Pilot Plant Model for Sequential CO2 Fixation and Storage
Abstract
In the previously developed method using low NaOH and CaCl2 concentrations, the atmospheric CO2 is simply fixed to CaCO3 and NaCl, which exist as coal or limestone, and a large chamber comprising spray nozzles to capture CO2 efficiently by mists or droplets of NaOH solution has been designed. In the present study, a sequential CO2 fixation and storage system was designed to efficiently capture a large amount of CO2 from the atmosphere and exhausted gases. Using NaOH and CaCl2, CO2 was converted to CaCO3, which is harmless and stable. This system with NaCl electrolysis can be applied to seawater instead of artificial NaCl solution and automatically fix CO2 without adding NaOH or CaCl2. Additionally, the process could potentially be integrated with existing generator systems based on atomic, thermal, solar, wind, hydro or wave power. This pilot plant is definitely practical and economical for direct air capture without environmental concerns. Thus, this system is consistent with the sustainable development goals (SDGs).
Introduction
It is scientific evidence that atmospheric CO2 concentration on the earth has increased since the Industrial Revolution which was started about 200 years ago, inventing the steam engines using fossil coal as fuel, and the internal combustion engines using oil, and that our present developed civilization has owed to these technical inventions. However, we have not paid a little attention to the effect of increase in atmospheric CO2 concentration for a long time, while the young generation represented by a Swedish High School student, Greta Thunberg, led climate change activities "Friday for Future" events as worldwide movements. Unfortunately, this movement would not expand all of peoples worldwide. One of reasons is due to our wide tolerance toward CO2 concentration in daily life. In fact, the atmospheric CO2 concentration at the house room is about 400 ppm, while the concentration easily reaches twice in the presence of several persons in the same room without ventilation. Even under high CO2 concentration around 1,000 ppm for certain time in the limited space, our life doesn't feel an abnormal symptom. Eventually, many people would be apparently insensitive to a small increase in atmospheric CO2 concentration, although this small change has clearly induced climate change crises on the earth. Indeed, certain many people have insisted that climate change is a myth. Based on scientific evidence for the relationship between global temperature, atmospheric CO2 increases and hydroclimate changes [1-3], the intergovernmental panel on climate change concluded on August 9th, 2021, that climate change has been caused by human activities that have produced carbon dioxide (CO2) since the Industrial Revolution [4].
Although Earth has undergone many periods of significant environmental change over time, the planet's environment has been unusually stable for the past 10,000 years [5]. During this time, various natural systems regulated the Earth's climate and maintained the conditions that enabled human development. However, these regulatory systems have been greatly disturbed, and the planet may be nearing a threshold beyond which unpredictable environmental changes may occur, such as increases in the mean global temperature [6]. To reduce atmospheric CO2 concentrations as a means of mitigating such effects, the so-called Paris Agreement was reached at the United Nations Climate Change Conference (COP20) in 2015. This agreement was based on the requirement to keep the increase in the mean global temperature below 2 °C relative to the temperature prior to the Industrial Revolution, and preferably less than 1.5 °C. At present, this goal is challenging based solely on the development of carbon-neutral energy systems.
The 7th G summit was held in Cornwall, England, on June 12-13, 2021, with climate change being one of the main themes. Electric vehicles have been developed and used instead of ordinary automobiles using gasoline, to reduce atmospheric CO2 concentrations. While electric vehicles do not exhaust CO2 directly into the atmosphere, the current electricity generated by renewable energy is insufficient to power electric vehicles. However, hydrogen vehicles have been developed, although the cost is expensive. Indeed, hydrogen usage does not exhaust CO2; however, its production process using brown coal and high-temperature water produces a significant amount of CO2, except for the electrolysis of water. Additionally, because nuclear power plants are one of energy sources that do not emit CO2 into the atmosphere, they can contribute to reducing the atmospheric CO2 concentration. However, nuclear plants cannot directly capture the accumulated CO2 in the present atmosphere. In other words, nuclear power plants cannot immediately contribute to improving climate change at present. Furthermore, the outbreak of the Ukrainian crisis has shown that nuclear power plants are under serious military threat.
CO2 can be captured from the atmosphere or from flue gas via several techniques, including absorption [7], adsorption [8-14], and membrane gas separation [9,15]. Absorption with amines is currently the dominant technology, while membrane and adsorption processes are still in the developmental stages with the construction of primary pilot plants anticipated in the future. Contrarily, the method based on amines is not widely used because of the toxicity of organic solvents. Synthetic membranes are useful for desalination, dialysis, sterile filtration, food processing, dehydration of air, and other industrial, medical, and environmental applications because of their energy requirements, compact design, and mechanical simplicity. In addition, biopolymer cellulose membrane can be used instead of synthetic membranes because they have similar characteristics [15-17]. Recently, we developed a novel method for CO2 fixation and storage [18]. This method is based on simple chemical reactions involving NaOH and CaCl2. Using low concentrations of these chemicals prevented the formation of Ca (OH)2 in the absence of CO2, but resulted in CaCO3 formation in the presence of CO2 bubbling. Additionally, a polytunnel-based improvement method for CO2 fixation with NaOH mist was reported as an "artificial forest" model [19,20].
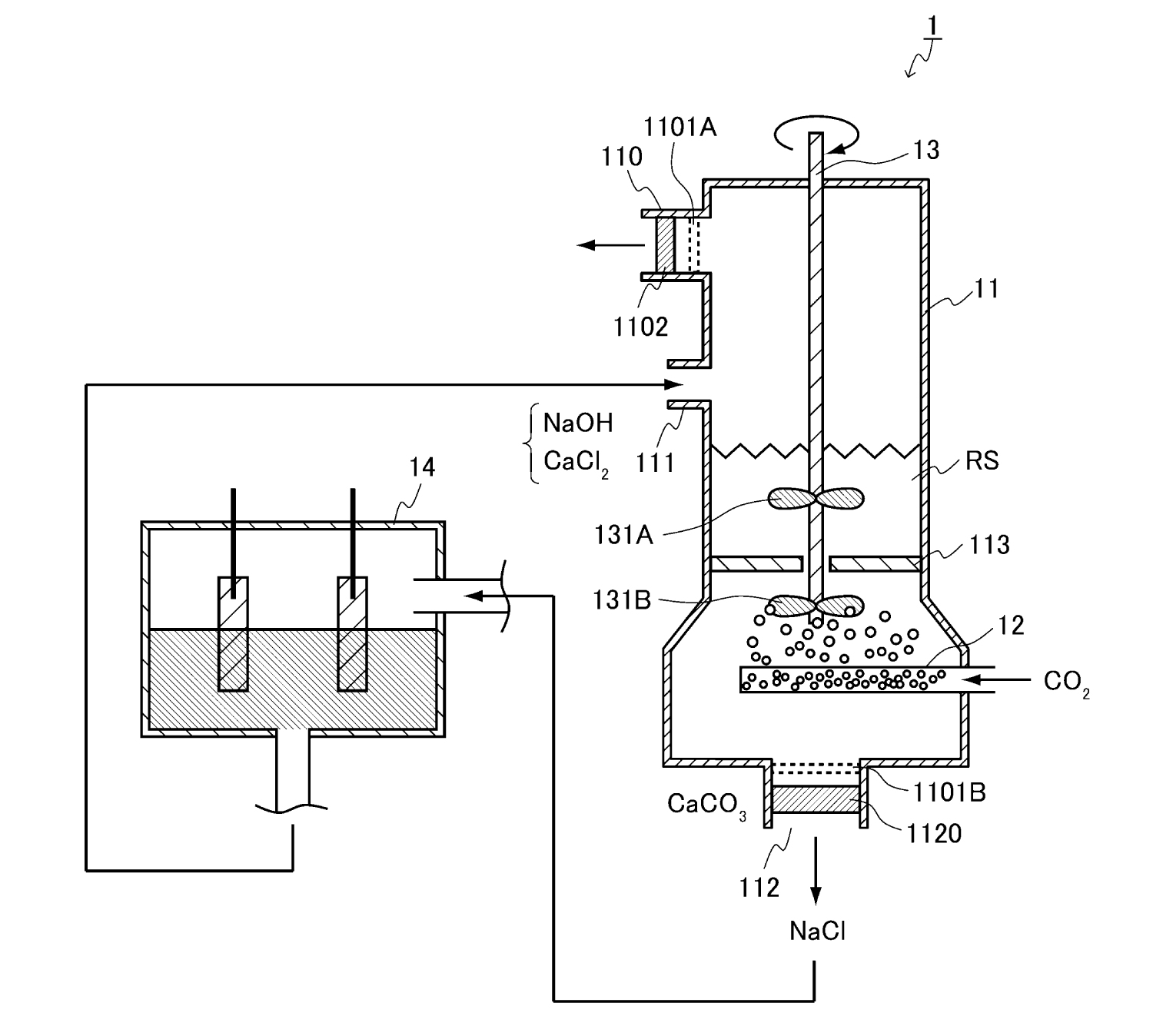
One crucial factor that determines the chemical reaction rate is substrate concentration. In the CO2 fixation reaction, CO2 gas molecules must first be efficiently absorbed by the NaOH solution. Therefore, the exhaust gas or atmosphere is formed as micro bubbles to increase the surface area of gas bubbles in contact with NaOH solution (Figure 1). Moreover, stirring the NaOH solution increases the probability of CO2 bubbles coming into contact with the NaOH solution. The high gas pressure increases the solubility of CO2 molecules in the NaOH solution, resulting in a higher reaction yield of CO2 with NaOH. It has also been found that deepening NaOH solution increases the contact time of CO2 bubbles with the solution. Hence, the liquid pressure increases with the liquid depth, showing a higher CO2 fixation. When multiple reaction towers are constructed, sequential CO2 fixation can be achieved.
The amount of CaCl2 that reacts with Na2CO3 can be determined from the measured concentration of Na2CO3 generated from CO2 and NaOH at the sensor. Furthermore, as the formation of Ca(OH)2 strongly inhibits the formation of CaCO3, the NaOH concentration in the reaction solution should also be measured to prevent Ca(OH)2 precipitation at high pH (> 0.2 N). Finally, the products, CaCO3 and NaCl, are separated by filtration or decantation.
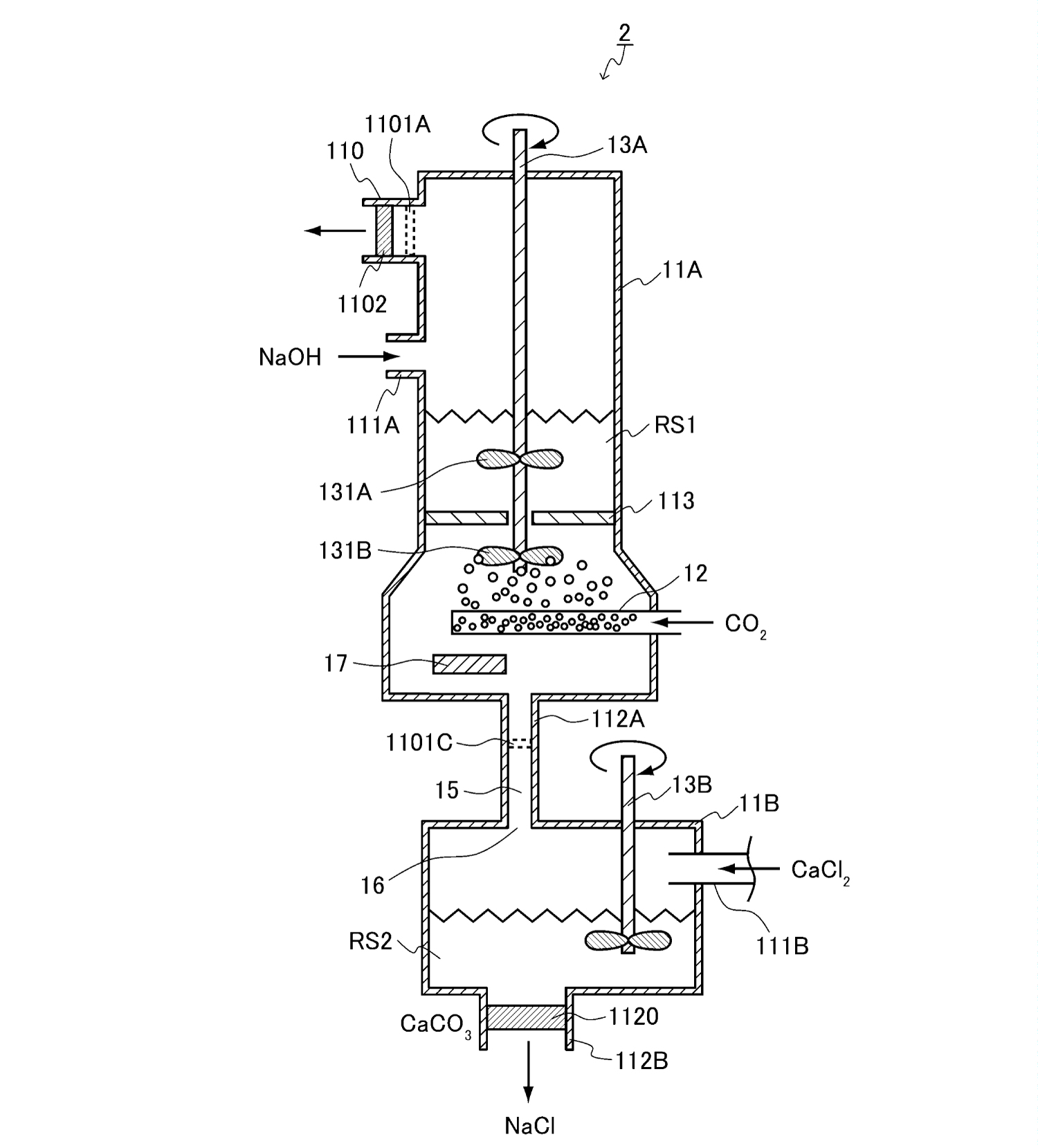
NaOH is practically produced from the artificial NaCl solution by electrolysis to generate H2 and Cl2. Therefore, it would be possible to use a filtrate containing NaCl produced by CO2 fixation instead of an artificial NaCl solution (Figure 2), indicating that NaOH can be generated in a cyclic process without adding NaOH. Furthermore, if seawater is used instead of artificial NaCl solution, CaCl2 can be supplied by seawater, eliminating the need for NaOH and CaCl2 [21]. This system has been patented already by our company (JP Patent#6906111). Furthermore, this process can be integrated with existing power generation systems such as nuclear, thermal, solar, wind, hydro, and wave power. Ultimately, the integrated system can capture CO2 and produce CaCO3 without external materials other than electricity.
A pilot plant was fabricated, as shown in Figure 3. Air was supplied into a 0.1-N NaOH solution through a Teflon microbubble former. When air with a CO2 concentration of approximately500 ppm was supplied to ~33-LNaOH solution at a flow rate of 30 L/min, the CO2 concentration in the ~43 L upper air space of the reaction tank reached 0 ppm within 2 minutes. When a stirrer was used, the CO2 disappeared more rapidly in the air space. This indicates that stirring is effective in fixing CO2 in a solution.
CO2 storage, geo-sequestration by injecting CO2 into underground geological formations, such as oil fields, gas fields, and saline formations, has been suggested [22,23], although these systems are still projects for the future. However, the proposed method can achieve both CO2 fixation and storage [18] simultaneously. The combination of large scale polytunnels that spontaneously absorb CO2 from atmosphere could be imitated like an "artificial forest" using simple and economical technology.
Our present civilization has been obviously created through the use of fossil fuels including coal, oil and natural gas, and this means that our daily lives are supported by a large cohort of fossil fuel workers. To make real change to renewable energy from fossil energy while maintaining life style and redeploying these workers. The smooth energy source transfer from fossil fuel to renewable energy source requires renewable energy development. It contributes not only to reduction of atmospheric CO2 concentration in the future, but also serves to reserve valuable natural energy sources for future generation. The drastic social changes imposed, however, may induce not only significant social concerns but also a slowdown of the accumulated atmospheric CO2 reduction.
Readers in the United States of America, the People's Republic of China, India and Japan declared that their countries would achieve the carbon-neutral society by 2050, 2060, 2070 and 2050, respectively. These achievements apparently would be effective to prevent a global climate crisis. However, we should recognize the fact that the present climate change, which has been caused by the present accumulated atmospheric CO2 exhausted not only from our daily activities including industrial activities but also from natural phenomena even before the Industrial Revolution. Thus, accumulated atmospheric CO2 should be reduced immediately to prevent the present ongoing rate of climate change. The concept of the carbon-neutral society by 2050 seems to be far too late.
Renewable energy production systems, such as solar radiation and wind power, can really reduce CO2 emission into the atmosphere but cannot reduce the present accumulated atmospheric CO2, which has induced the current climate change. Thus, using the method, which can capture most efficiently the atmospheric CO2 not only from the present atmosphere and exhaust gases but also from the future CO2, which will be emitted by human activities, is extremely important and should be developed as soon as possible.
The ultimate human evolution has been achieved based on the Industrial Revolution [24] which has resulted in climate change. Thus, as we are responsible for this crisis, we have amoral duty to address the situation through global cooperation beyond just making changes to our economy. Everybody must recognize that both wealth and civilization would be valueless on a ruined Earth without human prosperity.
Acknowledgents
The author thanks Enago (https://www.enago.jp) for editing a draft of this manuscript.
Competing Financial Interests
The author declares that the present data have been used to support applications to the Japan Patent Office (PCT/JP2019/03400, PTC/JP2019/045389, PCT/JP2019/045390, PCT/2020/026989, PCT/JP2019/048178, PCT/2020/002064, PCT/2020/026990, PCT/JP2020/029505, PCT/JP2020/029504, JP2020/79418, JP2021-090928, JP2021-126892, JP Patent #6783436, 6788170, 6878666, 6788169, 6830564, 6788162, 6739680, 6817485, 6906111, 6864143).
References
- Marvel K, Cook BI, Bonfils CJW, et al. (2019) Twenty-century hydroclimate changes consistent with human influence. Nature 569: 59-65.
- Friedlingstein P, Jones MW, O'Sullivan M, et al. (2019) Global carbon budget 2019. Earth Syst Sci Data 11: 1783-1836.
- Rick TC, Sandweiss DH (2020) Archaeology, climate, and global change in the age of humans. Pro Natl Acad Sci USA 117: 8250-8253.
- Allan RP, et al. (2021) Climate change 2021: The Physical Science Basis. Intergovernmental Panel on Climate Change (IPCC).
- Rioual P, Andrieu Ponel V, Rietti Shati M, et al. (2001) High-resolution record of climate stability in France during the last interglacial period. Nature 413: 293-296.
- Rockström J, Steffen W, Noone K, et al. (2009) A safe operating space for humanity. Nature 461: 472-475.
- Lv B, Guo B, Zhou Z, et al. (2015) Mechanisms of CO2 capture into monoethanolamine solution with different CO2 loading during the absorption/desorption processes. Environ Sci Technol 49: 10728-10735.
- Choi S, Drese JH, Jones CW (2009) Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. Chem Sus Chem 2: 796-854.
- Jones CW (2011) CO2 capture from dilute gases as a component of modern global carbon management. Ann Rev Chem Biomol Eng 2: 31-52.
- Nandi M, Okada K, Dutta A, et al. (2012) Unprecedented CO2 uptake over highly porous N-doped activated carbon monoliths prepared by physical activation. Chem Commun 48: 10283-10285.
- Hajra S, Biswas A (2020) Efficient chemical fixation and defixation cycle of carbon dioxide under ambient conditions. Sci Rep 10: 15825.
- Hiraide S, Sakanaka Y, Kajiro H, et al. (2020) High-throughput gas separation by flexible metal-organic framework with fast gating and thermal management capabilities. Nat Commun 11: 3867.
- Modak A, Nandi M, Mondal J, et al. (2012) Porphyrin based porous organic polymers: novel synthetic strategy and exceptionally high CO2 adsorption capacity. Chem Commun 48: 248-250.
- Qiao Z, Zhao S, Wang J, et al. (2016) A highly permeable aligned montmorillonite mixed-matrix membrane for CO2 Ange Chem Int Ed Engl 55: 9321-9325.
- Araujo T, Bernardo G, Mendes A (2020) Cellulose-based carbon molecular sieve membranes for gas separation: A review. Molecules 25: 3532.
- Xu S, Zhou H, Jia H, et al. (2021) Preparation and high performance of cellulose acetate films by grafting with imidazole ionic liquid. ACS Omega 6: 12500-12506.
- Ho NAD, Leo CP (2021) A review on the emerging applications of cellulose, cellulose derivatives and nanocellulose in carbon capture. Environ Res 197: 111100.
- Sorimachi K (2022) Innovative method for CO2 fixation and storage. Sci Rep 12: 1694.
- Sorimachi K (2022) Epoch-making discovery for CO2 characteristics: pseudo osmosis in the gas phase. Sci J Health Sci Res 1: 49-57.
- Sorimachi K (2022) Novel method for CO2 fixation and storage preventing climate crisis: an artificial forest model. Petro Chem Indus Intern 5: 1-4.
- Xie H, Liu T, Hou Z, et al. (2015) Using electrochemical process to mineralize CO2 and separate Ca2+/Mg2+ ions from hard water to produce high value-added carbonates. Environ Earth Sci 73: 6881-6890.
- Eccles J, Pratson LF, Chandel MK (2021) Effects of well spacing on geological storage site distribution costs and surface footprint. Environ Sci Technol 46: 4649-4656.
- Carroll SA, Iyer J, Walsh SDC (2017) Influence of chemical, mechanical, and transport processes on wellbore leakage from geologic CO2 storage reservoirs. Acc Chem Res 50: 1829-1837.
- Sorimachi K (2021) Study on ultimate human evolution: Cooperation of cerebral and five-fingernail development. New Visions in Biological Science.
Corresponding Author
Kenji Sorimachi, Bioscience Laboratory, Environmental Engineering Co., Ltd., Takasaki, Gunma, Japan, Tel/Fax: +81-27-352-2955
Copyright
© 2022 Sorimachi K. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.