Soil Ozonation for Nematode Disinfestation as an Alternative to Methyl Bromide and Nematicides
Abstract
Phytoparasitic nematodes are important pests that cause severe crop yield losses. In the past, methyl bromide and other proprietary nematicides have been used as management practices, but these practices are unsustainable and lead to atmospheric pollution and ozone layer destruction. Ozonation was studied as an alternative management practice since it is highly effective against microorganisms and degenerates quickly to oxygen. Soil samples that were naturally infested with nematodes were treated with different levels of gaseous ozone at 21 °C and 5 °C. Regression analysis results show that a medium level of ozonation (2.1 g O3 kg-1 for 15 min at a rate of ozonation 0.14 g O3 kg-1min-1) and low temperature (5 °C) resulted in 94% mean nematode inhibition. The data and analysis results imply that ozone may be an efficient and sustainable alternative to other practices.
Keywords
Methyl bromide alternatives, Nematicide alternatives, Nematodes, Ozone, Soil ozonation, Sustainability
Introduction
Plant-parasitic nematodes are microscopic, nonsegmented roundworm parasites that live in the soil and attack the plants through their roots. Endoparasitic nematodes infect and colonize the roots of plants (e.g. lance, root-lesion, and root-knot) while ectoparasitic nematodes remain outside of the root tissue (e.g. dagger, needle, spiral, sting, stubby root and stunt). Nematodes feed on the nutrients found in plant roots and vascular tissues, weakening the plant and leading to decreased yields. An international survey determined annual crop losses due to nematodes as follows: cotton, 10.7%; peanut, 12%; wheat, 7%; and soybean, 10.6% [1]. Nematodes can cause up to 75% yield loss in some crops, in addition to vectoring plant viruses and creating root wounds through which other pathogens can enter [2]. In 2000, global production losses to nematodes in all crops were estimated at US $121 billion, $9.1 billion of which in the U.S [3].
Currently, there are only a handful of chemicals registered for pre-plant nematode control [4,5]. The most important remaining nematicide, methyl bromide (MeBr), was the fourth most abundantly used pesticide in the U.S. in 1997 [6], but is now under phaseout due to its degradation of the stratospheric ozone layer. Approximately 25,000 to 27,000 metric tons of MeBr were still applied annually between 1990 and 1994 [7], with more than 75% of its use for pre-plant soil fumigation [8]. In 2013, only 562 metric tons of MeBr were allowed by the EPA as "critical use exemption", in compliance with the MeBr phaseout plan mandated by the Montreal Protocol [9] to protect the stratospheric ozone layer.
Ozone is a potent oxidant and it has been implemented successfully against numerous pathogens including viruses, bacteria, protozoa and also metazoa [10-12]. Ozone is often used to disinfect drinking water and waste water [13,14] and disinfest ships ballast water [15,16] due to its oxidizing properties.
In contrast to other disinfection methods and conventional pesticides used in the treatment of soil pests, such as soil fumigants MeBr, metam sodium and chloropicrin described above, the use of ozone as a disinfection method has the advantage that it does not produce pollutants, because its rapid decomposition produces oxygen only. The use of other nematicides is prohibited within 100 feet of drinking-water wells to protect groundwater from potential contamination [9], while ozone could be used safely near groundwater bodies.
Sopher, et al. [17,18] reported the successful use of gaseous ozone soil fumigation in increasing plant yield and reducing the detrimental effects of soil pathogens in a range of crops and soils under different climatic conditions. They reported positive effects of preplant ozone application, and theoretically attributed these effects to the decrease in soil pathogens and increased nutrient availability. However, they recommended further studies to confirm this theory and predict specific responses achieved from ozonation under different crops, soils, pathogens and climatic conditions. Nevertheless, to our knowledge, no further studies have been done in this regard.
The high oxidative power of ozone, its effectiveness in inhibiting pathogens without leaving toxic residues in the environment, and the limited research on ozone use in the domain of soil fumigation as alternative to nematicides inspired the current research. Furthermore, the economic importance of phytoparasitic nematodes, and the need for efficient and environmentally safe alternative treatments to the currently adopted fumigant nematicides, made treatment with ozone a realistic aim for further investigation. This study evaluated the effect of ozone on nematode viability in soil samples collected from a field in Iowa. The objectives were to evaluate (i) the effectiveness of different ozone doses and ozonation rates at reducing the viability of nematodes in the soil, and (ii) the efficacy of soil ozonation at low soil temperature (5 °C) versus high soil temperature (21 °C).
Background
Phytoparasitic nematodes survive in the soil or in plant roots, and active nematode stages are more susceptible to nematicides than resting stages [19,20]. Most systemic nematicides are needed in high concentrations (e.g. 1000 ppm of Vydate) to control nematodes within plant roots, which is impractical under field conditions [19]. Hence, it is difficult to deliver a nematicide in efficiently sufficient concentration directly in contact with nematodes within plant roots and root surroundings. Total eradication of nematode populations with a nematicide or fumigant is difficult to achieve due to the heterogeneous nature of soil that offers protection to some individuals or ova [3]. However, management should be aimed at inhibiting or deactivating the number of phytoparasitic nematodes in the soil below their economic threshold. Most nematicides are broad-spectrum, highly volatile fumigants that are able to move through the soil pores. Many of the most efficient volatile nematicides have been deregistered (e.g. ethylene dibromide and dibromochloropropane) [3], because they were associated with environmental and human health risks. Ethylene dibromide was the most abundantly used nematicide in the world, until 1983 when it was prohibited in the U.S. because of groundwater contamination and possible carcinogenicity [21,22]. Similarly, 1,3-dichloropropane was prohibited because it was classified as a probable carcinogen [6] while 1,2-dibromo-3-chloropropane (DBCP) was suspended in the U.S. in the late 1980s because it was found to cause male infertility and was a probable carcinogen [23]. Carbamates used as nematicides (i.e. aldicarb, carbofuran and oxamyl) are highly toxic to humans and animals [6,24] and organophosphates (ethoprop, fenamiphos, cadusafos, fosthiazate and phorate) have been reviewed by the U.S. Environmental Protection Agency (EPA), and several were withdrawn from use [25]. Some nematicides, however, have recently undergone re-registration eligibility decisions (REDs) by the U.S. EPA [26]. These include metam sodium, which has limited efficiency in controlling nematodes in some circumstances [21,27,28], and the fumigants chloropicrin, metam-potassium, and dazomet.
Many commodities have become dependent on MeBr for nematode control, which necessitates identifying effective alternatives [29]. Zasada, et al. [30] believed that it would be too difficult to manage phytoparasitic nematodes without MeBr. Methyl bromide is an effective pre-plant soil fumigant used to control soil pests (weed seeds, nematodes, insects, fungi, bacteria and viruses) [31], in many high-input, high-value crops in U.S. agriculture, including vegetables, nursery plants, ornamentals, fruit trees, strawberries and grapes [30]. This broad-spectrum pest control, along with its higher efficacy compared to other fumigants [32], and its volatility that enables it to penetrate treated soil [4], has made some crop production systems highly MeBr-dependent, e.g. strawberries and fresh market tomatoes, and led to reductions in crop rotation and in diversification of production [33].
Ozone has also been applied in mold prevention on stored corn [34,35]. Scanning electron microscopy showed that ozone causes damage to the surface of the ova of Toxocara canis, a nematode parasite of dogs and other canides [36]. Ozone is also capable of diffusing across bacterial membranes and reacting with cytoplasmic biomolecules, such as DNA, which results in cell death [37]. Furthermore, ozone reacts with biomolecules such as proteins, carbohydrates and polyunsaturated fatty acids bound to albumin, dyes, and is involved in lipid peroxidation [38,39].
Ozone has been approved by the U.S. Food and Drug Administration for direct use in human food, drugs, and cosmetics and also as compounds in food contact materials such as cutting boards and other surfaces that come in contact with unprotected food [40]. In addition, ozone is listed by the National Organic Program under the list of "The National List of Allowed and Prohibited Substances" with code (§205.605) referring to: "Nonagricultural (nonorganic) substances allowed as ingredients in or on processed products labeled as organic or made with organic (specified ingredients or food group(s))" [41].
Methods and Materials
Soil samples
Soil for this experiment was collected from the Hinds Farm (Iowa State University research farm, near Ames, Story County, Iowa). This soil belongs to the Clarion-Nicolett-Webster "principal association area", and Zenor soil series (Iowa Soil Properties and Interpretations Database-ISPAID). The soil was analyzed for texture and organic matter content and was found to contain 79% sand, 4.9% coarse silt, 4.7% fine silt and 10.4% clay. The soil had low organic matter content (1.4%) and low total carbon (0.7%). Accordingly, the soil texture is sandy loam, with fast draining rate, and low water retention and cation exchange capacity.
The species composition of nematodes present in the soil was determined by centrifugal flotation and species identification, with the aid of an inverted compound microscope, on four soil samples (100 g each). The soil contained an average of 225 non-plant parasitic nematodes, 2 spiral (Helicotylenchus sp.) and 0.5 ring (Criconemoides sp.) nematodes per 100 g soil. Non-plant parasitic species lack for feeding stylet, a mouth part necessary in plant parasitism. These nematodes belong to the group of free-living terrestrial nematodes, constituting 25% of all nematode species. Spiral nematode is one of the most common ectoparasites that occur in corn fields and floor of forests. Damage potential of spiral nematode is low, with a threshold of 500-1000 per 100 cm3 soil. Ring nematode is an ectoparasite with a damage threshold of 100 per 100 cm3 soil. Accordingly, both spiral and ring nematodes detected were well below damage thresholds.
Ozone treatment of soil
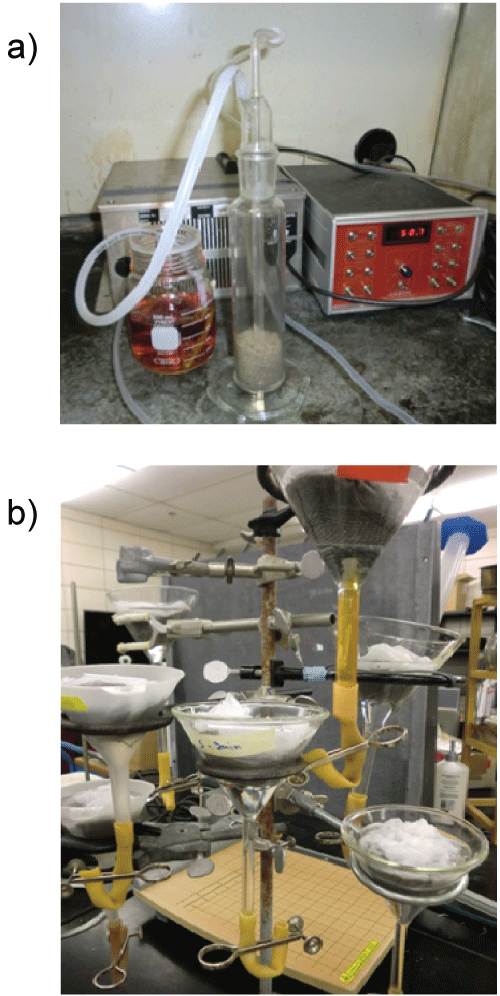
Prior to ozone treatment, the soil was sieved and mixed well. Samples of 100 g were treated with incrementally larger ozone doses (low, medium and high; Table 1) by increasing the ozone generation time (Figure 1a), at a flow rate of 0.1 L/min. Each experiment consisted of five samples of 100 g each: three ozonated at the same dose, and two nontreated control samples. Doses of ozone applied ranged from 0.35 to 3.9 g O3/kg soil. The effect of temperature on the efficacy of ozone to reduce the viability of nematodes was also tested. Two temperatures (5 °C and 21 °C) were tested for each ozone dose. For experiments at 5 °C, soil was kept in a refrigerator at 5 °C until the ozonation experiments. Increasing the level of ozonation was obtained by ozone from oxygen, while lower levels were generating ozone from air. After ozonation, the five samples were soaked in Baermann funnels (Figure 1b) [42] at room temperature. Since only viable nematodes migrate down through the soil sample, penetrate the filter and fall down into the distillate, nematode viability was easily determined by comparing nematode counts in the treated and untreated samples in the distillate after 24 h and 48 h. Nematodes were counted with the aid of an inverted compound microscope at x40 magnification. Viability was determined as the total number of nematodes in each treated sample divided by the average number of nematodes in the two control samples as a percentage. The experiment was repeated twice as shown in the experimental design (Table 1). As the experiments following the experimental design were carried out, it was found difficult to use the time of ozonation as a basis to achieve a certain ozone dose level. There were some fluctuations in the generated doses and some earlier experiments were repeated more times than others. Thus, the data from all of the experiments included some other experimental conditions not listed in Table 1, with a total of 106 obsevations (viability values as percentages).
Ozonation
The ozone generator used was a 1000BT-12 Triogen Model TOG C2B, generating a maximum of 1 g O3/h from pure oxygen by corona discharge. The reactor was made of glass (Figure 1a), and all tubing of silicone material. The operating volume in the reactor was 250 cm3. In each test the ozone flow rate was maintained at 1 L min-1L-1 gas-flow/liter volume of soil sample [43]. The excess and unreacted ozone was captured in a solution of 2% potassium iodide (KI). The amount of absorbed ozone by the soil sample was measured by the iodometric wet-chemistry method [44]. Well-established, standardized methods for ozonation and ozone measurement were used [45].
Data analysis
We analysed the data using multiple linear regression and R software (version 3.1.0, The R Foundation for Statistical Computing). The response variable is the percent nematode viability; the predictors are time (of ozonation), rate (of ozonation), dose, temperature (temp), time*temp, rate*temp, and dose*temp. Since dose = time*rate, only three of the predictors (time, rate, and temp) can vary their values independently; the other four are the interaction terms. We used leaps with Mallows's CP to select (among the 27 = 128 models) the best model. One observation (1.3 g O3 kg-1 for 7.5 min at 5 °C) with the highest viability of 90% was removed as an outlier from the final analysis, because it had an unusually large residual of 72% (compared with the next largest residual of 30%). For the remaining 105 observations, leaps with Mallows' CP gave the best model with an R-square = 0.364 and the F statistic = 11.34 (with the P-value = 1.16E-8) and the following estimated coefficients and their P-values:
| Intercept | Time | Dose | Time*Temp | Rate*Temp | Dose*Temp | |
|---|---|---|---|---|---|---|
| Estimate | 19.097 | -3.833 | 12.726 | 0.506 | 2.617 | -2.078 |
| P-value | 4.75E-4 | 0.0020 | 0.024 | 4.80E-6 | 0.0075 | 6.63E-5 |
We then used JMP software (Pro 12, The SAS Institute) to check the adequacy of the above model more conveniently. The plot of residuals versus predicted values, the normal probability plot, and the lack of fit test (with F = 0.73 and the P-value = 0.82) indicate that the model is adequate.
Results
The fitted regression model was used to obtain 95% confidence intervals for the mean percent nematode viability values at the experimental conditions listed in Table 1, and some additional selected experimental conditions used in the experiments. The results are given in Table 2. Based on the fitted model, we have the fitted equation for 21 °C as follows: viability = 19.097 - 1.301 time + 13.087 rate + 2.337 dose, and for 5 °C as follows: viability = 19.097 + 6.802 time + 54,967 rate - 30.910 dose.
As Table 2 shows, the estimated mean Nematode viability was between 6% to 7% at the ozone dose levels of 2.1 to 2.4 g O3 kg-1 for 15 min and at 5 °C, with the 95% upper confidence limits less than 16%, indicating that the viability of nematodes is reduced by 84% or more. A higher dose of ozone did not result in additional reduction in nematode viability. However, the reduction of nematode viability at low ozone dose level at 5 °C was somehow acceptable (more than 75% viability reduction as the upper confidence limits are less than 25%), and would be recommended in the case of plant parasitic nematode species with low virulence.
The table also shows that ozonation at 21 °C was less effective than at 5 °C. Nevertheless, the upper confidence limits for all the cases in Table 2 are still less than 45%, indicating that the viability of nematodes is reduced by 55% or more. At 21 °C, the dose levels of 2.2 to 2.4 g O3 kg-1 for 7.5 min gave the upper confidence limits less than 25%, indicating that the viability of nematodes is reduced by 75% or more.
It was noticed that the collected filtrate from treated samples was yellow in color (Figure 2), unlike that from untreated samples that was colorless.
Ozonated soil samples were analyzed for pH and the main oxidizable elements: P (Mehlich-3 extraction, showing P in its bioavailable form), Zn, Fe & Mn (analyses of the bioavailable forms by DTPA extraction method). Results did not show any correlation between ozonation dose (expressed in time of ozonation in min. and in dose in g.kg-1 O3 in the soil) and any of the analyzed parameters (Table 3).
Discussion
The overall results of this study clearly indicate that ozonating soil infected with nematodes at a dose of 1-2 g.kg-1 O3 at 5 °C is sufficient to kill 80% of the nematodes. Ozonation at low temperature (5 °C) was more efficient at killing soil nematodes than at high temperature (21 °C), which favors the application of this treatment at the beginning of the growing season. More than 50% of nematodes were inhibited at ozonation doses below 0.5 g.kg-1 O3 executed at all temperatures expected to be encountered. Accordingly, this level of disinfection might be enough to reduce the nematodes viability below damaging thresholds, without harming the soil biotic balance. Biotic balance is a crucial factor in maintaining the soil health and productivity, and non-plant parasitic nematodes and other beneficial microorganisms play an essential role in maintaining that through organic and non-organic nutrients recycling, and by competing with and suppressing, plant parasitic microorganisms. Hence, it is not recommended to use unnecessary higher ozone doses in the control of soil nematodes.
Ozone was more efficient at reducing nematode viability at lower temperature, similar to the observations of Patil, et al. [46]. This is attributed to the increasing ozone solubility ratio with decreasing temperature [47], and the slower ozone decomposition at lower temperature [48]. Hence, ozone is more stable at 5 °C, which prolongs its activity at oxidizing and inhibiting nematodes in the soil. Consistent with these physico-chemical ozone properties, the current study confirms a higher efficacy at a lower temperature. This effect of temperature efficacy does not occur with many nematicides (e.g. EDB and 1,3-D) [49] and fumigants (MeBr) [50]. This is an advantage for ozone use, because nematicides are usually applied at the beginning of the growing season, when temperatures are usually below optimal soil temperature range for nematode development and multiplication (21 °C to 27 °C). This qualification is an advantage over nematicides and other gas fumigants, because these latter are less efficient at low temperatures.
The results in Table 3 do not show any correlation between ozonation dose and the analyzed soil parameters (pH, Me-3 P, and DTPA- Zn, Fe, & Mn) in response to ozonation, which does not confirm the theory of Sopher, et al. [17,18] of increased nutrient availability by soil ozonation. A plausible explanation of the yellow coloration of ozonated soil filtrate might be the oxidation of soil organic matter. By oxidizing soil organic matter, the organic carbon content transforms from humine to humic acid and then to fulvic acid, which might explain the yellowish coloration of the filtrate. Fulvic acid is the most soluble and mobile form of organic carbon, and the most active form in chelating nutrients and rendering them available to plants. Hence, this could partially confirm Sopher, et al. [17] theory, since fulvic acid ameliorates the soil physical-chemical properties and increases plant productivity consequently. Moreover, these simple organic acids are effective in killing plant parasitic nematodes, while having little or no effect on free-living nematodes [51]. Accordingly, this suggests that the indirect effect of soil ozonation on plant parasitic nematodes is expected to be greater than the inactivation of free-living nematodes acquired in this experiment, through organic acids mobilization. The soil used in this experiment was sandy loam with low organic matter content. Ozone is known to be able to selectively oxidize colored matter and cause color changes [52,53].
Fumigants diffusion is faster in coarse-textured soil with high moisture [54], and these become less efficient in soils with high organic matter content [18]. Organic matter and metals increase the ozone demand because they are oxidizable. Hence, higher ozone doses will be required than in this research to reach similar nematode inhibition rates in heavier soils with higher organic matter and metal contents.
This study was not species-specific, since the observations were assessing the aggregate number of nematodes inhibited by the treatment unselectively amongst species. Therefore, further experimentation with species specificity is recommended, taking into consideration the significance of nematode inactivation by species. In addition, although the soil that was used in this research did not include significant numbers of plant parasitic nematodes, the high efficiency of ozone in inactivating non-parasitic nematodes could be an indicator for comparable effect on plant-parasitic nematodes as well. Hence, this could be a plausible confirmation of the Sopher, et al. [17,18] assumption that the increased crop yield after soil ozonation was attributed in part to a decrease in soil pathogens by ozone.
Since ozone does not leave toxic residues, and given that low doses are required to inactivate nematodes by half, which would control the nematodes without harming the soil biotic balance, ozonation could be used as a sustainable alternative to the conventional treatments that have been used to manage nematodes and other soil pathogens [55]. Thus, it could play an important role in organic agriculture. Furthermore, due to the complexity of ozone generation systems required in field application and the difficulty of bringing big ozone generators on site, the application of this technique is limited to small crop-lands. Lands that are suitable for soil ozonation are those usually treated with gas fumigants (e.g. MeBr), namely high-value crops and greenhouse crops. Finally, additional research is required to evaluate the economic feasibility of ozonation to control soil nematodes, the species-specific response to ozonation, and the application of soil ozonation at the field level.
Acknowledgements
The authors of this research acknowledge and express sincere thanks to the U.S. Department of State for providing an International Fulbright S&T Award to the first author for carrying out this research at Iowa State University. Also, we express appreciations and thanks to the U.S. DOE Ames Laboratory at Iowa State University for providing the lab facility needed to conduct this research, and to the reviewers for their valuable suggestions that significantly improved the quality of this paper.
References
- Sasser JN, Freckman DW (1987) A world perspective on nematology: The role of society. In: Veech JA, Dickson DW, Vistas on Nematol, Maryland, USA, 7-14.
- Barker KR, Koenning SR (1998) Developing sustainable systems for nematode management. Annu Rev Phytopathology 36: 165-205.
- Chitwood DJ (2002) Phytochemical based strategies for nematode control. Annu Rev of Phytopathol 40: 221-249.
- Duniway JM (2002) Status of chemical alternatives to methyl bromide for pre-plant fumigation of soil. Phytopathology 92: 1337-1343.
- Martin FN (2003) Development of alternative strategies for management of soil borne pathogens currently controlled with methyl bromide. Annu Rev Phytopathology 41: 325-350.
- Aspelin, Grube AH (1999) Pesticide industry sales and usage: 1996 and 1997 market estimates. Office of Pesticide Programs, U.S, Environmental Protection Agency.
- (1995) United States Department of Agriculture. Agricultural chemical usage: 1994 Vegetable summaries, National Agricultural Statistics Service, Economic Research Service, Washington.
- (1992) United Nations Environment Programme. Report of the fourth meeting of the parties to the Montreal Protocol on substances that deplete the ozone layer, Copenhagen.
- (2001) United States Environmental Protection Agency.
- Orta-de Velasquez MT, Rojas-Valencia MN (2002) Destruction of helminth eggs (Ascaris suum) by ozone: second stage. Water Resources 3: 227-233.
- Orta-de Velasquez MT, Martinez JL, Monje-Ramirez I, et al. (2004) Destruction of helminth (Ascaris suum) eggs by ozone. Ozone Science & Engineering 26: 359-366.
- Ramirez-Cortina CR, Alonso Gutierrez MS, Bautista JD, et al. (2005) Ozonation: An effective process for Toxocara canis eggs inactivation. In: 17th World Ozone Congress, Strasbourg.
- Van Leeuwen J (Hans) (1996) Reclaimed water-an untapped resource. Desalination 106: 233-240.
- Van Leeuwen J (Hans), Pipe-Martin C, Lehmann RM (2003) Water reclamation at South Caboolture, Queensland, Australia. Ozone Science & Engineering 25: 107-120.
- Oemcke DJ, van Leeuwen J (Hans) (2004) Sea water ozonation of Bacillus subtilis spores: implications for the use of ozone in ballast water treatment. Ozone Science & Engineering 26: 389-401.
- Oemcke DJ, van Leeuwen J (Hans) (2005) Ozonation of the marine dinoflagellate alga Amphidinium sp implications for ballast water disinfection. Water Res 39: 5119-5125.
- Sopher CD, Graham DM, Rice RG, et al. (2002) Studies on the use of ozone in production agriculture and food processing. Proceedings of the International Ozone Association, Pan American Group 1-15.
- Smelt JH, Leistra M (1992) Nematology: From molecule to ecosystem. In: Gommers FJ, Maas PWT, European Society of Nematologists. Invergowrie, Scotland, 266-280.
- Evans AA (1973) Mode of action of nematicides. Annals of Applied Biology 75: 469-473.
- McKenry MV, Thomason IJ (1974) 1,3-dichloropropene and 1,2-dibromoethane compounds: II. Organism-dosage response studies in the laboratory with several nematode species Hilgardia 42: 422-438.
- Hague NG, Gowen SR (1987) Principles and practices of nematode control in crops. In: RH Brown, BR Kerry, Academic Press, Sydney, Australia, 131-178.
- Nordmeyer D (1992) Nematology: from molecule to ecosystem. In: Gommers FJ, Maas PWT, European Society of Nematologists. Invergowrie, Scotland, 281-293.
- Thrupp LA (1991) Sterilization of workers from pesticide exposure: the causes and consequences of DBCP-induced damage in Costa Rica and beyond. Int J Health Serv 21: 731-757.
- Hartwig J, Sikora RA (1991) Mode-of-action of the carbamate nematicides cloethocarb, aldicarb and carbofuran on Heteroderu schachtii. Revue de Nematologie 14: 525-530.
- Norris FA, Jordan EG, Guardigli A (1988) Analytical methods for pesticides and plant growth regulators. Ethoprop 16: 3-20.
- (2008) United States Environmental Protection Agency.
- Csinos AS, Sumner DR, Johnson WC, et al. (2000) Methyl bromide alternatives in tobacco, tomato and pepper transplant production. Crop Protection 19: 39-49.
- Locascio SJ, Dickson DW, Mitchell DJ, et al. (1999) Alternative treatments to methyl bromide for strawberry. Proc Fla State Hort Soc 112: 297-302.
- Carpenter J, Gianessi L, Lynch L (2000) The economic impact of the scheduled phase-out of methyl bromide in the U.S. National Center of Food and Agricultural Policies. Washington, 466.
- Zasada IA, Halbrendt JM, Kokalis-Burelle N, et al. (2010) Managing nematodes without methyl bromide. Annu Rev Phytopathol 48: 311-328.
- Ragsdale NN, Wheeler WB (1995) Methyl bromide: risks, benefits, and current status in pest control. Review of Pesticide Toxicology, 21-44.
- McKenry MV (1994) Nematicides. Encyclopedia of Agriculture Science. In: CJ Arntzen, Academic Incorporation, San Diego, 87-95.
- Braun AL, Supkoff DM (1994) Options to methyl bromide for the control of soil borne diseases and pests in California with reference to the Netherlands. In: Pest Management and Analysis Division Department of Pesticide Regulation.
- White SD, Murphy PT, Bern CJ, et al. (2010) Controlling deterioration of high-moisture maize with ozone treatment. J of Stored Products Research 46: 7-12.
- White SD, Murphy PT, Leandro LF, et al. (2013) Mycoflora of high-moisture maize treated with ozone. J of Stored Products Research 55: 84-89.
- Ooi HK, Lin CL, Wang JS (1998) Effect of ozone treatment on Toxocara canis eggs. J of Veterinary Medicine Science 60: 169-173.
- Ishizaki K, Kazuyuki S, Kazunobu M, et al. (1987) Effect of ozone on plasmid DNA in Escherichia coli in situ. Water Research 21: 823-827.
- Bocci V (2005) Ozone, a new medical drug. Springer, Dordrecht, The Netherlands, 315.
- Zhu W, Koziel JA, Cai L, et al. (2013) Ozonation-based decolorization of food dyes for recovery of fruit leather wastes. J Agric Food Chem 61: 8198-8206.
- Kobayashi F, Ikeura H, Ohsato S, et al. (2011) Disinfection using ozone microbubbles to inactivate Fusarium oxysporum f. sp. melonis and Pectobacterium carotovorum subsp. carotovorum. Crop Protection 30: 1514-1518.
- (2005) National Organic Program. Agricultural Marketing Service, USDA.
- Viglierchio DR, Schmitt RV (1983) On the methodology of nematode extraction from field samples: Baermann funnel modifications. J Nematol 15: 438-444.
- Sankaran S, Khanal SK, Pometto AL, et al. (2008) Ozone as a selective disinfectant for nonaseptic fungal cultivation on corn-processing wastewater. Bioresour Technol 99: 8265-8272.
- Iodometric method for the deter-mination of ozone in process gas (1987) International Ozone Assoc., Standardization Committee-Europe. Intl. Ozone Assoc, European-African Group, Paris, France.
- van Leeuwen, (2015) Proposed OS&E Requirement: Measuring ozone dosage. Ozone Sci and Eng 37: 191-192.
- Patil S, Cullen PJ, Kelly B, et al. (2009) Extrinsic control parameters for ozone inactivation of Escherichia coli using a bubble column. J Appl Microbiol 107: 830-837.
- Bablon G, Bellamy WD, Bourbigot MM, et al. (1991) Ozone in water treatment: Application and Engineering. In: Langlais G, Reckhow DA, Brink DR, Fundamental aspects. Lewis Publishers, Inc Chelsea, MI, USA, 11-132.
- Rice RG, Robson CM, Miller GW, et al. (1981) Uses of ozone in drinking water treatment. Journal (American Water Works Association) 73: 44-57.
- Tacconi R, Santi R, Gironi R (1999) Population dynamics of Aphelenchoides besseyi on rice and effect of seed treatments on seed for propagation. Nematologia Mediterranean 27: 291-293.
- Grove IG, Haydock PPJ (2000) Toxicity of 1,3-dichloropropene to the potato cyst nematodes Globodera rostochiensis, G. pallida. Annals of Applied Biology 59: 103-108.
- Kimenju J, Sibanda Z, Talwana H, et al. (2004) Nematology training manual. Nematology initiative for Eastern and Southern Africa.
- Van Leeuwen J (Hans), Sridhar A, Esplugas M, et al. (2009a) Improving biodegradation of organic pollu-tants with ozonation during biological wastewater treatment. Ozone Science & Engineering 31: 63-70.
- Van Leeuwen J (Hans), Sridhar A, Esplugas M, et al. (2009b) Ozonation within an activated sludge sys-tem for azo dye removal by partial oxidation and biodegradation. Ozone Science & Engineering 31: 279-286.
- Bromilow RH (1973) Break down and fate of oximecarbonate nematicides in crops and soils. Annals of Applied Biology 75: 473-479.
- Msayleb (2014) Soil ozonation as a sustainable alternative to fumigation with methyl bromide and treatments with synthetic pesticides Doctoral Dissertation. Iowa State University, USA.
Corresponding Author
J (Hans) van Leeuwen, Department of Agricultural & Biosystems Engineering, Civil, Construction & Environmental Engineering, Food Science and Human Nutrition, Iowa State University, 476 Town Engineering, IA 50011-3232, Ames, USA, Tel: +1-515-294-5251.
Copyright
© 2017 Msayleb N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.