An Overview of the Effects of Heavy Metals Content in Wastewater on Anammox Bacteria
Abstract
The application of Anammox process in treating nitrogen rich wastewater had been more preferable since the discovery of Anammox process and Anammox bacteria at 1999 due to the advantages of energy saving and cost reduction compared to the conventional nitrification/denitrificationi process. However, often nitrogen-laden wastewater such as metal refinary wastewater, swine, industrial wastewater, and landfill leachate contain various concentrations of heavy metal ions such as Cadmium, Copper, Lead, Mercury, Nickel, Zinc, Silver and Ferrous iron. Trace amount of Zn(II), Co(II), Mn(II), Cu(II) and Ni(II) are recommended to be added in substrate during cultivation of Anammox bacteria as essential micronutrients. These metals are crucial co-factors for certain enzymes and metalloproteinase. Nevertheless, regardless of stimulation effect of some metals on the growth of bacteria at low concentration, a high concentration of metals ions might cause a negative effect on long term. The inhibition effects of various heavy metals were compared in this study. It was found that nine heavy metal, Pb2+ has the lowest inhibition effect on Anammox process, Cu2+ might be having the most inhibition effect on Anammox with the IC50 inhibition concentration. The IC50 inhibition concentration of Fe (II) was found at 0.20 mM. It was found Pb2+ has the lowermost inhibition effect, AT > 75 mg/L of Pb2+, while, Cu2+ might be having the supreme inhibition effect with the IC50 inhibition concentration at 1.9 mg/L. The IC50 of Fe (II) was found at 55.6 mg/L. More effort should be dedicated to understand the profound knowledge of heavy metal on Anammox bacteria.
Keywords
Anammox, Wastewater, Heavy metal, Inhibition, IC50, Stimulation
Abbreviations
Anammox: Anaerobic Ammonium Oxidizing; AOB: Ammonia Oxidizing Bacteria; COD: Chemical Oxygen Demand; EDX: Energy Dispersive X-ray; Hao: Hydroxylamine Oxidoreductase; HDH: Hydrazine Dehydrogenase; hh: Hydrazine Hydrolase; HZO: Hydrazine Oxidizing Enzyme; HZS: Hydrazine Synthase; IC50: Half Inhibition Concentration; N: Element Nitrogen; NH4⁺: Ammonium; NO: Nitric Oxide; N2O: Nitrous Oxide; NOB: Nitrite-Oxidizing Bacteria; SAA: Specific Anammox Activity; TN: Total Nitrogen
Introduction
The concept where ammonium can be oxidized under anoxic condition initially came from calculations based on theoretical thermodynamic [1] and the Redfield ratio in marine ecosystems [2]. The concept was proven 20 decades later by Mulder, et al. [3] who discovers the anaerobic ammonium oxidizing (anammox) process (Eq. (1)) and Strous, et al. [4] who found the responsible microorganisms, anammox bacteria. Anammox bacteria are Planctomycete type bacterium with anaerobic (no need oxygen) and autotrophic (no need organic carbon) metabolism, which combines ammonium (as electron donor) and nitrite (as an electron acceptor) to generate dinitrogen gas in the absence of oxygen.
NH4+ + 1.32NO2- + 0.066HCO3- + 0.13H+ → 1.02N2 + 0.256NO3- + 0.066CH2O0.5N0.15 + 2.03H2O (1)
The process of Anammox has become essential at the global level as it contributes to oceanic nitrogen loss level and has multiple advantages compared to the conventional nitrification/denitrification process in the removal of nutrient from wastewater [5]. In addition, Anammox process is critical in the ocean's nitrogen cycle, wherein the continental shelf sediment, Anammox contributes up to 67% of the nitrogen gas and only 33% of nitrogen gas formed due to denitrification process [6]. Anammox process is considered as most cost effective biological nitrogen removal processes, and the advantages of Anammox process over conventional nitrification/denitrification process are: i) Save 90% operation cost (reduces 64% aeration, 80-90% sludge production and 100% exogenous electron donor (e.g organic carbon)) [7-11] reduced CO2 or N2O emissions [5]. Anammox process is a promising method for treating nitrogen rich and low chemical oxygen demand (COD) content wastewater such as landfill leachate and metal refinery wastewater. Table 1 shows various concentration of nitrogen in different type of wastewater. However, these type of wastewaters contain a high concentration of heavy metals [12-14]. Heavy metals such as iron, zinc, copper, nickel, and cobalt can either cause stimulation, inhibition or even toxic effect in biochemical reactions dependent on the concentrations and species [13]. Therefore, it is crucial to obtain more knowledge about the effect of heavy metal on the microbial activity and the performance of the reactor. This aims to provide a compressive review concerning the stimulation and inhabitation effects of heavy metals on anammox process and anammox bacteria activities.
Removal of Nitrogen Compounds from Wastewater
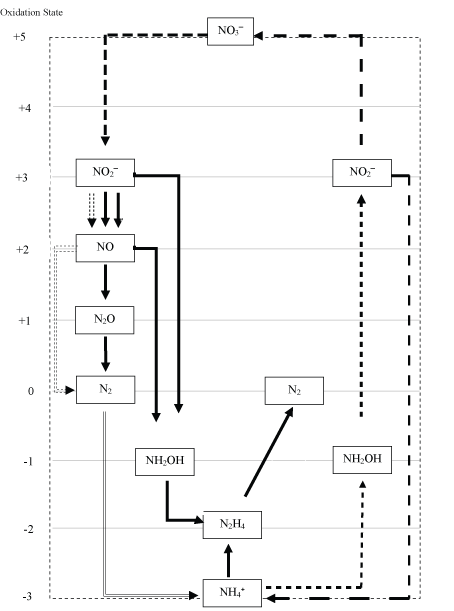
The element nitrogen (N) in its various redox forms are essential macronutrients for all living organisms on earth. Our land's atmosphere is made up of 78% of dinitrogen gas (N2). However, living organisms cannot access the nutrients of nitrogen from the atmosphere directly. A specialized group of bacteria can fix dinitrogen gas from the air to ammonium (NH4⁺), then, the fixed nitrogen, in the form of NH4⁺, will either enter food chain directly by assimilation process for macromolecule biosynthesis or it will be used as a substrate for bacteria which acquired energy for growth throughout the oxidation. Figure 1 shows the nitrogen cycle in nature that is responsible for all the reaction of the bacteria that convert nitrogen to its various redox forms, from +5 (NO3-) to -3 (NH4⁺/NH3). Nitrification (autotrophic conversion of ammonium to nitrite then further to nitrate), is a stepwise oxidation process where ammonia oxidizing bacteria (AOB) oxidize NH4⁺ to NH3‾ and nitrite oxidizing bacteria (NOB) oxidize NO2‾ to NH3‾. Denitrification (heterotrophic conversion of nitrate to dinitrogen gas) is a stepwise reduction process where denitrifying bacteria reduced NH3‾ to N2 with NO2‾, nitric oxide (NO) and nitrous oxide (N2O) as intermediate. Furthermore, in the process of nitrogen fixation, some non-symbiotic and some symbiotic with leguminous plants will fixate the atmospheric N2 to NH4⁺. In Anammox process, autotrophic bacteria utilize NH4⁺ as an electron donor and NO2‾ as an electron acceptor and convert into nitrogen gas and some nitrate under anaerobic conditions in the absence organic carbons.
Conventional nitrification/Denitrification process
Due to the elevation of industry or/and agriculture activities, and discharge of excess nitrogen from wastewater directly into water bodies such as river and lake without advanced treatment, nature suffered from eutrophication and hypoxia. Nowadays worldwide environmental authorities enforcing stringent nitrogen discharge criteria. The conventional nitrogen removal system implemented nitrification and denitrification process to helped in treating nitrogen-rich wastewater before discharging to nature. In autotrophic nitrification process, aeration is necessary as both AOB and NOB need either oxygen or sulfate and ferric as other oxidized compounds, as an electron acceptor to oxidize NH4⁺ to NH3‾ and oxidize NO2‾ to NH3‾. While in heterotrophic denitrification process (from NH3‾ to N2), addition external electron donor (e.g. organic carbon) such as methanol (in majority case) is required for the denitrifying bacteria. Intermediate such as NO2‾, NO and N2O will be produced in denitrification process. When applying nitrification/denitrification in the process of nitrogen removal, a significant amount of oxygen is needed, large amount of sludge produced, and the considerable volume of N2O generated a greenhouse gas with a potential roughly 300 times higher than CO2.
Anaerobic ammonium-oxidizing (Anammox) process
Anammox bacteria is a coccoid-shaped Planctomycete type bacterium with anaerobic (absence of oxygen) and autotrophic (not require organic carbon) metabolism, which utilize ammonium (NH4⁺) (as electron donor) and nitrite (NO2‾) (as electron acceptor) in a ratio of 1:1.3 during the growth phase to generate dinitrogen gas (N2) and some nitrate (NH3‾). Anammox used bicarbonate as carbon sources to produce biomass (CH2O0.5N0.15) (see Eq. (1)), which also acts as a buffering agent in Anammox process. Nitrite not only functions as an electron acceptor for ammonium oxidation but also as an electron donor for the reduction of carbon dioxide. The central catabolic Anammox metabolism can be described as a three-step reaction. It involves nitric oxide (NO) and hydrazine (N2H4), a well-known rocket fuel, as intermediates [15]. First, nitrite (NO2‾) will be reduced to nitric oxide (NO), catalyzed by nitrite reductase (Nir) (which also named nitrite reducing enzyme (NR)) (Eq (2)). Then the nitric oxide (NO) and ammonium (NH4⁺) will be condensed to hydrazine (N2H4) through hydrazine synthase (HZS) enzyme (Eq (3)), followed by oxidation of hydrazine (N2H4) to nitrogen gas (N2) catalyzed by hydrazine oxidizing enzyme (HZO) (Eq (4)).
NO2‾ + 2H⁺ + e → NO + H2O (E0' = +0.38 V) (2)
NO + NH4+ + 2H+ + 3e → N2H4 + H2O (E0' = +0.06 V) (3)
N2H4 → N2 + 4H+ + 4e (E0' = -0.75 V) (4)
NO2‾ → NO¯ + 2H+ +2e (E0' = +0.42 V) (5)
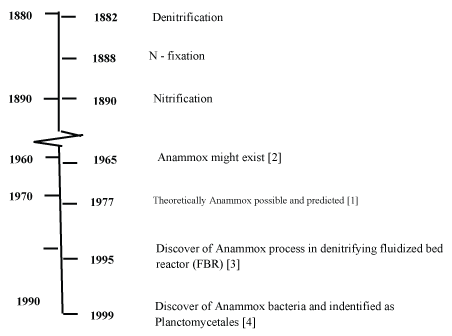
Until now five genomes have been identified: 'Candidatus Brocadia', 'Candidatus Kuenenia', 'Candidatus Scalindua', 'Candidatus Anammoxoglobus’ and 'Candidatus Jettenia'. Together, they form the monophyletic order Brocadiales that branches deeply in the phylum Planctomycetes [11]. Timeline of discovery of Anammox and Electro-dense Anammoxosome particles containing iron are illustrated in Figure 2.
Conventional nitrification/denitrification vs. Anammox process
Before the discovery of Anammox process and Anammox bacteria, conventional nitrification/denitrification process was one of the milestones in nitrogen removal technology that helped to prevent environmental disasters such as eutrophication and hypoxia. However, there are several drawbacks in conventional nitrification/denitrification process in treating nitrogen rich wastewater include: i) Requirement of aeration for conversion of NH4+ to NH3- in autotrophic nitrification process, ii) Heterotrophic denitrification process requires external carbon source (e.g., electron donor) such as methanol (in majority cases), that ultimately derived from fossil fuel and iii) Both process release N2O, which is the primary cause of ozone depletion [16] and iv) Produce large amount of sludge. In addition, besides being a novelmethod, Anammox process are environmentally friendly and cost effective compared to conventional nitrification/denitrification. Anammox directly convert ammonium, NH4+ (electron donor) and nitrite, NO2- (electron acceptor) to nitrate, NH3- and dinitrogen gas, N2 without both oxygen and organic carbon.
Genera and Species of Anammox
Ten species of Anammox bacteria had been identified (Table 2) based on culture dependent methods and 16S rRNA analysis, which belong to five genera which together form the order of Brocadiales, branching deeply in the bacteria phylum Planctomycetes. Among five genera of Anammox bacteria, Brocadia, Kuenenia, Anammoxoglobus, and Jettenia are belong to fresh water species while Scalindua belong to marine species [17].
Morphology of anammox and Anammoxosome
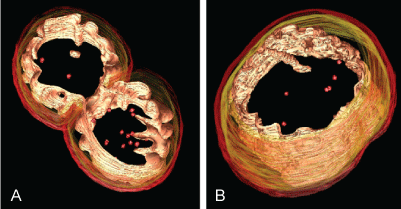
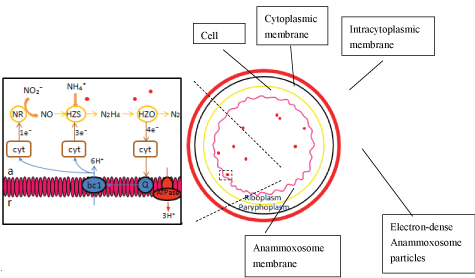
In Anammox cell, there are three compartments divided by bilayer membrane; they are from inside to the outside: The Anammoxosome, riboplasm and paryphoplasm [18]. While the function of paryphoplasm remains unknown [19]. Inside riboplasm, there isribosomes and nucleoid and the role of riboplasm is similar to the cytoplasm of other bacteria, in which the translation and transcription will take place. The mechanism that sorted and transports the protein synthesized in riboplasm to other compartment remain unknown [20]. Anammoxosome is a membrane-bounded intracytoplasmic compartment in Anammox bacteria, with the majority of its membrane in a curved configuration as shown in Figure 3. Anammoxosome occupied most of the volume of Anammox cell [21], and the catabolism metabolism of Anammox bacteria are assumed to take place inside this compartment [20]. The researcher had discovered the existence of the iron-containing electron-dense Anammoxosome particles in the Anammoxosome compartment [21]. The storage of iron inside Anammoxosome compartment was for the excess supply of iron for further Heme c synthesis [22].
Effect of Heavy Metals on Anammox Bacteria
Stimulation effect of heavy metals on Anammox
It was found that the existance of limited amount of several heavy metals during the cultivation of Anammox cell will enhance anammox activity [23]. The common trace element solutions addded to substrate of Anammox bioreactor (1.25 ml/L) contained EDTA (15 mg/L), FeSO4•7H2O (5 mg/L), NiCl2•6H2O (0.05 mg Ni/L), CuSO4•5H2O (0.06 mg Cu/L), CoCl2•6H2O (0.06 mg Co/L), ZnSO4•7H2O (0.10 mg Zn/L), NaMoO4•2H2O (0.10 mg Mo/L), MnCl2•4H2O (0.28 mg Mn/L), NaSeO4•10H2O (0.05 mg Se/L), and H3BO3 (0.014 mg/L) [24]. Heavy metal ions can enhance Anammox activity through stimulating the metabolism of Anammox cell by the fact that those metal ions can either be the component of many enzymes or the co-enzyme. Both molybdenum and copper are vital constituents of enzyme associated with the catabolism of Anammox cell, such as nitrite oxidoreductase [25] and nitrite reductase [23]. Besides that, enzyme of Anammox bacteria such as nickel-dependent hydrogenase [23] zinc-containing dehydrogenase [24] and ATP-dependent zinc metalloprotease Fts Hare also reliant on heavy metal. When starvation for those required heavy metal occurs, specific metal transport can be induced via different tranporter families suah as those driven by ATP.
In addition, Fe (II) is one of the essential nutrients for growth of Anammox. Since the discovery of Anammox process by Van de Graff, et al. [26], the Fe (II) concentration was set as 0.03 mM or 0.04 mM in most of the feeding medium of enriched Anammox sludge system. Energy dispersive x-ray (EDX) analysis revealed that several electron-dense Anammoxosome particles contained iron. In which the possible function of these Anammoxosome particles are for energy generation and as an iron storage facility for the heme-c enzyme involved in electron transport chain [21]. Therefore, Fe (II) can be the potential factor to affect the growth and activity of Anammox bacteria. Electron-dense An anammoxosome particles containing iron was found within the Anammoxosome compartment of Anammox [21]. Fe (II) is an essential nutrient for Anammox as it helps synthesize of heme c-containing enzyme of Anammox by forming the active region of heme c-containing enzyme. Hydrazine synthase (HZS), also named hydrazine hydrolase (hh) and hydrazine oxidizing enzyme (HZO) also called hydroxylamine oxidoreductase (hao), or hydrazine dehydrogenase (HDH) are two of the heme c-containing enzymes. In HZS enzyme catalyze formation of hydrazine (N2H4), the intermediates of Anammox from ammonia (NH4⁺) and nitric oxide (NO), while hydrazine oxidizing enzyme (HZO) helps in the formation of dinitrogen gas (N2) by oxidizing hydrazine, at the same time providing electrons needed for hydrazine synthase and nitrite reduction Figure 4. shows the morphology of Anammox cell and model for catabolic Anammox reaction.
Since 2013, the researcher had evaluated the effect of different concentration of Fe (II) on Anammox. Liu, et al. [22] had study the relationship between the effect of various Fe (II) concentration and the growth rate of Anammox by batch test, and they found that growth rate (i.e. 0.172 d‾1) was maximum at 0.09 mM of Fe (II). Similarly, Zhen, et al. [27] had investigated Anammox start-up period and they found that the shortest start-up (i.e. 50 d) was attained at 0.09 mM of Fe (II). Sen, et al. [28] investigated the total nitrogen (TN) removal percentage by batch test at different concentrations of Fe (II), and they found that the maximum total nitrogen removal percentage (63%) was observed at 0.09 mM of Fe (II) concentration. From the above studies, it can be presumed that 0.09 mM Fe (II) concentration has the most positive effect on Anammox.
The inhibition factors of heavy metal on Anammox bacteria
After the Anammox process and Anammox bacteria had been discovered, researcher from around the world have been tried to study Anammox due to the advantages of low cost and its capability of removing high ammonium nitrogen wastewater when comparing to conventional nitrification/denitrification process [29]. The Anammox process have been successfully applied from the lab-scale reactor to full-scale treatment plant to treat ammonium or nitrogen rich wastewater since the last two decades [30]. On the other hand, the application and operation of Anammox process in treating real wastewater can be restricted by few factor such as the slow growth rate of Anammox cell and the present of some inhibition factor in wastewater such as heavy metals, substrate, etc.
The inhibitory effects of heavy metals may show great variations in synthetic wastewater, contaminated wastewater and natural environment based on the type and concentration [31].
Toxicity occurs when microorganism's uptake excess amount of heavy metals or metal partitioning through extracellular sorption, transmembrane transport, and intracellular accumulation [10,32,33]. Depending on the viability of the biomass and concentrations of the heavy metals, the transmembrane transport can active, passive or both. The toxicity of heavy metals towards Anammox cell was through their bioaccumulation in cells. When there is present of heavy metals, diffusion of metals will occur across the outer wall of Anammox bacteria through porins and subsequently enter transport across the cytoplasmic membrane in various ways. When the metal ions are inside the Anammox cell, they can interact with both nucleic acids, enzyme active sites and lead to a rapid decline in membrane integrity, which is usually demonstrated as leakage of mobile cellular solutes and cell death.
Zhen Bi, et al. [27] had investigated the inhibition effects of Cd, Ag, Hg and Pd on Anammox activity. Result of the study illustrated that deterioration of crude enzyme activity occurred due to the accumulation of heavy metals inside Anammox cell, which eventually lead to the decrease of nitrogen removal rate of the Anammox system. Furthermore, the heme c concentration also susceptible to short-term exposure to heavy metals. Therefore, investigations are necessary for the comprehend of the inhibitors so that the inhibition effect can be minimize at the same time improve Anammox process.
Specific Anammox Activity (SAA) Test
According to Daverey, et al. [34] the effect of heavy metals on Anammox bacteria is more prominent when the biomass concentration is low (< 2000 mg-MLVSS L-1). Most of the specific anammox activity (SAA) tests perform according to Dapena-Mora, et al. [35] methods. The production of N2 gas is normally tracked bymeasuring the overpressure in the headspace with a time-frequency depending on the biomass activity in each batch test.
Evaluation of SAA
The total amount of N2 gas produced is normallycalculated from the overpressure measured in the headspace of each serum bottle at the end of the assay by using the ideal gas law equation. The N2 gas production rate, dN2/dt can be calculated from the maximum slope of the curve describing the pressure increase in the vial along the time (α) (Eq. (6)) [35]
α = slope of pressure increase in the bottle along the time (atm)
VG = volume of gas phase (0.01 L)
R = ideal gas constant 0.0820575 (atm L mol-1 K-1)
T = temperature (K)
Then, SAA determined by dividing the N2 gas production rate, dN2/dt by the concentration of biomass in the serum bottle, X (g VSS L-1) (Eq. (7)) [35]
28 = molecular weight of N2 (g N/mol)
24 = unit conversion factors from hour to days
X = biomass concentration in the bottle (g VSS L-1)
VL = volume of liquid phase in the bottle
Percentage of activity increase and IC50
Comparison had been done between inhibition effect, IC50 inhibition concentration of various heavy metals on Anammox bacteria based on specific anammox activity (SAA) as shown in Table 3. The inhibition effects of nine various heavy metals had been compared included ferrous ion (Fe2+), Cadmium (Cd2+), Copper (Cu2+), Lead (Pb2+), Mercury (Hg2+), Molybdate (MoO42+), Nickel (Ni2+), Silver (Ag+) and Zinc (Zn2+) respectively. Among the nine heavy metal, Lead ion, Pb2+ has the lowest inhibition effect on Anammox process, in which even 75 mg/L of Lead ion do not cause any inhibition effects on Anammox bacteria, while copper ion, Cu2+ might be having the most inhibition effect on Anammox with the IC50 inhibition concentration ranged start from 1.9 mg/L. The IC50 inhibition concentration of Fe (II) was found to be approximately 0.20 mM, which, after conversion is equal to 55.6 mg/L [36]. Thus, from the comparison, the inhibition effects of Fe (II) on Anammox process is not as intense as most of the heavy metals tested and the inhibition effects of Fe (II) was found lower than Cadmium (Cd2+), Copper (Cu2+), Nickel (Ni2+), Silver (Ag+) and Zinc (Zn2+).
Recovery
Achlesh, et al. [37] had studied the long term effect of zinc on SNAD system of Anammox bacteria, the system was able to recovered the nitrogen (total nitrogen and ammonium nitrogen) removal efficiency to around 90% after inhibited by 20 mg/L of zinc which had reduce the nitrogen (total nitrogen and ammonium nitrogen) removal efficiency to around 70%, due to the fact that the microbial communities in the reactor were well acclimated. In the study of Kimura and Isaka [38] a continuous Anammox bioreactor (lab-scale) with gel-carrier had been operated to investigate the effects of Ni, Cu, Co, Zn and Mo on Anammox activity. It was demonstrated that high concentrations of those heavy metal is inhibitory to Anammox cell, however the effects were reversible. Yet, the inhibition effect of Mo on Anammox cell was irreversible. Therefore, it is suggested that extra attention should be paid to Mo concentrations in the wastewater subject to Anammox bacteria. In addition, Anammox activity and performance subjected to copper inhibition for long time (almost 200 days) were restorable and the recovery process lasted for short time (nearly 50 days) due to the accumulation of Anammox cell [39].
Conclusion
Anammox process has become essential in treating ammonium rich wastewater due to its multiple advantages compared to the conventional nitrification/denitrification process. Anammox process is a promising method for treating nitrogen rich and low COD content wastewater. The presence of heavy metals in wastewater can either affect stimulation, inhibition or even toxic in biochemical reactions. It was found that heavy metal ions can improve Anammox activity through stimulating the metabolism of Anammox cell as those metal ions can either be the component of many enzymes or the co-enzyme. Nevertheless, toxicity occurs when microorganism uptake extra quantity of heavy metal through extracellular sorption, transmembrane transport, and intracellular accumulation. In this review, IC50 inhibition concentration of various heavy metals on Anammox bacteria based on SAA. Based on literature review, it was found that Lead ion, Pb2+ has the lowest inhibition effect, in which even 75 mg/L of Pb2+ do not cause any inhibition effects on Anammox bacteria, while copper ion, Cu2+ might be having the most inhibition effect on Anammox with the IC50 inhibition concentration of 1.9 mg/L. The IC50 of Fe (II) was found at 55.6 mg/L. It can be concluded that that the high concentrations of those heavy metal has inhibitory effect on Anammox cell, on the other hand the effects are generally reversible.
Acknowledgements
Our sincere acknowledgement goes to the financial support of National Chiao Tung University. Taiwan and Universiti Tunku Abdul Rahman, Malaysia by allowing the completion of this study under UTAR research fund "IPSR/RMC/UTARRF/2016-C2/M01".
References
- Broda E (1977) Two kinds of lithotrophs missing in nature. Z Allg Mikrobiol 17: 491-493.
- Richard FA (1965) Anoxic basins and Fjords. Chemical Oceanography 1: 611-643.
- Mulder A, Van de graaf AA, Robertson LA, et al. (1995) Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor. FEMS Microbiology Ecology 16: 177-183.
- Strous M, Fuerst JA, Kramer EH, et al. (1999) Missing lithotroph identified as new Planctomycete. Nature 400: 446-449.
- Op den Camp HJ, Kartal B, Guven D, et al. (2006) Global impact and application of the anaerobic ammonium-oxidizing (anammox) bacteria. Biochem Soc Trans 34: 174-178.
- Arrigo KR (2005) Marine microorganisms, and global nutrient cycles. Nature 437: 349-355.
- Chamchoi N, Nitisoravut S (2007) Anammox enrichment from different conventional sludges. Chemosphere 66: 2225-2232.
- Terada Akihiko, Sheng Zhou, Masaaki Hosomi (2011) Presence and detection of anaerobic ammonium oxidizing (Anammox) bacteria and appraisal of Anammox process for high-strength nitrogenous wastewater treatment: a review. Clean Technologies and Environmental Policy 12: 759-781.
- Jin RC, Yang GF, Yu JJ, et al. (2012) The inhibition of the Anammox process: a review. Chemical Engineering Journal 197: 67-79.
- Van Loosdrecht MC, Salem S (2006) Biological treatment of sludge digester liquids. Water Sci Technol 53: 11-20.
- Jetten MSM, Op den Camp H, Kuenen GJ, et al. (2010) Bergey's Manual of Systematic Bacteriology. (2nd edn), Springer, New York, 4: 918-926.
- Horan NJ, Gohar H, Hill B (1997) Application of a granular activated carbon biological fluidized bed for the treatment of landfill leachate containing high concentrations of ammonia. Water Science and Technology 36: 369-375.
- Wang XH, Gai LH, Sun XF, et al. (2010) Effects of long- term addition of Cu(II) and Ni(II) on the biochemical properties of aerobic granules in sequencing batch reactors. Appl Microbiol Biotechnol 86: 1967-1975.
- Kieu HT, Muller E, Horn H (2011) Heavy metal removal in anaerobic semi continuous stirred tank reactors by a consortium of sulfate-reducing bacteria. Water Res 45: 3863-3870.
- Kartal B, Maalcke WJ, De Almeida NM, et al. (2011) Molecular mechanism of anaerobic ammonium oxidation. Nature 479: 127-130.
- Ravishankara AR, Daniel JS, Portman RW (2009) Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326: 123-125.
- Jetten MS, Niftrik Lv, Strous M, et al. (2009) Biochemistry and molecular biology of anammox bacteria. Crit Rev Biochem Mol Biol 44: 65-84.
- Van Teeseling MC, Neumann S, Van Niftrik L (2013) The Anammoxosome organelle is crucial for the energy metabolism of anaerobic ammonium oxidizing bacteria. J Mol Microbiol Biotechnol 23: 104-117.
- Van Niftrik L (2013) Cell biology of unique anammox bacteria that contain an energy conserving prokaryotic organelle. Antonie Van Leeuwenhoek 104: 489-497.
- Van Niftrik L, Jetten MS (2012) Anaerobic ammonium-oxidizing bacteria: unique microorganisms with exceptional properties. Microbiol Mol Biol Rev 76: 585-596.
- Van Niftrik L, Geerts WJ, Van Consular EG, et al. (2008) Combine structure and chemical analysis of the anammoxosome: a membrane-bounded intracytoplasmic compartment in anammox bacteria. J Struct Biol 161: 401-410.
- Liu Yi, Ni Bing Jie (2015) Appropriate Fe (II) addition significantly enhances anaerobic ammonium oxidation (Anammox) activity through improving the bacteria growth rate. Sci Rep 5: 8204.
- Hira D, Toh H, Migita CT, et al. (2012) Anammox organism KSU-1 expresses a Nir K-type copper-containing nitrite reductase instead of a Nir S-type with cytochrome cd1. FEBS Lett 586: 1658-1663.
- Strous M, Pelletier E, Mangenot S, et al. (2006) Deciphering the evolution and metabolism of an anammox bacterium from a community genome. Nature 440: 790-794.
- Kartal B, Van Niftrik L, Rattray J, et al. (2008) Candidatus 'Brocadia fulgida': an autofluorescent anaerobic ammonium oxidizing bacterium". FEMS Microbiol Ecol 63: 46-55.
- Van de Graaf AA, de Bruijn P, Robertson LA, et al. (1996) Autotrophic growth of anaerobic ammonium-oxidizing microorganisms in a fluidized bed reactor. Microbiology 142: 2187-2196.
- Zhen Bi, Qiao S, Zhou J, et al. (2014) Fast start-up of Anammox Process with appropriate ferrous iron concentration. Bioresour Technol 170: 506-512.
- Sen Qiao, Zhen Bi, Jiti Zhou, et al. (2013) Long-term effects of divalent ferrous ion on the activity of anammox biomass. Bioresource Technology 142: 490-497.
- Van Hulle SWH, Vandeweyer HJP, Meesschaert BD, et al. (2010) Engineering aspects and practical application of autotrophic nitrogen removal from nitrogen rich streams. Chemical Engineering Journal 162: 1-20.
- Shalini SS, Joseph K (2012) Nitrogen management in landfill leachate: application of Sharon, Anammox and combined Sharon-Anammox process. Waste Manag 32: 2385-2400.
- Lars L, Rudolf R (2004) Metals in society and in the environment: a critical review of current knowledge on fluxes, speciation, bioavailability and risk for adverse effects of copper, chromium, nickel and zinc. Environmental Pollution 8: 407.
- Chang D, Fukushi K, Ghoosh S (1995) Stimulation of activated sludge cultures for enhanced heavy metal removal. Water Environ Res 67: 822-827.
- Christopher W, Simon CW, Geoffrey MG (1995) The role of microorganisms in biosorption of toxic metals and radionuclides. International Biodeterioration and Biodegradation 35: 17-40.
- Daverey A, Chen YC, Liang YC, et al. (2014) Short-term effects of monoethanolamine and copper on the activities of Anammox bacteria. Sustain Environ Res 24: 325-331.
- Dapena Mora A, Ferenandez I, Campos JL, et al. (2007) Evaluation of activity and inhibition effects on Anammox process by batch tests based on the nitrogen gas production. Enzyme and Microbial Technology 40: 859-865.
- Mak CY, Lin JG, Bashir MJK, et al. (2017) Short term effect of Fe (II) on anammox activity. 2nd International Symposium on Green and Sustainable Technology (ISGST 2017), Kampar, Malaysia, 10-13.
- Achlesh D, Yi-Chian C, Shihwu S, et al. (2014) Effect of zinc on anammox activity and performance of simultaneous partial nitrification, anammox, and denitrification (SNAD) process. Bioresource Technology 165: 105-110.
- Kimura Y, Isaka K (2014) Evaluation of inhibitory effects of heavy metals on anaerobic ammonium oxidation (anammox) by continuous feeding tests. Appl Microbiol Biotechnol 98: 6965-6972.
- Zhang Qian-Qian, Yang Guang-Feng, Wang Hui, et al. (2013) Estimating the recovery of ANAMMOX performance from inhibition by copper (II) and oxytetracycline (OTC). Separation and Purification Technology 113: 90-103.
- Stankovic V, Bozic D, Gorgievski M, et al. (2009) Heavy metal ions adsorption from mine waters by sawdust. Chemical Industry and Chemical Engineering Quarterly 15: 237-249.
- Subbiah RM, Sastry CA, Agamuthu P (2000) Removal of zinc from rubber thread manufacturing industry wastewater using chemical precipitant/flocculant. Environmental Progress & Sustainable Energy 19: 299-304.
- Davis AP, Shokouhian M, Ni S (2001) Loading estimates of lead, copper, cadmium and Zinc in urban runoff from specific sources. Chemosphere 44: 997-1009.
- Santos A, Barton P, Cartmell E, et al. (2010) Fate and behavior of copper and zinc in secondary biological wastewater treatment processes: II Removal at varying sludge age. Environ Technol 31: 725-743.
- Kjeldsen P, Barlez MA, Rooker AP, et al. (2002) Present and long-term composition of MSW landfill leachate: a review. Critical Reviews in Environmental Science and Technology 32: 297-336.
- Renou S, Givaudan JG, Poulain S, et al. (2008) Landfill leachate treatment: review and opportunity. Journal of Hazardous Materials 150: 468-493.
- Nicholson FA, Chambers BJ, Williams JR, et al. (1999) Heavy metal contents of livestock feeds and composition of MSW landfill leachate: a review. Crit Rev Environ Sci Technol 32: 297-336.
- Nicholson MB, Szogi AA, Hunt PG, et al. (2007) Development of environmentally superior treatment system to replace anaerobic swine lagoons in the USA. Bioresour Technol 98: 3184-3194.
- Oshiki M, Shimokawa M, Fujii N, et al. (2011) Physiological characteristics of the anaerobic ammonium-oxidizing bacterium 'Candidatus Brocadia sinica'. Microbiology 157: 1706-1713.
- Schmid M, Walsh K, Webb R, et al. (2003) Candidatus "Scalindua brodae", sp. nov., Candidatus "Scalindua wagneri", sp. nov., two new species of anaerobic ammonium oxidizing bacteria". Syst Appl Microbiol 26: 529-538.
- Woebken D, Lam P, Kuypers MM, et al. (2008) A microdiversity study of anammox reveals a novel Candidatus Scalindua phylotype in marine oxygen minimum zones. Environ Microbiol 10: 3106-3119.
- Van de Vossenberg J, Woebken D, Maalcke WJ, et al. (2013) The metagenome of the marine anammox bacterium 'Candidatus Scalindua profunda' illustrates the versatility of this globally important nitrogen cycle bacterium. Environ Microbiol 15: 1275-1289.
- Kartal B, Rattray J, Van Niftrik LA, et al. (2007) Candidatus "Anammoxoglobus propionicus" a new propionate oxidizing species of anaerobic ammonium oxidizing bacteria". Syst Appl Micrbiol 30: 39-49.
- Quan ZX, Rhee SK, Zuo JE, et al. (2008) Diversity of ammonium-oxidizing bacteria in a granular sludge anaerobic ammonium-oxidizing (anammox) reactor. Environ Microbiol 10: 3130-3139.
- Hu BL, Rush D, Van der Biezen E, et al. (2011) New anaerobic, ammonium-oxidizing community enriched from peat soil. Appl Environ Microbiol 77: 966-971.
- Li H, Zhou S, Ma W, et al. (2014) Long-term performance and microbial ecology of a two-stage PN-ANAMMOX process treating mature landfill leachate. Bioresour Technol 159: 404-411.
Corresponding Author
Mohammed JK Bashir, Faculty of Engineering and Green Technology (FEGT), Universiti Tunku Abdul Rahman, 31900 Kampar, Perak, Malaysia, Tel: 605-4688888, Fax: 605-4667449.
Copyright
© 2018 Mak CY, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.