Landslide Effect on an Oribatid-Mite Community in a Monsoon Forest
Abstract
Landslide-caused erosion of soil alters above ground plant cover and primary production, and is a major disturbance in forests and agro ecosystems in Taiwan. Bimonthly litter collections were conducted for one year, with oribatid mites studied at landslide sites and on the undisturbed forest floor. We hypothesized that landslide soil erosion would decrease oribatid-mite abundance and diversity and alter its community structure compared with control site. Eighty-three oribatid morphospecies belonging to 47 families were sampled, with higher density of oribatid mites in the landslide site, and clear correlation with rainfall. In landslide areas, the density of total adult and juvenile oribatid mites was significantly higher in the wet than dry season. Contradictory to our hypothesis, the diversity of oribatid-mite morphospecies was not significantly different between seasons and sampling sites. Density of Peloribates sp. and Scheloribates sp. was dominant in both sampling sites and seasons. Density of Peloribates sp. in the control sites was 2.9- and 2.3- fold higher than in landslide sites in dry and wet seasons, respectively. Density of Scheloribates sp. was 2.8- and 4.6- fold higher in the landslide than control site in dry and wet seasons, respectively. The exact mechanisms controlling oribatid-mite community structure were not yet determined, but a predictable trend was found for the effects of rainfall and landslide soil erosion on total oribatid-mite density and morphospecies number. A natural process such as a landslide is known to have a devastating effect on the aboveground community, but for the belowground soil biota, it could trigger new trophic organization.
Keywords
Disturbance, Microarthropod, Soil erosion, Subtropical forest, Taiwan
Introduction
Soil erosion caused by landslide frequently occurs in terrain areas where the anthropogenic factors play an important role. The phenomenon is widespread and adversely affects all agricultural and forestry ecosystems in Taiwan, as well as in many other parts of the world [1]. As a result of this nature-driven destructive force, the triggering of soil erosion ranks among the most significant environmental problems in the world [2-4].
Erosion rates are found to be relatively lower in forest ecosystems, with a relatively high vegetation cover, than in agro- and pasture ecosystems [5,6], where loss of vegetation cover is high due to inadequate use of crop residue [7].
Diverse soil biotas play a major role in nutrient supply, affecting the diversity of above and belowground primary productivity. They also facilitate soil formation and as a result of erosion will affect soil quality in agro- and forestry ecosystems. Odum [8] elucidated the importance of vegetation as the main component of ecosystem biomass and demonstrated that it provides the resources needed by the biotic components: the microflora, micro, meso and macrofauna [9]. Moreover, Lee and Foster [10] and Condron [11] showed that one square meter of productive soil can support about 200,000 individual arthropods and enchytraeids and billions of soil bacteria and fungi. Since an increase in biodiversity is generally correlated with productivity and environmental components, it is logical to assume that soil erosion may decrease the diversity and abundance of soil micro and mesofauna and microflora organisms.
Soil microarthropods are known to be among the most dynamic soil-biotic populations. They are sensitive to abiotic fluctuation, soil quality and plant cover [12,13]. Numerous studies have described the effect of plant cover as one of the factors enhancing the soil microarthropod population [14-20], changes that are mainly due to plant organic-matter contribution to the soil. Studies on soil arthropod populations in tropical forests found that density and diversity are correlated to the litter depth, soil organic matter and C/N ratio [21,22].
Our overall objective was to determine the density and diversity of an oribatid-mite community in forest litter in samples collected at a landslide site of one of the most widely dispersed natural phenomena in Taiwan. Based on the above, we hypothesize that the landslide will strongly affect litter-dwelling oribatid-mite density and diversity, with a significant change in the Shannon-Wiener diversity index (H') in samples collected during the wet, high-rainfall period.
Materials and Methods
Study site
The study site was located in the Nanjenshan Nature Reserve subtropical deciduous forest (22°02'51''N, 120°51'37''E, 300-500 m above sea level), in Taiwan, with a slope approximately 9° to 12°. The mean annual temperature is 22.3 ℃ with the highest mean temperature of 26.5 ℃ in the summer (July) and the lowest of 17.7 ℃ in the winter (January). The mean annual rainfall is 3310 mm, with 75% of the precipitation (including large amounts of rainfall carried by tropical cyclones) occurring in summer (May to August) and fall (September-November). The northeast monsoon winds, which bring some precipitation in the winter, prevail from October to late February, therefore, there are generally two distinct dry (February, April and June) and wet seasons (August, October and December) throughout the year. The prevailing monsoon winds cause chronic stress to local vegetation and result in a large cumulative effect on forest structure and plant species composition [23].
The soil at the study site is an ultisol (red clay composition), its depth ranges from 60 to 130 cm, with clay texture on top and clay loam beneath. Soil organic-matter content is low, and the pH value is lower than 4.5 [24]. The litter layer that covers the forest floor is usually less than 5 cm, where the vegetation is dominated by Bischofia javanica, Michelia compressa, Castanopsis indica and Machilus kusanoi trees [25].
Rainfall determination
Rainfall was measured using the tipping bucket automated precipitation gauge, by which output was recorded by an automatic counter that showed the accumulated precipitation from each precipitation event.
Litter collection
We selected three sites where intermediate landslide perturbations occurred in December 2002, with distances of more than 500 m between each site. The landslide plots were selected first and a control plot adjacent to the landslide plot was set up later. Three pairs of control and landslide plots were randomly selected and at each one of the sampling sites at the Nanjenshan Natural Forest Reserve, random samples were collected.
The landslide event occurred less than two years before the study was initiated, with a disturbance that covered an area of over 100 m2. From each plot, litter samples (n = 3) were collected from an area of 30 × 30 cm, placed in individual plastic bags, and placed in a cooler in order to avoid overheating during transportation to the laboratory.
Litter samples were collected in the first week of each sampling period, once every two months (a total of 6 times) between February 2003 and December 2003, from each plot. One hundred grams litter was extracted by Tullgren funnels over a period of 7 days in order to determine the litter fauna. The extracted fauna were kept in vials containing 75% ethanol at room temperature throughout faunal taxonomical identification.
Oribatid mites were identified under the Leica MZ 125 dissecting microscope or the Nikon Eclipse 50i light microscope. The specimens were identified to the genus level and then sorted into morphospecies according to Balogh and Balogh [26]. Juveniles with distinct structures present in the samples were classified into morphospecies. Juveniles lacking distinguishable characteristics were lumped into one group. The litter from the extraction funnels was later used for dry mass determination by oven-drying at 75 ℃ for 48 hours.
Statistical analysis
All data obtained during the study period were subjected to General Linear Model (GLM) analysis using the Statistical Analysis System (SAS) model. Duncan's Multiple Range Tests (MRT) was used to determine differences between separate means. Significance was defined at a level of P < 0.05. A paired t-test analysis for comparison of densities in the control and landslide plots was conducted. The Shannon-Wiener diversity index (H') was used to determine the effect of abiotic and site parameters on the oribatid community. All the above-mentioned procedures were performed with the Minitab v.14 software (Minitab Inc., State College, PA).
Redundancy Analysis (RDA) was used to relate environmental factors, such as rainfall and landslide treatment with the density (total, only adults and only juveniles) and diversity (H') of litter-dwelling oribatid mites [CANOCO, version 4.54; [27]. RDA analysis produced graphic illustrations, with arrows connecting the different components to their corresponding measured parameters. Arrows pointing in the same direction indicate a positive correlation, while arrows pointing in opposite directions indicate a negative one. The longer the arrow, the greater the significance of the relationship.
Results
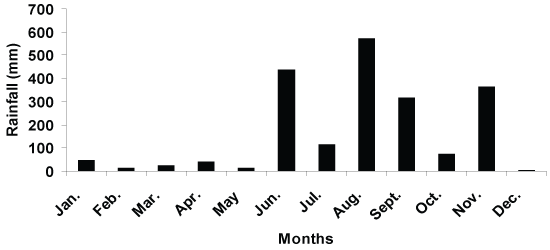
A total amount of rainfall of 2016.8 mm was recorded during the study period, with 71% falling in June-September (Figure 1). However, a total amount of 1008 mm rainfall was recorded during the litter sampling occasion of June and August, and this sampling occasion was, therefore, defined as a wet season. In the sampling occasion of February, April, October and December, the monthly rainfall ranged between 4.1 to 72.9 mm, with a mean of 32.5 mm per month. Based on the significant difference (p < 0.005) in rainfall distribution during the sampling occasion, data analysis had to be taken into account and the data were obtained as two main groups: (a) wet and (b) dry season.
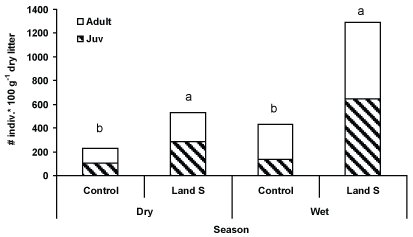
Total mean density of oribatid mites in forest litter reached a mean maximal value of 530 and 1290 individuals in 100 g of dry litter for the dry and wet seasons, respectively, at the landslide sampling sites (Figure 2). The total number of oribatid-mites at the control site reached 43% and 34% of the quantities found in the landslides during the dry and wet season, respectively. The difference between the two sampling sites was significant at a level of p < 0.001 for each of the seasons (Figure 2). The paired t-test results (Table 1) showed a significant (P < 0.004) difference in oribatid-mite density between the control and landslide, where the significant sampling occasions were February (p < 0.049) and December (p < 0.013). Moreover, significant (p < 0.001) seasonal and sampling effects were obtained (Table 2). Patterns similar to the above were obtained in adult and juvenile communities, where significant differences in seasonal and sampling site effects were found in adult and juvenile oribatid mites and no significant interaction effects were found (Table 1).
A total of 83 oribatid morphospecies belonging to 47 families were found. The Shannon-Wiener diversity index (H') was calculated for each soil-sample community. In the wet season, the Shannon-Wiener diversity index (H') of the oribatid community from the control and landslide sites was 2.96 and 1.97, respectively. In the dry season, the index was 2.82 at the control site and 2.65 at the landslide site. No significant diversity differences were detected between seasons (p = 0.139) or between sampling sites (p = 0.143). However, one morphospecies of the genus Scheloribates, which belongs to Scheloribatidae, reached a maximal value of 302 per 100 g dry litter, and was present in all samples during the study period (Table 3). Calculating the percentage of each individual morphospecies from the total population, we found that between 36 and 41 morphospecies represent a maximum of 1% of the total number of individuals in each sample. The percentage of individual morphospecies in each sample increased from 1% to 5%, with a decrease in the number of morphospecies to a value between 10 and 21 morphospecies for the wet landslide and dry control, respectively. Only four morphospecies were found to reach over 10% of the total individuals for the dry control, dry landslide, wet control and wet landslide litter samples.
Peloribates sp. 1 and Scheloribates sp. 1 were common in the control and landslide sampling sites in both the dry and wet seasons. Peloribates sp. 1 was found to have 2.9- and 2.3- fold higher densities in the control sampling sites than in the landslide sampling sites during the dry and wet seasons, respectively. Scheloribates sp. 1 was 2.8- and 4.6- fold higher in the landslide sites than in the control sites in the dry and wet seasons, respectively (Table 3). Lamellobates sp. had a high density (53 individuals per 100 g dry litter) only in the control wet site, followed by 1 individual per 100 g-1 dry litter in the dry landslide litter samples, whereas it was absent in the two remaining sampling sites (Table 3).
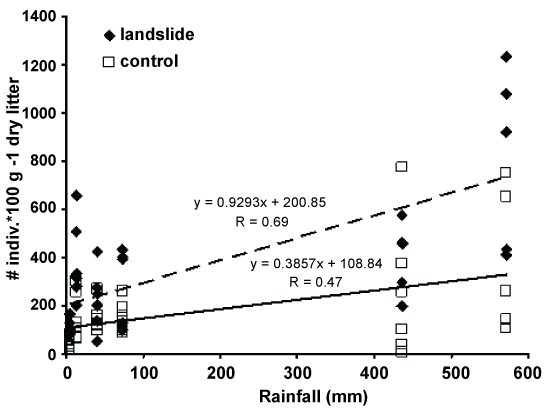
Based on the above classification, the correlation between the total numbers of oribatid mites in each sample at each sampling site to the total monthly amount of rainfall was determined (Figure 3). The results indicated a convincing correlation between the number of individuals and rainfall in the landslide sampling sites, reaching an r value of 0.69, whereas the r value was 0.47 in the control sampling site. Moreover, running a multiple linear regression to test the effect on both variables (control and landslide), a significant (p = 0.0109) difference between the two the slopes was found, elucidating the different effects of rainfall on each of the variables.
RDA analysis
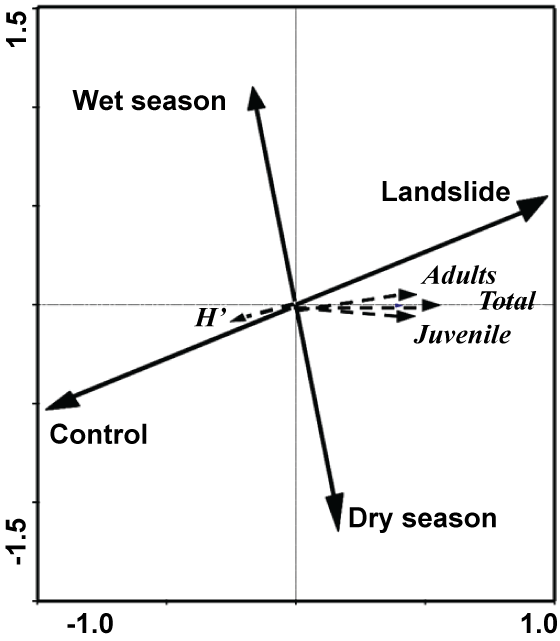
In integrating all the data using RDA, we found that rainfall is one of the main triggers affecting moisture availability. It enhances aboveground primary production and, together with the litter sampling locations, has a major effect on oribatid-community components (Figure 4). The arrow representing moisture availability (wet season) and the landslide arrow are of similar lengths, with an orientation opposite that of the dry season and control, respectively. The total oribatid community is clearly defined-bordered between the control and the landslide sampling site. The Shannon-Wiener diversity index (H') values indicated a temporal and spatial effect strongly related to the control site, contrary to the oribatid-population densities.
Discussion
During the present study, litter samples were collected from a disturbed landslide site and an undisturbed control site over a period of one year. In the course of the study period, oribatid mites increased at the family and morphospecies levels, along with one of the most frequent anthropogenic effects in the southern part of Taiwan.
Landslide erosion, one of the most well-known soil-losing phenomena as a result of rain and wind, changes the soil nutrient levels and organic matter by redistribution, thus providing new microhabitats for fast-growing communities. Pimentel and Kounang [1] and Restrepo, et al. [28] found significant alteration in land cover that can be related to distribution and increases in biomass, which are correlated with an increase in biodiversity. Landslide perturbation will enhance the opposite trend, although new microhabitats will be formed. Our results showed a similar pattern in oribatid-mite diversity in the undisturbed control site in both dry and wet seasons. However, the abundance of oribatid mites was 2-3-fold higher in the landslide site than in the control site, which may indicate higher biomass and was contrary to the findings of Pimentel and Kounang [1]. Accordingly, it can be further hypothesized that landslide perturbation has an impact on the development and composition of microbial communities that will serve as a food source for the microarthropod community.
Badejo, Erdmann, et al. and Wehner, et al. [29-32] explained higher reproductive activities after disturbances which he found by age structure differences between forest and disturbed plots. Forest plots had more adults than juveniles, whereas disturbed plots had more juveniles. Norton and Palmer [33] reported that morphospecies belonging to the Trhypochthoniidae family were completely parthenogenetic, whereas in our study, juvenile representatives of this family were present, suggesting a higher reproductive rate of parthenogenetic morphospecies in the landslide site. This phenomenon could be based on the reproductive cycle, in which an unfertilized egg develops into a new individual, known as a time- and energy-saving process, and which is similar to Maraun’s [34] findings for Desmonomata, including the Trhypochthoniidae, who showed that such reproduction occurs in response to disturbance. In addition, the relatively high number of juvenile oribatid mites in disturbed sites may be due to a decrease in the number of predators in the more disturbed habitats [35-37]. However, landslide sites were found to be beneficial for other morphospecies, such as Scheloribates sp. 1 and Peloribates sp. 1, which are known to become successfully established in the first stage of the succession process on new land surfaces. In addition, they tend to invade open terrain, even though they reproduce sexually [34,38].
RDA analysis enabled determining that the sampling location (landslide) has a greater effect on age structure composition, whereas the wet season had a greater effect on the adult fraction, and the two together equally determine the total number of individuals in the litter samples. We can also assume that the physical movement-landslide-may affect food-source availability by increasing primary-production availability to the detritus trophic-group level (decomposers), increasing microflora (bacteria and fungi) biomass and diversity, which became a trigger for the micro (protozoa, enchytraeids) and mesofauna (microarthropods), as elucidated in our study. Moreover, since landslide disturbances alter the soil nutrients and habitat availability [39], decrease temperature fluctuation, and can increase food sources [40], these parameters will impose changes on the community structure, composition, and diversity of oribatid mites, as presented in this study. Similar findings for mechanical perturbation effects in a microcosm experiment were shown by Maraun, et al. [34].
The present study illuminates the importance of the landslide effect on soil biota in general, and particularly on oribatid-mite density and morphospecies abundance and composition, which were found to be clearly related. The obvious follow-up question is what the exact role is played by the microbial communities in the food web as a result of habitat alterations in this sensitive, subtropical forest ecosystem.
Acknowledgements
This paper is dedicated to the memory of Dr. Ping-Chun Lucy Hou, who supported her students throughout their study. Unfortunately, she passed away on January 18, 2012. We lost a great scientist, teacher and friend. We are terribly sorry that she did not live to see this paper published. Special thanks to Ms. Sharon Victor for her comments and for preparing the manuscript for publication.
References
- D Pimentel, N Kounang (1998) Ecology of soil erosion in ecosystems. Ecosystems 1: 416-426.
- Pimentel D, Harvey C, Resosudarmo P, et al. (1995) Environmental and economic costs of soil erosion and conservation benefits. Science 267: 1117-1123.
- Pimentel D (2006) Soil erosion: A food and environmental threat. Environ Dev Sustain 8: 119-137.
- C Baxter, JS Rowan, BM McKenzie, et al. (2013) Understanding soil erosion impacts in temperate agroecosystems: bridging the gap between geomorphology and soil ecology using nematodes as a model organism. Biogeosciences 10: 7133-7145.
- R Lal (1994) Water management in various crop production systems related to soil tillage. Soil and Tillage Research 30: 169-185.
- Vanacker V, von Blanckenburg F, Govers G, et al. (2007) Restoring dense vegetation can slow mountain erosion to near natural benchmark levels. Geology 35: 303-306.
- S Begueria (2006) Validation and evaluation of predictive models in hazard assessment and risk management. Natural Hazards 37: 315-329.
- Odum EP (1971) Fundamentals of ecology. (3rd edn), WB Saunders Company, Philadelphia.
- Coleman DC, Crossley DAJ, Hendrix PF (2004) Fundamentals of Soil Ecology. (2nd edn), Elsevier, London.
- Lee KE, Foster RC (1991) Soil fauna and soil structure. Australian Journal of Soil Research 29: 745-775.
- Condron LM (2017) The marvel of soil biodiversity. Autumn edition, NZ Turf Management J, 24-25.
- Pankhurst CE, Hawke BG, McDonald HJ, et al. (1995) Evaluation of soil biological properties as potential bioindicators of soil health. Aust J Exp Agr 35: 1015-1028.
- Gbarakoro TN, Umoren EI (2016) Soil microarthropod- Indicators of effects of living mulches in sole and mixed cropping systems at University Park Farm, Port Harcourt, Nigeria. European Journal of Agriculture and Forestry Research 4: 13-21.
- PF Santos, J Phillips, WG Whitford (1981) The role of mites and nematodes in early stages of buried litter decomposition in a desert. Ecology 62: 664-669.
- JA Wallwork (1983) Oribatids in forest ecosystems. Annu Rev Entomol 28: 109-130.
- DE Walter (1988) Nematophagy by soil arthropods from the shortgrass steppe, Chihuahuan Desert and Rocky Mountains of the central United States. Agric Ecosyst Environ 24: 307-316.
- Whitford WG, Stinnet K, Steinberger Y (1988) Effects of rainfall supplementation on microarthropods on decomposing roots in the Chihuahuan Desert. Pedobiologia 31: 147-155.
- WG Whitford (1989) Abiotic controls on the functional structure of soil food webs. Biol Fertil Soils 8: 1-6.
- Steinberger Y, Aldon EF, Whitford WG (1990) Soil microarthropod fauna of 4 habitats of the Rio Puerco watershed, New Mexico. The Southwestern Naturalist 35: 279-284.
- Whitford WG (2002) Ecology of desert systems. Academic Press, New York, London.
- Lee YF, Kuo YM, Lu SS, et al. (2009) Trampling, litter removal, and variations in the composition and relative abundance of soil arthropods in a subtropical hardwood Forest. Zoological Studies 48: 162-173.
- Sayer EJ, Sutcliffe LME, Ross RIC, et al. (2010) Arthropod abundance and diversity in a lowland tropical forest floor in Panama: The role of habitat space vs. nutrient concentrations. Biotropica 42: 194-200.
- Sun IF, Hsieh CF, Hubbell SP (1998) The structure and species composition of a subtropical monsoon forest in southern Taiwan on a steep wind-stress gradient. In: Turner CHDIM, Lim SSL, Ng PKL, Biodiversity and the dynamics of ecosystems. DIWPA, Kyoto, Japan, 147-169.
- Lee KY, Liao CL (1996) Evaluation of soil nutrition and nutrition cycle. In: Global Change: Nanjenshan Forest Ecosystem Studies. National Science Council Research Project Report, Division of Life Science.
- Hou PCL, Zou XM, Huang CY, et al. (2005) Plant litter decomposition influenced by soil animals and disturbance in a subtropical rainforest of Taiwan. Pedobiologia 49: 539-547.
- Balogh J, Balogh P (1992) The oribatid mites genera of the world. Hungarian National Museum Press, Budapest.
- Ter Braak CJF (2005) Biometris - Quantitative Methods in the Life and Earth Sciences Plant Research International, Wageningen University and Research Centre, Wageningen, The Netherlands.
- Restrepo C, Vitousek P, Neville P (2003) Landslides significantly alter land cover and the distribution of biomass: an example from the Ninole ridges of Hawai'i. Plant Ecology 166: 131-143.
- Badejo MA (1990) Seasonal abundance of soil mites (Acarina) in 2 contrasting environments. Biotropica 22: 382-390.
- Badejo MA (1994) Effect of accidental fire on soil mite density in a forest reserve in Nigeria. Experimental & Applied Acarology 18: 703-710.
- Erdmann G, Floren A, Linsenmair KE, et al. (2006) Little effect of forest age on oribatid mites on the bark of trees. Pedobiologia 50: 433-441.
- Wehner K, Scheu S, Maraun M (2014) Resource availability as driving factor of the reproductive mode in soil microarthropods (Acari, Oribatida). PLoS One 9: e104243.
- Norton RA, Palmer SC (1991) The distribution, mechanisms, and evolutionary significance of parthenogenesis in oribatid mites. In: Schuster R, Murphy PW, The Acari: Reproduction, development and life-history strategies. Chapman and Hall, London, 107-136.
- Maraun M, Salamon JA, Schneider K, et al. (2003) Oribatid mite and collembolan diversity, density and community structure in a moder beech forest (Fagus sylvatica): effects of mechanical perturbations. Soil Biology and Biochemistry 35: 1387-1394.
- Brust GE, House GJ (1988) Weed seed destruction by arthropods and rodents in low-input soybean agroecosystems. American Journal of Alternative Agriculture 3: 19-25.
- House GJ, Brust GE (1989) Ecology of low-input, no-tillage agroecosystems. Agric Ecosys Environ 27: 331-345.
- Siemann E, Tilman D, Haarstad J, et al. (1998) Experimental tests of the dependence of arthropod diversity on plant diversity. Am Nat 152: 738-750.
- Woas S (2002) Acari. In: Adis J, Amazonian Arachnida and Myriapoda, Pensoft, Sofia, Moscow, 21-291.
- Minor MA, Norton RA (2004) Effects of soil amendments on assemblages of soil mites (Acari : Oribatida, Mesostigmata) in short-rotation willow plantings in central New York. Canadian Journal of Forest Research 34: 1417-1425.
- Gergocs V, Hufnagel L (2009) Application of oribatid mites as indicators. Applied Ecology and Environmental Research 7: 79-98.
Corresponding Author
Prof. Yosef Steinberger, The Mina & Everard Goodman Faculty of Life Sciences, Bar-Ilan University, Ramat-Gan, Israel, Tel: 972-3-5318571.
Copyright
© 2017 Hao-Chiang C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.