Pseudokirchneriella subcapitata, Ceriodaphnia dubia and Rainbow Trout Responses to Uranium Exposure in Combination with Metals, Nutrients and Total Dissolved Solids Mixtures Using Site Water Collected from Two Creeks Located in the Yukon
Abstract
This study evaluates the effects of uranium (U) in combination with metals (arsenic, copper, chromium and zinc), nutrients (ammonia, sulphate) and total dissolved solids (TDS) on Pseudokirchneriellasubcapitata, Ceriodaphniadubia and rainbow trout (Oncorhynchus mykiss).Water was collected from two creeks in the Yukon and spiked with a mixture of U, metals, nutrients and TDS. Water was collected at two times of the year (summer and winter). Tests included: a - Metal Mixture, b - Metal Mixture + Nutrients and c - Metal Mixture + Nutrients + TDS with three treatment levels representing low, moderate and high concentrations per test. No adverse effects were noted in the algae after exposure to any of the tests and treatments (i.e., low, moderate and high). A stimulatory effect was observed in the algae which may have implications for enrichment of aquatic environments downstream mines.
For C. dubia, no effects occurred for the a - Metal Mixtures test. However, addition of nutrients to the solutions (i.e., test b) produced reproductive effects in winter water for both creeks. Effects occurred at U concentrations ranging from 100 to 180 μg U/L and mostly due declines in the invertebrate osmotic tolerances. The addition of TDS to the mixture (i.e., test c) augmented the appearance of reproductive effects in C. dubiaand further supports the hypothesis that adverse effects are related to declines in the invertebrate's osmotic tolerance. For rainbow trout, reductions in fish growth occurred after addition of TDS (i.e., test c)at nominal concentrations of 800 and 1,000 mg TDS/L.
Introduction
Uranium (U) concentrations in Canadian freshwater systems are generally below 1 μg/L (Canadian Council of Ministers of the Environment [CCME] 2011)[1]. However, some areas in Canada have naturally occurring U rich deposits. For example, areas located in the Yukon (YT) are characterized by the presence of uraniferous ore deposits which lead to background stream levels of U significantly higher (>100 μg/L) than the applicable short-term(33μg/L) and long-term (15μg/L)water quality guidelines (WQGs) (CCME 2011) [1]. These mineralized areas hold several operational mines and otherplanned developments, such as placer mining.Due to the mineralized characteristics of these areas in the YTand the naturally elevated U concentrations generally found in streams, there is potential for adverse impacts to aquatic life from increased U mobility released from mines and mills into the receivingenvironment. Therefore, it is crucial to understand U behaviour in aquatic systemsas well as to determine the levels that could adversely affect aquatic biota in these U-rich areas.
For aquatic biota relevant to Canadian waters, invertebrates (Hyalellaazteca and Ceriodaphniadubia) and algae (Pseudokirchneriellasubcapitata) are generally more sensitive to U exposure than northern temperate fish (e.g., rainbow trout (Oncorhynchus mykiss); CCME 2011)[1]. For algae, P.subcapitata) reported inhibition concentrations (IC10) for growth ranged from 5.4 to 120 μg U/L Acute toxicity in fish ranged from 1,670 to 59, 000 μg/L (CCME2011)[1]. Reported acute endpoints (e.g., Lethal Concentration [LC]50) for invertebrates including crustaceans, worms, hydra, insects, and bivalves exposed to U generally range from 150 to 10,000 μg U/L, (Poston, et al. 1984; Khangarot 1991; Bywater et al. 1991; Hyne et al. 1993a, b; Goulet et al. 2015) [2-6]. However, Ceriodaphniadubia emerged as the most sensitive invertebrate to acute U exposure based on the reported 48h LC50 of 60-89 μg U/L (low water hardness [< 6 mg/L as CaCO3]) by Pickett, et al. (1993) [7]. Long-term exposure to U can also result in chronic effects to aquatic biota. For fish, no effect observed concentrations (NOEC) for U range from 260 to 14, 300 μg/L (CCME2011)[1]. The reported NOEC for invertebratesranged from 1.5 to 2,250 μg U/L (CCME 2011; Muscatello and Liber 2009; Burnett-Seidel and Liber 2006)[1,8,9]with H. azteca and C. dubiaidentified as the two most sensitive invertebrate species to long-term aqueous U exposure (CCME 2011) [1].The sensitivities of these two aquatic invertebrates to U exposure generally overlaps and is dictated mainly by differences in water characteristics, a factor that have the capacity to affectmetal toxicity, including U (Poston, et al. 1984; Riethmuller et al. 2001; Markich 2002; van Dam et al. 2012) [2,10-12].
Uranium is known to form a variety of complexes with inorganic ligands (e.g., uranyl carbonate, sulphate and/or phosphate), humic and fulvic acids in the form of dissolved organic carbon (DOC), clay and silt particles, iron and manganese oxides (Trenfield, et al. 2011; Crawford et al. 2016) [13]. For example, recently Muscatello, et al. (2020)[14] evaluated the toxicity of U to C. dubia exposed to water collected from two creeks in the YT spiked with U and reported chronic endpoints significantly above the CCME water quality guideline (WQG) of 15 μg U/L (no observed effect concentration [NOEC] = 381 μg U/L; lowest observed effect concentration [LOEC] 524 μg U/L). Concentrations of DOC in these creeks during the ice-free period can exceed 10 mg/L and itwas postulated that the DOC concentrationspresent in the collected site water reduced the availability of U and hence, ameliorated its toxicity to C. dubia. Dissolved organic carbon is known to complex metals, including U in aquatic systems and the presence of DOC has been demonstrated to reduce U toxicity to freshwater organisms (Trenfield, et. al. 2011) [13]. In addition, U solubility is also affected by pH, alkalinity and tolesser degrees, by hardness (Riethmuller, et al. 2001; Goulet et al. 2015) [10,6]. The presence of other metals in solution is known to affect toxicity responses by either antagonistic, or synergistic processes. However, limited data are available on U interactions with other metals, regarding its toxicity to aquatic organisms. Markich (2002) [11], reported a synergistic effect between U and manganese on the valve movement of the tropical freshwater bivalve,Velesunioangasi. Charles, et al. (2006)[15] reported an antagonistic toxic effect between copper (Cu)and U in the tropical duckweed, Lemnaaequinoctialis. Furthermore, data on the interactions of U and other elements, such as sulphate, ammonia and total dissolved solids (TDS), known to generally occur in natural aquatic environments and in mine discharges, is lacking. All these factors have the potential to confound the responses of aquatic biota to U exposure; therefore, characterizing site-specific factors in natural waters (e.g., DOC, sulphate, ammonia, presence of other metals) and understanding U dynamics in aquatic systems is key to establishing protective water quality guidelines and objectivesfor U downstream operational mines.
The main objective of this research is to evaluate the responses of a common freshwater algae (P. subcapitata), the water flea C. dubiaand rainbow trout exposed to U in combination with elements commonly found in mine discharges, includingmetals (arsenic [As], chromium [Cr], Cu and zinc [Zn]), nutrients (ammonia and sulphate) and TDS, using spiked site-water collected from two un-impacted streams in a proposed gold mine development area in the YT. The algae, invertebrate and fish species selected for these tests are good candidates for assessing the aqueous toxicity of U since as previously mentioned, P. subcapitata and C. dubiaare known to be sensitive aquatic organisms to U exposure and all three species are known to be widely distributed in Canadian waters.
Material and Methods
Site water collection
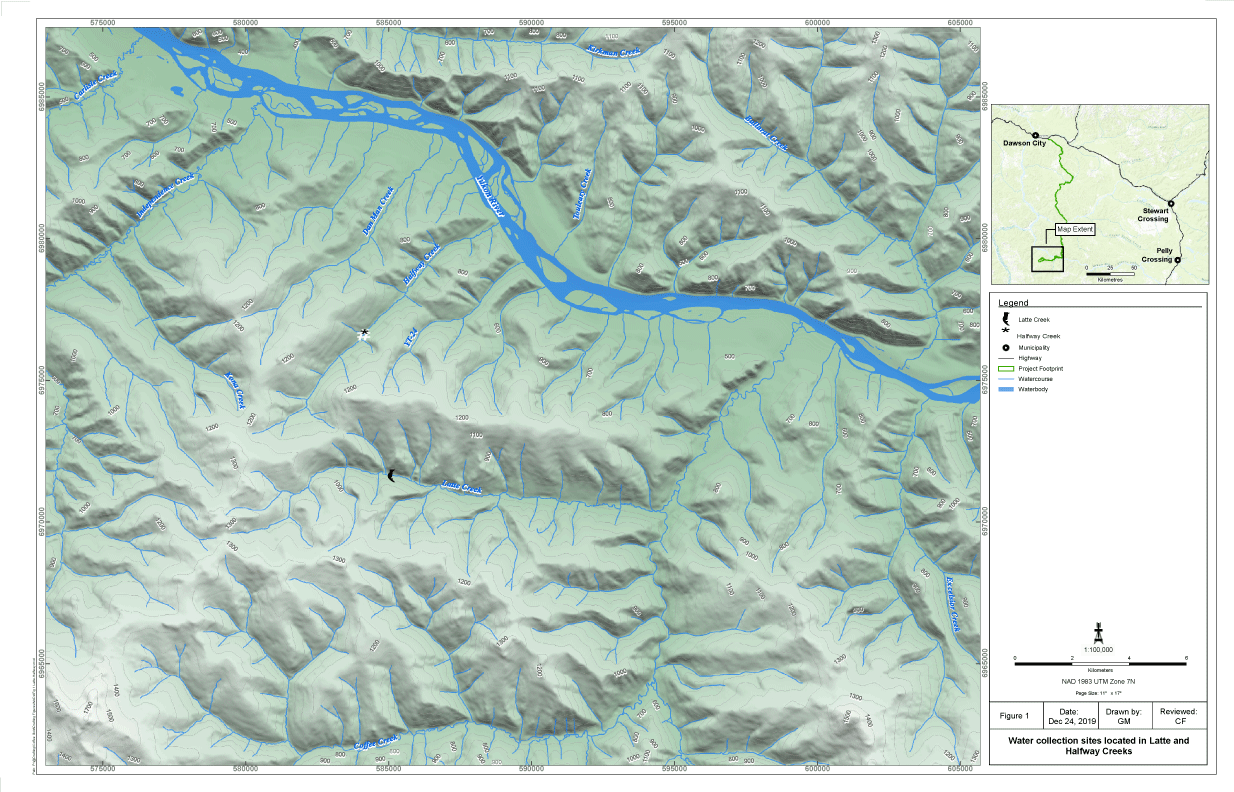
Toxicity tests were performed using site water collected from Latte Creek and Halfway Creek, located in the White Gold District of west-central YT, approximately 130 km south of Dawson City (Figure 1). The creeks are predominantly erosional with rocky, cobble and boulder substrates and limited pool areas. Flows in this area tend to be seasonal, and mostly driven by spring freshet (high-flow) in surface water and groundwater sources at winter times (low-flow). Complete winter ice cover generally occurs in the upper creeks, with aufeisobserved in some areas of the creeks.During winter low-flow periods and influenced mostly by groundwater input, U concentrations in these creeks have consistently been above water quality guidelines for the protection of aquatic life (15 μg/L [long-term], CCME [2011][1]).
Site water was collected from Latte and Halfway Creeks on March 17, 2018 and August 20-21, 2018.Water was collected during open-water and ice-covered water to represent characteristics of seasonal flows (summer-high and winter-low flows, respectively). Samples were delivered to the toxicological laboratory (where toxicity tests were performed) within 72-hrs after collection (recommended holding time by Environment Canada 2007a, b) [16,17]. Samples were transported in 20-L plastic containers and inside coolers containing freezer packs to keep temperature during transport at approximately 4 ℃. After arrival, samples were stored in the dark at 4 (± 2) ℃ until use in the tests. Toxicity testing commenced within 48-hrs after water arrival. A subsample of collected site water along with a sample of laboratory water were sent for general chemistry determinations (e.g., total suspended solids [TSS]) and total and dissolved metals analysis after arrival to the laboratory facilities (Figure 1).
Toxicity Tests
Three aquatic species were used in the toxicity tests, algae (P. subcapitata), the invertebrate C. dubiaand rainbow trout all of which are widely used in laboratory tests and considered sensitive to U exposure. Test species were also chosen on the basis of providing a suitable representation of aquatic biota present in Latte Creek and Halfway Creek. All organisms were obtained from the same population and sourced from well established laboratory colonies at Nautilus Environmental Company (Nautilus); Burnaby, BC. Toxicity tests included a) singly exposure to Metal Mixtures (As, Cu, Cr, and Zn), b) Metal Mixtures +Nutrients (sulphate and ammonia) and c) Metal Mixtures+ Nutrients + TDS (Table 1) to evaluate the potential for interactions between these elements and U. Exposure solutions were prepared using analytical grade chemical (SCP Science [Quebec, Canada]) diluted with site water collected from Latte Creek and Halfway Creek during winter and summer flows periods (with the exception of test c, which was only performed using summer collected water) (Figure 1; Table 1). Test concentrations for U were based on calculated 95th percentile background values known to occur in the creeks (31 μg U/L and 86 μg U/L for Latte and Halfway Creek, respectively [Muscatello, et al. 2020]) [14]. Uranium was added to these mixtures in incremental concentrations to achieve 3 test solutions: Low (to include the lower background value of 31 μg U/L), moderate (to include the highest background value 86 μg U/L) and high (to represent concentrations generally above background). Selection of additionalmetals(e.g., As, Cu, Cr and Zn) for inclusion in the test solutions werebased on professional experience of elements of concern and concentrations with the potential to occur downstream of operational minesin the YT (Table 1). In addition, nutrients, such as sulphate and ammonia were added to the test solutions as these two elements are known tooccur in mining operations as a result of operational/treatment activities and explosives use in blasting, respectively.
Experimental Procedures
A summary of the test conditions is shown in (Table S1). Exposures were conducted in an environmental chamber with a set photoperiod and controlled temperature. P. subcapitata tests were performed with 3-7 day old logarithm growth phase algae in microplates. The duration of tests was 72-hrs, static and followed protocols described in Environment Canada (2007b) for algae exposures. For C. dubia, tests were started with < 24-hrs old neonates;test duration was 7-days and followed protocols described by Environment Canada (Environment Canada [2007a]) [16]. Exposure of organisms to test solutions was conducted in 20-ml glass tubes following a static renewal protocol. Test organisms were fed daily using a 3:1 ratio of P. subcapitata cells and yeast-cerophyl-trout chow mix (YCT; Aquatic Biosystems [ABS], Fort Collins, CO). Rainbow trout tests were performed with 2-6 days post-swim-up fry as required by established protocols (Lazorchak and Smith 2007) [18]. Tests were conducted in 1-L glass containers following static-renewal protocol. Fish were fed twice daily with newly hatched brine shrimp nauplii (Artemia sp.) from an in-house culture. Aeration was not provided for any of the tests; however dissolved oxygen (DO) levels were recorded daily. Toxicity tests were performed by Nautilus, a certified laboratory by the Canadian Association for Laboratory Accreditation (CALA) and in accordance with applicable protocols (Environment Canada 2007a,b;Lazorchak and Smith 2007) [16-18].
Measurements of mortality, dissolved oxygen (DO), conductivity, pH and temperature were performed daily for the C.dubia and rainbow trout tests. Temperature and DO were recorded daily for the static algae test. For static-renewal tests, water was replaced in its totality daily to maintain adequate water quality and adequate exposure concentrations. Samples for determinations of As, Cu, Cr, Zn,ammonia, sulphate andTDS were collected from randomly selected test tubes at test initiation, halfway and finalization prior to water change for the invertebrate and fish tests and at test initiation for the algae test.ALS Laboratories (Burnaby, BC) a CALA certified laboratory performed all elemental analyses.
All testing included a negative (i.e., laboratory control prepared with dechlorinated laboratory water) and positive control (prepared using a reference toxicant; Table S1) to evaluate the quality and reproducibility of the performed test. In addition, site controls prepared with collected site water were used to evaluate potential effects of un-spiked site water on exposed organisms. Evaluated toxicity endpoints included growth inhibition in the algae P. subcapitataevaluated using the number of algal cells/mL, survival and reproduction (evaluated as the number of produced broods) in the water flea C. dubia, and survival and growth (evaluated as dry weight [d.w]) in rainbow trout. To evaluate fish d.w, rainbow trout were euthanized, and specimens dried for 24-hrs at 60℃ before weight determinations.
Statistical Analysis
Statistical analyses were conducted using Comprehensive Environmental Toxicity Information System (CETIS) statistical software package (Tidepool Scientific Software, 2013)[19] based on measured metal dissolved concentrations and used un-amended site water as the control value. For statistical analyses of toxicological data, significance level was established at α = 0.05. Statistical analysis included Fisher Exact Test followed by Bonferroni-Holm comparisons for analysis of survival data, and analysis of the variance (ANOVA) on Ranks followed by Dunnett's multiple comparisons for analysis of reproduction data.
Results
Site water characterization
General water quality parameters for controls including laboratory and un-amended Latte and Halfway Creeks waters are summarized in the Supplemental Material (Tables S2 to Table S3). The main noticeable differencesin site waters between the two evaluated seasonal flow periodswere the concentration of U, DOC, conductivity and hardness. Latte Creek water is generally characterized by low concentrations of TSS (< 3 mg/L), hard water and circumneutral pH. Concentrations of U were higher in the winter months along with conductivity and hardness. Uranium concentrations ranged from 8 μg/L and 24 μg/L for summer and winter, respectively. Conductivity and hardness values were approximately two times higher in the winter season (conductivity = 550 μS/cm; hardness = 390 mg/L [as CaCO3]) than those recorded in summer (conductivity = 308 μS/cm; hardness = 151 mg/L [as CaCO3]). Alkalinity was 86 to 92 mg/L (as CaCO3) for summer and winter collected water, respectively. The concentrations of DOC ranged from 4.8 mg/L and 8.2 mg/L for winter and summer, respectively.
For Halfway Creek, water is generally characterized by low concentrations of TSS (< 3 mg/L), hard water and circumneutral pH. Similar to Latte Creek, concentrations of U in Halfway Creek were higher in winter (74 μg/L)than in summer (23.μg/L). Conductivity and hardness values were approximately two times higher in the winter season (conductivity = 340μS/cm; hardness = 300 mg/L [as CaCO3]) than those recorded in summer (conductivity = 195μS/cm; hardness = 88 mg/L [as CaCO3]). Concentrations of DOC were 4.3 mg/L and 9.4 mg/L for winter and summer periods, respectively.Alkalinity was 66 to 78 mg/L (as CaCO3) for summer and winter collected water, respectively. This creek appears to generally have slightly lower hardness and conductivity values than those recorded for Latte Creek (Supplemental Material Tables S2 and S3).
Test conditions
A summary of experimental conditions including pH, temperature, DO, hardness and conductivity measurements throughout the test is provided in (Tables S4 to S7)of Supplemental Material. Measured DO was consistently >7 mg/Lfor all tests and values for the other parameters remained consistent for the length of the experiment. Measured As, Cu, Cr, U, Zn, sulphate, ammonia and TDS concentrations in exposure water resembled targeted nominal concentrations (TablesS7). With exception of arsenic, all metals exceeded applicable WQGs (CCME 1999)[20] whereas ammonia and sulphate concentrations fell within guideline values. Brief descriptions of exposure water parameters are provided for each test: a - Metal Mixtures, b - Metal Mixtures + Nutrients, and c - Metal Mixtures + Nutrients +TDS in the following sections.
Exposure to the reference toxicant conducted during this testing program fell within the acceptable range for organism performance of mean and two standard deviations in all tests. Mean sensitivity values based on laboratory historical data ranged from 1.8 - 2.0 g Sodium [Na]/L and 0.8 - 1.9 g Na/L for C. dubiasurvival [LC50] and reproduction [inhibitory concentration (IC50)], respectively; 26.0 - 40.2μg Zn/L for P.subcapitatagrowth (IC50) and29.1 - 153.2 μgCu/L and 28.8 - 148.4μgCu/Lfor rainbow trout survival (LC50) and growth (IC50), respectively. Thus, the sensitivity of the organisms used in these tests is considered appropriate. All laboratory controls passed the test validity requirements listed in (Table S1).
Exposure tests
a) Metal mixtures: General water quality parameters for Latte and Halfway Creeks exposures are summarized in Tables S4 and S7 (Supplemental Material) and briefly described below. Water quality remained constant through the tests and was within acceptability requirements for all evaluated species. Exposure water was generally characterized by circumneutral pH with maxima value of 8.0. Dissolved oxygen levels ranged from 7.7 to 9.8 mg/L throughout the test. Hardness values ranged from 117 to 126mg/L (as CaCO3) and from 314 to 322 mg/L (as CaCO3) forLatte Creek in summer and winter collected water, respectively. For Halfway Creek hardness values were < 100 mg/L (as CaCO3) in the summer and approximately 200 mg/L (as CaCO3) in winter collected water. Conductivity was approximately 250 μS/cm and 580 μS/cm for Latte Creek in summer and winter, respectively. For Halfway Creek conductivity values were160 μS/cm in summer and 400 μS/cm in winter collected water. Generally, Halfway Creek conductivity and hardness values were slightly lower than those recorded in Latte Creek (Tables S4 and S7).
Toxicity test resultsusing metal spiked site water collected from Latte and Halfway Creek during winter and summer periods are summarized in Table 2 to Table 4. For algae, an increase in the number of algal cells that was significantly different (p< 0.05) from laboratory control was observed in both Latteand Halfway Creek waters. This stimulatory effect was more noticeable in winter collected water for both creeks.No other significant differences were observed in algal growth between creeks and/or between treatments (i.e., low, moderate, and high) (Table 2).
Generally, no reductions were recorded on the survival and reproduction ofC. dubiaexposed to any of the treatmentsolutions (Table 3). Although not significant,a slightreduction in reproduction seemed to occurin Latte Creek site water collected during the summer compared to laboratory control. No statistical differences were found in C. dubiareproduction between evaluated site waters. For rainbow trout, no adverse effects were observed in survival and/or growth (expressed as d.w) after exposure to low, moderate and hightest solutions (Table 4). In addition, significant differences in survival and growth between creeks and seasons were not apparent.
b) Metal Mixtures + Nutrients: General water quality parameters in Latte and Halfway Creeks are summarized in the Supplemental Material (Tables S5 and S7) and briefly described below. Water quality remained constant through the tests and was within acceptability requirements for all evaluated species. Exposure water was generally characterized by circumneutral pH with amaxima value of 8.3. Dissolved oxygen levels ranged from 7.6 to 9.6 mg/L throughout the test (Table S5, Supplemental Material). Concentrations of ammonia in site water were approximately 0.8 mg/L (as nitrogen [N]) and < 0.005 mg/L (as N) for creek waters collected in winter and summer, respectively (Tables S2 and S3, Supplemental Material). Concentrations of sulphate for Latte Creek were 163 mgsulphate/L collected in winter and 71 mgsulphate/L in summer. For Halfway Creek sulphate concentrations were 78 and 29 mgsulphate/L for winter and summer, respectively (Tables S2 and S3, Supplemental Material). Hardness values ranged from 385 to 422 mg CaCO3/Land from 591 to 632 mgCaCO3/L for Latte Creek in collected summer and winter water, respectively (Table S7 Supplemental Material). For Halfway Creek hardness values ranged from 331 to 412 mgCaCO3/L in summer andfrom 490 to 564 mgCaCO3/L in winter collected water. Conductivity values in Latte and Halfway Creek exposure waters ranged approximately from 700 and 1,000 μS/cm for summer and winter, respectively (Table S7 Supplemental Material). Conductivity values increased in the low, moderate and high exposure treatments relative to site control due to salts and other inorganic chemicals added to achieve targeted exposure concentrations. Hardness and conductivity values are higher in winter than those measured in summer collected water for both creeks.
Results for the Metals Mixtures + Nutrients toxicity test using Latte and Halfway Creeks waters collected during the winter and summer period are summarized in (Tables 5 to 7). A significant (p< 0.05) stimulatory effect of site waters compared to laboratory controls was noticeable for algae growth in all treatments, which was consistent with the response recorded in the a - Metal Mixture Test Section 3.3.1; (Table 5). Although similar phosphorus and magnesium concentrations were measured in the creeks between seasons (Tables S2 and S3 Supplemental Material), the recorded stimulatory effect was slightly more noticeable in winter collected water for both, Latte and Halfway Creeks (Table 5). A significant reduction in growth (p< 0.05; ~ 15 % reduction compared to site control) was observed for algae exposed to low test concentrations using winter water collected from Latte Creek. However, these differences were not apparent at higher test concentrations (i.e., moderate and high; Table 5).
No effects on C. dubiasurvival were observed at any of the evaluated test concentrations (Table 6). A reduction in the number of produced broods (~25-30%) was observed for C. dubiaexposed to moderate and high treatment concentrations for Latte Creek and Halfway Creek, respectively. Significant effects in winter water were not observed in the high exposure treatment in Latte Creek water, however a reduction in reproduction relative to site control is apparent. No effects occurred in rainbow trout survival and reproduction at any of the evaluated treatments and site waters (Table 7).
c) Metal mixture + Nutrients + TDS: General water quality parameters in Latte and Halfway Creeks are summarized in the Supplemental Material (Tables S6 and S7) and briefly described below. Water quality in exposure treatments remained constant through the tests and was within acceptability requirements for all evaluated species (as those shown in Table S1). Exposure water was generally characterized by circumneutral pH with maxima of 8.1. Dissolved oxygen levelswere approximately 9.0 mg/L throughout the test (Table S6, Supplemental Material). Concentrations of ammonia and sulphate are described in the previous Section (3.3.2). The ammonia concentration was similar between creeks and seasons, however sulphate values appeared to be higher during the winter period for both creeks (Tables S2 and S3, Supplemental Material). Hardness values ranged approximately from 500 to 900 mgCaCO3/Lfor Latte Creek and Halfway Creek waters collected in summer (Table S7 Supplemental Material). Conductivity values in the creek's exposure waters ranged approximately from 1,000 to 2,000μS/cm (Table S6, Supplemental Material). Similar to the b - Metal Mixtures and Nutrients study, conductivity values in exposure solutions increased relatively to site control due to salts and other inorganic chemicals added to achieve targeted treatment concentrations.
Results of the toxicity test using mixture solutions in Latte and Halfway Creek waters collected during the open water period are summarized in (Table 8). A stimulatory effect of site waters was noticeable for algal growth, which was consistent with the response recorded in previous tests (Section 3.3.1; Section 3.3.2). For C. dubia, no survival effects occurred in any of the evaluated treatment solutions however, reproductive impairment was evident in the moderate and high exposure in both Latte Creek and Halfway Creek waters. For rainbow trout, survival was not affected however, growth effects were evident in all low, moderate and high treatments in Latte Creek and Halfway Creek waters.
Discussion
The differences in background U concentrations between winter and summer collected site waters are related to naturally U-enriched groundwater discharges into the Latte and Halfway Creek systems. As previously reported (Muscatello, et al. 2020)[14]naturally elevated U concentrations are known to occur in groundwater within the Latte and Halfway Creek drainages. In winter, the baseflows of the creeks aredominated by groundwater inputs, with negligible surface runoff input. Conversely, during the ice-free periods of the year (e.g., May to September), snowmelt and surface runoff inputs dilute the groundwater signature, resulting in considerably lower U concentrations in these creeks. These seasonal changes also have effects on other parameters that have relevance to U bioavailability such as DOC. Most notably, concentrations of DOC are highest during the ice-free months (e.g., May to August in particular) due to terrestrial runoff.
In this study, a stimulatory growth effect was observed in all tests (i.e., tests a, b, c) for the algae P. subcapitata. This effect is mostly related to the natural presence of nutrients and more suitable growth media present in site water compared to laboratory water. The addition of nutrients such as ammonia and sulphate to the testwater did not produce an increase in algal growth, with similar growth values reported for the a - Metal Mixture, b -Metal Mixture + Nutrients and c - Metal Mixture + Nutrients + TDS experiments. Winter water stimulated algae growth slightly more than collected summer water for both, Latte and Halfway Creeks. However, elements that may encourage algal growth such as for example magnesium and phosphorous, P. subcapitata exposed to spiked Latte and Halfway Creeks water is not immediately clear. A significant reduction in growth was showed similar values between seasons. Thus, the reason behind the observedstimulatory enhancement observed for algae exposed to the low concentration treatment using winter collected water from Latte Creek. However, these differences were not apparent at the high treatment concentrations and thus, assumed to be an artifact of the statistical analysis.The lack of adverse effects found in P. subcapitata exposed to metal mixtures (including U), sulphate, ammonia and TDS suggests that the algae may not be a suitable choice to evaluate (or derive)U thresholds applicable to aquatic environments downstream of planned (or operational) mines. Although no adverse effects were observed in P. subcapitataforallthe evaluated tests (i.e., tests a, b, c) and exposure concentrations (i.e., low, moderate, high), the stimulatory growth enhancement observed to occur in site water may have implications for enrichment in receiving aquatic environments. This enrichment factor should be considered when evaluating potential effects in aquatic environments downstream of proposed and/or operational mines.
Previous studiesreported by Muscatello, et al. (2020)[14] using Latte and Halfway Creek water spiked with U, postulated that the increased in DOCconcentration present in the collected site water reduced the availability of U and hence, ameliorated its toxicity to C. dubia. For example, calculated toxicity endpoint concentrations for C. dubia(e.g., LC50 = 799 μg U/L; NOEC [reproduction] = 381 μg U /L) were several orders of magnitude higher than the applicable CCME WQGs for this element (short-term = 33 μg U/L; long-term = 15 μg U/L). Therefore, based on the highest exposure concentrations for U used in the present study (nominal concentration = 100 μg U/L) effects on the aquatic invertebrate were not trulyexpected to occur. It was postulated, however that additional metals in solution may alter the detoxification capacity in C. dubiaat the expense of reproduction. When organisms face a trade-off between reproduction and survival imposed by a toxicant, in this case several metals in solution, they may reallocate resources toward detoxification mechanisms instead of reproduction and thus, achieve low mortality at the expense of reproductive fitness (Holloway, et al. 1990). It was expected that additional stressors in the a -Metal Mixtures test such as As, Cu, Cr and Zn may trigger detoxification mechanisms in C. dubia. These detoxification mechanisms are likely to be energy consuming and occur only at the expense of reproductive fitness (Holloway, et al. 1990; Muscatello and Liber 2009)[8] with reproductive effects occurring at lower U concentrations than those reported in previous studies (i.e., Muscatello et al. 2020) [14]. However, this did not appear to be the case in the present study as no adverse effects were recorded for C. dubiaregardless of exposure to low, moderate and high metal mixtures. The concentrations of DOC in site waters are known to have a protective role against U toxicity responses in C. dubia(Muscatello, et al. 2020)[14]. Although not relevant for U (Goulet, et al. 2015)[6], it is known that metal toxicity is generally decreased by hardness (Borgmann, et al. 2005)[21]. It is plausible that DOC and hardness ranges in site waters play a protective role in ameliorating the toxicity response of C. dubiato metal mixtures of As, Cu, Cr, U and Zn. In contrast to single metals exposure, knowledge about metal mixture effects remainsconfounded. Interactions between the metals in treatment solutions, could also ameliorate toxicity. For example, effects on growth rate of Lemnaaequinoctialis (duckweed) were lower when Cu and U were present in mixtures, relative to single/individual metal exposures, suggesting an antagonistic effect between these two elements (Charles, et al. 2006)[15]. It could be then concluded that mixtures of As, Cu, Cr and Znwith U at and/or below concentrations such as those reported in the high treatment exposure, are not expected to cause adverse effects toC. dubiain waters with similar chemistry characteristics (e.g., hardness, DOC) than those recorded forLatte and Halfway Creeks.
With the addition of sulphate and ammonia into the metal mixture (i.e., b - Metal Mixtures and Nutrients test) reproductive effects in C. dubiawere apparent in winter water specifically for the moderate and high treatment in Latte Creek and Halfway Creek, respectively. Sulphate, and in minor degree nitrogen forms (i.e., ammonia), are contributors to the ionic composition of the water. Other major contributors to ionic strength are calcium and magnesium which play a main role in water hardness. The exposure water collected in winter from both creeks shows higher hardness (~600 mg CaCO3/L) and conductivity(i.e., a measure of the ionic strength of water; ~1,000μS/cm) than those in summer (hardness ~400mg CaCO3/L; conductivity ~700 μS/cm). It seems that C. dubiaexhibited increased sensitivity to metal exposure in this winter water due to addition of sulphate and ammonia relative to the a -Metal Mixtures test. It is plausible the increased ionic strength of the test solutions generated using winter water (i.e., hard water and high conductivity) resulted in osmotic and ionic regulation challenges to the invertebrate that, combined with the evaluated metal concentrations, resulted in adverse reproductive effects at lower U concentrations (measured concentrations ~ 100 to 180 μg U/L for the moderate and high treatments, respectively) than those previously reported (NOEC ~ 300 μg U/L, Muscatello, et al. 2020 [14]. This has been reported to occur for other elements, for example, Elphick, et al. 2011,[22] reported increased sensitivity to sulphate in C. dubiaat hardness values of 160 to 320 mgsulphate/L due to increased ionic strength of the water and osmotic challenges in the invertebrate. The addition of TDS (at nominal concentrations of 600, 800 and 1,000 mg TDS/L for the low, moderate and high treatments, respectively) to the metal and nutrient mixture (i.e., c -Metal Mixtures + Nutrients + TDS) producedthe appearance of effects in C. dubiawith reproductive impairment occurring in the moderate (~1,360and 1,220 mg TDS/L for Latte Creek and Halfway Creek, respectively) and high (~1,650and 1,580 mg TDS/L for Latte Creek and Halfway Creek, respectively) treatments for both creeks in summer collected water.These effect concentrations are in agreement with Weber-Scannell and Duffy (2007)[23] whereTDS valuesranging from 735 to 1,910 mg TDS/L produced adverse effects in exposedC. dubia. Two individual tests using summer collected Halfway Creek water spiked with TDS (nominal concentrations 200 to 1,500 mg TDS/L) and sulphate (nominal concentrations 50 to 1,000 mg/L) in combination with ammonia (at nominal concentrations of 2 mg/L [as N]) found no effects in exposed C. dubiawith reported IC50 values above 1,500 mg/L and 1,000 mg/L, for TDS and sulphate, respectively (Muscatello and Flather 2021)[24]. This further substantiates the hypothesis that the effects observed inC. dubiaexposed to a mixture of metals, ammonia, sulphate and TDS are the result ofa decline in osmotic tolerances due to the increased ionic strength of exposure water, in associationwith an increased sensitivity to metal exposure triggered by the osmotic challenges faced by the invertebrate.
Exposure of swim-up rainbow trout fry to low, moderate and high metal mixtures, sulphate and ammonia solutions prepared using Latte Creek and Halfway Creek site water produced no observed reductions in survival or growth in winter or summer collected waters. Therefore,U in mixturesformulations of As, Cu, Cr, Zn, sulphate and ammonia at and/or below concentrations such as those reported in the high exposure treatment, are not expected to cause adverse effects to the fishspecifically in waters with similar chemical characteristics (e.g., hardness, DOC) tothose existing for Latte Creek and Halfway Creek. Rainbow trout is one of the most sensitive fish species to TDS exposure, particularly at the egg hardening stage and embryo developmental period (Weber Scannell and Jacobs 2001) [25]. Not surprisingly, the addition of TDS to Latte Creek and Halfway Creek waters (i.e., c -Metal Mixtures + Nutrients + TDS tests) triggered the appearance of significant growth reductions for all treatments (i.e., low, moderate and high) at measured TDS concentrations ranging from ~ 800 to 1,700 mg TDS/L. These effects are believed to be related to TDS rather than a result of exposure to metals (including U), sulphate and ammonia mixtures given the lack of effects recorded in these exposures (i.e., tests a and b). Tests using TDS in exposed salmonids have yielded mixed results, depending on site-specific conditions, ionic composition of exposure water, life stage and species used in the tests. For example, Chapman, et al. (2000),[26] reported no effects in rainbow trout (eggs and swim-up fry) at TDS concentrations > than 2,000 mg TDS/L whereas, Ketola, et al. (1988)[27] reported effects in survival of rainbow trout post fertilized eggs exposed to 1,500 mg TDS/L. Other authors reported reduction in the that fertilization and/or embryonic development of rainbow trout with IC25 values of 1,200 mg TDS/L and 600 mg TDS/L after 7 and 15 days of exposure, respectively. (Weber Scannell and Jacobs 2001)[23]. Based on the available scientific literature for rainbow trout exposed to TDS, the results reported in the present study fall within the concentration ranges expected (600 to 2,000 mg TDS/L) to cause adverse effects in this fish (independent of life stages evaluated) (Weber Scannell and Jacobs 2001)[23]. It should be noted that WQGs are not currently available for TDS. Guideline derivation should thus, incorporate evaluation of TDS effects to sensitive life stages (e.g., egg hardening) and sensitive species such as, rainbow trout. In addition,consideration of the ionic composition and strength of site waters should be considered as this may have an effect in toxicity responses.
Conclusion
Few studies have investigated interactions of U with other metals and elements, known to occur downstream of operational mines such as sulphate ammonia and TDS. The goal of the present study was to determine the effects of metal mixtures, ammonia, sulphate and TDS on the toxicity of U inP. subcapitata, C. dubiaand rainbow trout. Three independent tests including a -b - Metal Mixtures, Metal Mixtures + Nutrients and c Metal Mixtures + Nutrients + TDS using spiked water collected from Latte Creek and Halfway Creek located in the YT, in summer and winter were performed to evaluate the presence of adverse effects. Organisms were exposed to three treatment solutions low, moderate and high per test.P. subcapitata showed no adverse effects for any of the evaluated tests (i.e.., tests a, b, c) and treatments (i.e., low, moderate and high). However, growth stimulation occurred in site water which may have implications for the enrichment of aquatic systems downstream planned and/operating mines. For C. dubia, no adverse effects were noted for the a -Metal mixtures test suggesting that U concentrations in combination with metals such as, As, Cu, Cr, Zn at and/or belowconcentrations such as those reported in the high exposure treatment, are not expected to cause adverse effects in waters with similar chemistry characteristics (e.g., hardness, DOC) than those existing for Latte Creek and Halfway Creek. The addition of ammonia and sulphate to the test solutions (i.e., b - Metal Mixtures + Nutrients) produced reproductive effects in winter collected water in both, Latte and Halfway Creek test solutions due to osmotic pressures faced by the invertebrate. Responses occurred at U concentrations lower (~100 to 180 μg U/L) than those previously reported to cause adverse effects in the invertebrate (NOEC ~ 300 μg U/L and postulated to be a result of increased sensitivity to metal exposures triggered by declines in the invertebrate osmotic tolerances. The addition of TDS to the mixture (i.e., c- Metal Mixtures + Nutrients + TDS) increasedthe appearance of reproductive effects in C. dubiaand further supports the hypothesis that adverse effects are the result of a decline in the invertebrate's osmotic tolerances. For rainbow trout, adverse effects were only noticed for the c- Metal Mixtures + Nutrients + TDS test, and mostly a resultof TDS in the moderate (measured concentration ~ 800 mg TDS/L) and high (measured concentration ~ 1,700mg TDS/L) treatments rather than metal exposure. Consideration of both the freshwaterorganism's sensitive life stages and the ionic strength of the water should be considered when deriving TDS WQGs.
Acknowledgments
The authors would like tothank Newmont/Goldcorp personnel for their technical supportand Cheng Kuang for her assistanceduring this study.
Funding: Not applicable.
Conflicts of interest/competing interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Availability of data and material: All data utilized in the generation of this paper, has been submitted (supplemental material).
Code availability: Not applicable.
Authors' contributions: JorgelinaMuscatello: Conceptualization, Methodology, Experimental Design, Data analysis, Manuscript generation;Mimi Tran: Test performance, Data management and general Laboratory tasks; David Flather: Supervision, Review; Curtis Eickhoff: Supervision of laboratory tasks; Jennie Gjertsen: Field support, Review, Regulatory input.
Ethics approval: Not applicable.
Consent to participate: Not applicable.
Consent for publication: Authors agree to publish this original work on Advances in Environmental Studies.
References
- CCME (2011). Canadian Water Quality Guidelines for the Protection of Aquatic Life: Uranium. In: Canadian Environmental Quality Guidelines, 1999, Canadian Council of Ministers of the Environment.
- Poston TM, Hanf RW Jr, Simmons MA (1984) Toxicity of uranium to Daphnia magna. Water Air Soil Pollut 22: 289-298.
- Khangarot BS (1991) Toxicity of metals to a freshwater tubificid worm, Tubifex tubifex (Muller). Bull Environ Contam Toxicol 46: 906-912.
- Bywater JF, Banaczkowski R, Bailey M (1991) Sensitivity to uranium of six species of tropical freshwater fishes and four species of cladocerans from northern Australia. Environ Toxicol Chem 10:1449-1458.
- Hyne RV, Padovan A, Parry DL, et al. (1993) Increased fecundity of the cladoceran Moinodaphnia macleayi on a diet supplement with a green algae and its uses in uranium toxicity test. Aust J Mar Freshwater Res 44: 389-399.
- Goulet R, Thompson PA, Serben K, et al. (2015). Impact of environmentally based chemical hardness on uranium speciation and toxicity in six aquatic species. Environ Toxicol Chem 34: 562-574.
- Pickett JB, Specht WL, Keyes JL (1993). Acute and chronic toxicity of uranium compounds to Ceriodaphnia -Daphnia dubia. Savannah River, Aiken, South Carolina, Westinghouse Savannah River Co. WSRC-RP-92-995.
- Muscatello JR and Liber K (2009). Accumulation and chronic toxicity of uranium over different life stages of the aquatic invertebrate Chironomus tentans. Arch Environ Contam Toxicol 57: 531-539.
- Burnett Seidel C, Liber K (2012). Evaluation of sediment quality guidelines derived using the screening-level concentration approach for application at uranium operations in Saskatchewan, Canada. Environ Monit Assess 184: 1593-1602.
- Riethmuller N, Markich SJ, van Dam RA, et al. (2001). Effects of water hardness and alkalinity on the toxicity of uranium to a tropical freshwater hydra (Hydra viridissima). Biomarkers 6: 45-51.
- Markich SJ (2002) Uranium speciation and bioavailability in aquatic systems: An overview. The Scientific World Journal 2: 707-729.
- Van Dam RA, Trenfield MA, Markich SJ, et al. (2012). Reanalysis of uranium toxicity data for selected freshwater organisms and the influence of dissolved organic carbon. Environ Toxicol Chem 31: 2606-2614.
- Tren field MA, Ng J, Noller BN, et al. (2011) Dissolved organic carbon reduces uranium bioavailability and toxicity. 2. Uranium [VI] speciation and toxicity to three tropical freshwater organisms. Environ Sci Technol 45: 3082-3089.
- Muscatello J, Flather D, Gjertsen J (2020). Survival and reproductive effects in the aquatic invertebrateCeriodaphnia dubia exposed to uranium spiked site water collected from two creeks in the Yukon, Canada. Arch Environ Contam Toxicol 79: 80-88.
- Charles AL, Markich SJ, Ralph P (2006). Toxicity of uranium and copper individually, and in combination, to a tropical freshwater macrophyte (Lemna aequinoctialis). Chemosphere 62: 1224-1233.
- Environment Canada. 2007a. Biological test method: test of reproduction and survival using the clado ceran Ceriodaphnia dubia. Environmental Protection Series. Report EPS 1/RM/21, Second Edition, February 2007. Environment Canada, Method Development and Application Section, Environmental Science and Technology Centre, Science and Technology Branch, Ottawa.
- Environment Canada. 2007b. Biological test method: growth inhibition test using the fresh water alga. Environmental Protection Series, Report EPS 1/RM/25. Second Edition, March 2007.Environment Canada, Method Development and Application Section, Environmental Science and Technology Centre, Science and Technology Branch, Ottawa.
- Lazorchak JM and Smith ME (2007). Rainbow Trout (Oncorhynchus mykiss) and Brook Trout (Salvelinus fontinalis) 7-day Survival and Growth Test Method. Arch Environ Contam Toxicol 53: 397-405.
- Tidepool Scientific Software (2013). CETIS comprehensive environmental toxicity information system, version 1.8.7.16 Tidepool Scientific Software, McKinleyville, CA.
- Canadian Council of Ministers of the Environment (CCME) (1999). Canadian water quality guidelines for the protection of agriculture, irrigation and livestock.
- Borgmann U, Couillard Y, Doyle P, et al. (2005). Toxicity of sixty-three metals and metalloids to Hyalella azteca at two levels of water hardness. Environ Toxicol Chem 24: 641-652.
- Elphick JR, Davies M, Gilron G, et al. (2011). An aquatic toxicological evaluation of sulfate: The case for considering hardness as a modifying factor in setting water quality guidelines. Environ Toxicol Chem 30: 247-253.
- Weber Scannell P and Duffy LK (2007). Effects of total dissolved solids on aquatic organisms: A Review of literature and recommendation for salmonid species. Am J Environ Sci 3: 1-6.
- Muscatello J and Flather D (2021). Two independent tests evaluating the effects of total dissolved solids and sulphate in combination with ammonia in Ceriodaphnia dubia using spiked site water collected from two creeks in the Yukon, Canada.
- Weber Scannell P, Jacobs L (2001) Technical Report NO. 01-06, Effects of total dissolved solids on aquatic organisms: A literature review. Alaska Department of Fish and Game Division of Habitat and Restoration, Juneau, AK.
- Chapman PM, Bailey H, Canaria E (2000) Toxicity of total dissolved solids associated with two mine effluents to chironomid larvae and early life stages of rainbow trout. Environ Toxicol Chem 19: 210-214.
- Ketola HG, Longacre D, Greulich A, et al. (1988) High calcium concentration in water increases mortality of salmon and trout eggs. Progressive Fish-Culturist 50: 129-135.
Corresponding Author
JorgelinaMuscatello, Lorax Environmental Services Ltd, 2289 Burrard Street Vancouver,BritishColumbia,CanadaV6J3H9,Tel: 604-688-7173.
Copyright
© 2022 Muscatello J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.