lancet 387: 1109-1122.
- Bannister MJ, Freeman S (2000) Adult-onset atopic dermatitis. Australas J Dermatol 41: 225-228.
- Silvestre Salvador JF, Romero Pérez D, Encabo Durán B (2017) Atopic dermatitis in adults: A diagnostic challenge. J Investig Allergol Clin Immunol 27: 78-88.
- Rönmark EP, Ekerljung L, Lötvall J, et al. (2012) Eczema among adults: Prevalence, risk factors and relation to airway diseases. Results from a large-scale population survey in Sweden. Br J Dermatol 166: 1301-1308.
- Vinding GR, Zarchi K, Ibler KS, et al. (2014) Is adult atopic eczema more common than we think? - A population-based study in Danish adults. Acta Derm Venereol 94: 480-480.
- Mortz CG, Andersen KE, Dellgren C, et al. (2015) Atopic dermatitis from adolescence to adulthood in the TOACS cohort: Prevalence, persistence and comorbidities. Allergy 70: 836-845.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. (2019) Atopic dermatitis in America study: A cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol 139: 583-590.
- Irvine AD, McLean WH, Leung DY (2011) Filaggrin mutations associated with skin and allergic diseases. N Engl J Med 365: 1315-1327.
- Paternoster L, Standl M, Waage J, et al. (2015) Multi-ancestry genome-wide association study of 21,000 cases and 95,000 controls identifies new risk loci for atopic dermatitis. Nat Genet 47: 1449-1456.
- Kantor R, Silverberg JI (2017) Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev Clin Immunol 13: 15-26.
- Strachan DP (2000) Family size, infection and atopy: The first decade of the "hygiene hypothesis". Thorax 55: S2-S10.
- Czarnowicki T, He H, Krueger JG, et al. (2019) Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol 143: 1-11.
- Kelleher M, Dunn-Galvin A, Hourihane JO, et al. (2015) Skin barrier dysfunction measured by transepidermal water loss at 2 days and 2 months predates and predicts atopic dermatitis at 1 year. J Allergy Clin Immunol 135: 930-935.
- Weidinger S, Beck LA, Bieber T, et al. (2018) Atopic dermatitis. Nat Rev Dis Primers 4: 1.
- Frazier W, Bhardwaj N (2020) Atopic Dermatitis: Diagnosis and treatment. Am Fam Physician 101: 590-598.
- Agarwal US, Besarwal RK, Gupta R, et al. (2014) Hand eczema. Indian J Dermatol 59: 213-224.
- Albrecht M (2021) Turning off the alarm - Targeting alarmins and other epithelial mediators of allergic inflammation with biologics. Allergol Select 5: 82-88.
- Garcovich S, Maurelli M, Gisondi P, et al. (2021) Pruritus as a distinctive feature of type 2 Inflammation. Vaccines 9: 303.
- Geoghegan JA, Irvine AD, Foster TJ (2018) Staphylococcus aureus and Atopic Dermatitis: A complex and evolving relationship. Trends Microbiol 26: 484-497.
- Kong HH, Oh J, Deming C, et al. (2012) Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res 22: 850-859.
- Kim JP, Chao LX, Simpson EL, et al. (2016) Persistence of atopic dermatitis (AD): A systematic review andmeta-analysis. J Am Acad Dermatol 75: 681-687.
- Drucker AM, Wang AR, Li WQ, et al. (2017) The burden of atopic dermatitis: Summary of a report for the national eczema association. J Invest Dermatol 137: 26-30.
- Eichenfield LF, Tom WL, Chamlin SL, et al. (2014) Guidelines of care for the management of atopic dermatitis: Section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol 70: 338-351.
- Coenraads PJ (2012) Hand eczema. N Engl J Med 367: 1829-1837.
- Beltrani VS (2001) Eyelid dermatitis. Curr Allergy Asthma Rep 1: 380-388.
- Pugliarello S, Cozzi A, Gisondi P, et al. (2011) Phenotypes of atopic dermatitis. J Dtsch Dermatol Ges 9: 12-20.
- Tarbox M, Walker K, Tan M (2018) Scabies. JAMA 320: 612.
- Naldi L, Rebora A (2009) Clinical practice. Seborrheic dermatitis. N Engl J Med 360: 387-396.
- Mowad CM, Anderson B, Scheinman P, et al. (2016) Allergic contact dermatitis: Patient diagnosis and evaluation. J Am Acad Dermatol 74: 1029-1040.
- Vahlquist A, Fischer J, Törmä H (2018) Inherited nonsyndromic ichthyoses: An update on pathophysiology, diagnosis and treatment. Am J Clin Dermatol 19: 51-66.
- Cohen SN, Baron SE, Archer CB (2012) Guidance on the diagnosis and clinical management of psoriasis. Clin Exp Dermatol 1: 13-18.
- Zic JA (2021) Diagnosis and management of cutaneous lymphomas including cutaneous t-cell lymphoma. Med Clin North Am 105: 737-755.
- Pichard DC, Freeman AF, Cowen EW (2015) Primary immunodeficiency update: Part I. Syndromes associated with eczematous dermatitis. J Am Acad Dermatol 73: 355-364.
- Eichenfield LF, Tom WL, Berger TG, et al. (2014) Guidelines of care for the management of atopic dermatitis: Section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol 71: 116-132.
- Hebert AA, Rippke F, Weber TM, et al. (2020) Efficacy of nonprescription moisturizers for atopic dermatitis: An updated review of clinical evidence. Am J Clin Dermatol 21: 641-655.
- Sher LG, Chang J, Patel IB, et al. (2012) Relieving the pruritus of atopic dermatitis: A meta-analysis. Acta Derm Venereol 92: 455-461.
- Gutman AB, Kligman AM, Sciacca J, et al. (2005) Soak and smear: A standard technique revisited. Arch Dermatol 141: 1556-1559.
- Chiang C, Eichenfield LF (2009) Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol 26: 273-278.
- Akers WA (1980) Risks of unoccluded topical steroids in clinical trials. Arch Dermatol 116: 786-788.
- Simpson EL (2010) Atopic dermatitis: A review of topical treatment options. Curr Med Res Opin 26: 633-640.
- Paller AS, Tom WL, Lebwohl MG, et al. (2016) Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol 75: 494-503.
- Pescitelli L, Rosi E, Ricceri F, et al. (2021) Novel therapeutic approaches and targets for the treatment of atopic dermatitis. Curr Pharm Biotechnol 22: 73-84.
- Sidbury R, Davis DM, Cohen DE, et al. (2014) Guidelines of care for the management of atopic dermatitis: Section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol 71: 327-349.
- Amor KT, Ryan C, Menter A (2010) The use of cyclosporine in dermatology: Part I. J Am Acad Dermatol 63: 925-946.
- Bangert CA, Costner MI (2007) Methotrexate in dermatology. Dermatol Ther 20: 216-228.
- Bieber T (2020) Interleukin-13: Targeting an underestimated cytokine in atopic dermatitis. Allergy 75: 54-62.
- Schmitt J, Schmitt N, Meurer M (2007) Cyclosporin in the treatment of patients with atopic eczema - a systematic review and meta-analysis. J Eur Acad Dermatol Venereol 21: 606-619.
- Sowden JM, Berth-Jones J, Ross JS, et al. (1991) Double-blind, controlled, crossover study of cyclosporin in adults with severe refractory atopic dermatitis. Lancet 338: 137-140.
- Granlund H, Erkko P, Remitz A, et al. (2001) Comparison of cyclosporin and UVAB phototherapy for intermittent one-year treatment of atopic dermatitis. Acta Derm Venereol 81: 22-27.
- Haeck IM, Knol MJ, Ten Berge O, et al. (2011) Enteric-coated mycophenolate sodium versus cyclosporin A as long-term treatment in adult patients with severe atopicdermatitis: A randomized controlled trial. J Am Acad Dermatol 64: 1074-1084.
- Schram ME, Roekevisch E, Leeflang MM, et al. (2011) A randomized trial of methotrexateversus azathioprine for severe atopic eczema. J Allergy Clin Immunol 128: 353-359.
- Chavez-Alvarez S, Herz-Ruelas M, Villarreal-Martinez A, et al. (2020) Azathioprine: Its uses in dermatology. An Bras Dermatol 95: 731-736.
- Meggitt SJ, Gray JC, Reynolds NJ (2006) Azathioprine dosed by thiopurine methyltransferase activity for moderate-to-severe atopic eczema: A double-blind, randomised controlled trial. Lancet 367: 839-846.
- Schram ME, Borgonjen RJ, Bik CM, et al. (2011) Off-label use of azathioprine in dermatology: A systematic review. Arch Dermatol 147: 474-488.
- Gooderham MJ, Hong HC, Eshtiaghi P, et al. (2018) Dupilumab: A review of its use in the treatment of atopicdermatitis. J Am Acad Dermatol 78: S28-S36.
- Parmar NV, Abdula MA, Al Falasi A, et al. (2022) Long-term real-world experience of the side effects of dupilumab in 128 patients with atopic dermatitis and related conditions aged 6 years and above: Retrospective chart analysis from a single tertiary care center. Dermatol Ther 35: e15415.
- Silverberg JI, Simpson EL, Guttman-Yassky E, et al. (2021) Dupilumab significantly modulates pain and discomfort in patients with atopic dermatitis: A post hoc analysis of 5 randomized clinical trials. Dermatitis 32: S81-S91.
- Narla S, Silverberg JI, Simpson EL (2022) Management of inadequate response and adverse effects to dupilumabin atopic dermatitis. J Am Acad Dermatol 86: 628-636.
- Halling AS, Loft N, Silverberg JI, et al. (2021) Real-world evidence of dupilumab efficacyand risk of adverse events: A systematic review and meta-analysis. J Am Acad Dermatol 84: 139-147.
- Solimani F, Meier K, Ghoreschi K (2019) Emerging topical and systemic jak inhibitors in dermatology. Front Immunol 10: 2847.
- Li C, Sun X, Zhao K, et al. (2021) Efficacy and safety of Janus kinase inhibitors for the treatment of atopic dermatitis: A systematic review and meta-analysis. Dermatology 1-11.
- Simpson EL, Flohr C, Eichenfield LF, et al. (2018) Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: A randomized, placebo-controlled phase II trial (TREBLE). J Am Acad Dermatol 78: 863-871.
- Wollenberg A, Howell MD, Guttman-Yassky E, et al. (2019) Treatment of atopic dermatitis with tralokinumab, an anti-IL-13 mAb. J Allergy Clin Immunol 143: 135-141.
- Bilsborough J, Leung DY, Maurer M, et al. (2006) IL-31 is associated with cutaneous lymphocyte antigen-positive skin homing T cells in patients with atopic dermatitis. J Allergy Clin Immunol 117: 418-425.
- Ruzicka T, Hanifin JM, Furue M, et al. (2017) Anti-interleukin-31 receptor a antibody for atopic dermatitis. N Engl J Med 376: 826-835.
- Xiao X, Lin L, Zhu C, et al. (2021) Efficacy and safety of Nemolizumab for treatment of adult atopic dermatitis: A meta-analysis of randomized clinical trials. J Investig Allergol Clin Immunol 31: 190-192.
Corresponding Author
John R Baumann, University of Missouri, Columbia School of Medicine, Columbia, Missouri, USA.
Copyright
© 2023 Baumann JR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.