Associations between Serum 25-Hydroxyvitamin D Levels and Metabolic Syndrome in Male Shift Workers
Abstract
Objective: We investigated the association between serum 25-hydroxyvitamin D (25(OH)D) levels and metabolic syndrome (MetS) in male shift workers vulnerable to serum 25(OH)D deficiency.
Materials and methods: Laboratory measurements and self-questionnaire survey were conducted on 985 daytime and 755 shift workers. Shift workers comprised two-shift (n = 38) and three-shift (n = 717). Variables between daytime and shift workers were compared, the correlation between these variables and serum 25(OH)D levels and differences of variables according to quartiles of serum 25(OH)D levels were analyzed. Odds ratios (OR) of MetS and metabolic components depending on serum 25(OH)D levels were determined using multiple logistic regression analysis.
Results: The prevalence of Met S in daytime and shift workers was 24.7% and 23.8%, respectively. In daytime workers, mean serum 25(OH)D level was lower and age, the number of alcohol drinker, worker who lack exercise and sleep, married worker was higher than shift workers with significance. After correction of confounding factors including lifestyle, sociodemographic factors and seasonal variation, MetS (OR = 1.580, 95% CI = 1.004-2.488, P = 0.048) and waist circumference (OR = 1.915, 95% CI = 1.217-3.013, P = 0.005) were statistically significant in daytime workers, but not in shift workers. Both groups with serum 25 (OH) D deficiency showed hypertriglyceridemia regardless of MetS.
Conclusions: Associations between serum 25(OH)D levels and MetS in shift workers could be affected by confounding factors and working condition. Serum 25(OH) D deficiency is a risk factor of hypertriglyceridemia in male workers.
Keywords
Vitamin D, Shift, Daytime, Workers, Metabolic syndrome, Hypertriglyceridemia
Introduction
Metabolic syndrome (MetS) includes abdominal obesity, hyperglycemia, low high-density lipoprotein cholesterol (HDL-C), high triglyceride (TG), hypertension and is related to cardiovascular disease, type 2 diabetes mellitus (DM), stroke, and cardiovascular mortality [1,2]. Insulin resistance (IR) related to abdominal obesity is the main cause of MetS, as well as lifestyle, environment, and genetic factors [3,4]. In South Korea, the prevalence of MetS increased from 24.5% in 2008 to 28.1% in 2017 in males and decreased from 20.5% to 18.7% in females [5].
For vitamin D synthesis, 7-dehydrocholesterol is converted into cholecalciferol in the skin keratinocyte from ultraviolet B of the sunlight and is absorbed by food intake. Next, 25-hydroxyvitamin D (25(OH)D) is produced by 25-hydroxylation in the liver, and then 1,25-dihydroxyvitamin D (1,25(OH)2D) is synthesized by 1α-hydroxylation in the kidney. Additionally, 25(OH)D is a clinically useful measurement target because of its relatively longer half-life than 1,25(OH)2D [6].
Vitamin D is involved in bone metabolism and plays an essential role in cell differentiation control and cell proliferation, immune function, and anticancer effect, and its insufficiency causes rickets and osteoporosis [7]. Clinically, vitamin D is related to hypertension, cardiovascular disease, diabetes mellitus, cancer, and MetS.
Vitamin D deficiency is one of the major health problems worldwide. In South Korea, vitamin D deficiency is considered as a serum 25(OH)D level of < 20 ng/mL, with 52.8% and 68.2% prevalence in males and females in 2008, which was 75.2% and 82.5% in 2014, respectively [8]. Decreased outdoor activities due to the increased urbanization cause inadequate sunlight exposure and decreased amount of ultraviolet B rays reaching the ground due to air pollution, leading to a decreased vitamin D synthesis in the skin [9].
Workers are affected by vitamin D concentration depending on working conditions, including indoor or outdoor, night or daytime, and office or manufacturing workers [10]. Shift work is a common form of work outside of working hours and varies depending on the duration of the shift, the change of working time, night or day, and the number of workers [11]. Circadian rhythm changes due to shift work cause various physical diseases, such as depression, cardiovascular diseases, and cancers [12]. Furthermore, developing type 2 DM is a risk with lifestyle factors, such as smoking, obesity, and lack of exercise [13]. Therefore, the development of MetS may be vulnerable to shift workers. However, many studies on the relationship between shift work and MetS have revealed inconsistent results [14].
This, this study aimed to compare the prevalence of MetS between daytime and shift workers and analyzed the relationship between serum 25(OH)D and MetS in two groups.
Methods
Subjects
This retrospective cross-sectional study included 2,003 workers who underwent an annual health check-up from March to December 2021. Of these, 243 females, 6 individuals who did not undergo clinical questionnaires or laboratory measurements, and 14 individuals taking vitamin D were excluded. A total of 1,740 males were finally enrolled. The numbers of daytime and shift workers were 985 and 755, respectively. Daytime workers refer to workers who never work at night in their usual work schedule. The time work schedule was from 09:00 to 18:00 h. Shift workers are divided into two-shift (n = 38) and three-shift workers (n = 717). The starting time of two-shift work is 08:00 h or 20:00 h, and the three-shift work begins at 08:00, 16:00, and 24:00 h. This study protocol was approved by the Institutional Review Board of Seoul National University Bundang Hospital, Seongnam-si, Gyeonggi-do, Korea (B-1703-385-003). Written informed consent was obtained from all participants.
Laboratory measurements
Blood collection of participants took place in the morning after fasting for at least 8 h. Weight, height, and blood pressure (BP) measurements were taken using standard techniques. Body mass index (BMI) was obtained by dividing weight (kg) by height squared (m2). Waist circumference (WC) was measured at the level midway between the lower ribs and the highest area of the iliac crest. Waist-BMI ratio (WBR) was calculated as WC in centimeters divided by BMI [15]. Fasting plasma glucose (FPG), HDL-C, TG, and serum creatinine (Cr) levels were measured. Serum 25(OH)D (Atellica IM 1600 analyzer, Siemens, Germany) was measured using the Chemiluminescence immunoassay (CLIA) method. The seasonal data of serum 25(OH)D examination was categorized into four groups: Spring (March-May), summer (June-August), autumn (September-November), and winter (December-February).
Definition of MetS
We defined MetS by the 2009 harmonization criteria [16]. Subjects who meet at least three of the following criteria were diagnosed with MetS: (1) WC of ≥ 90 cm; (2) current use of anti-hypertensive medications or BP of ≥ 130/85 mmHg; (3) receiving treatment for previously diagnosed type 2 DM or FPG of ≥ 100 mg/dL; (4) HDL-C of < 40 mg/dL; (5) receiving medical treatment for elevated TG levels or TG of ≥ 150 mg/dL. The WC cut-off was set following the abdominal obesity criteria in Koreans under the Korean Society for the Study of Obesity [17].
Clinical questionnaires
We conducted self-assessment questionnaire for the workers who participated in the study. Alcohol drinkers were defined as alcohol intake more than once a week. Smoking status was classified as current smoker, former smoker, and no smoker. The exercise was considered as above-recommended physical activity (PA), including walking for 20 min daily; moderately intense activity (e.g., brisk walking or doubles tennis) for at least 20 min 5 days a week; or vigorously intense activity (e.g., jogging or singles tennis) for at least 20 min 4 days a week. Less intense levels were defined as below recommended PA. Sleep duration was classified into three groups, including 5 h, 6-7 h, and 8-9 h based on data answered for "In the past year, on average, how many hours per day did you sleep, including naps?" in the survey. The three subjects who slept more than 10 hours were included in the 8-9 h group. Marital status was classified as single or married. One worker was widowed and 12 workers who divorced were classified as single workers. Education level was divided into four levels: high school, college, university, and graduate school.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation. The Kolmogorov-Smirnov test was performed for the normality of continuous variables. Student's t-test and Mann-Whitney U test were performed to determine differences between two groups for continuous variables, respectively and Chi-square test for categorical variables expressed as percentage or frequency. For the correlations between serum 25(OH)D level and variables, continuous variables were performed using the Spearman's correlation analysis. Serum 25(OH)D levels were logarithmically converted and categorized into quartiles (Q1, 1.91-2.72 ng/mL; Q2, 2.73-2.99 ng/mL; Q3, 3.00-3.24 ng/mL; Q4, 3.25-4.29 ng/mL). Continuous variables according to the serum 25(OH)D levels were compared using the Kruskal-Wall is test and categorical variables using the Chi-square test. Multiple logistic regression analysis, including potential confounders, was performed to analyze the relationship between serum 25(OH)D quartiles and the prevalence of MetS and its components. The Statistical Package for the Social Sciences for windows version 26.0 (SPSS Inc., Chicago, IL, USA) was used for all analyses. P-values of < 0.05 was considered statistically significant.
Results
Characteristics of the study population
Table 1 shows the clinical characteristics of both daytime and shift workers. The prevalence of MetS was 24.7% and 23.8% in daytime and shift workers, respectively, but without statistically significant differences (P = 0.690). Age, BP, HDL-C, and Cr were significantly increased in daytime workers. The mean serum 25(OH)D level (P = 0.002) was significantly lower, and the number of workers with serum 25(OH)D levels of < 20 ng/mL was higher in daytime workers than in shift workers (P < 0.001). Additionally, daytime workers showed significantly higher marital status, education level, alcohol consumption, lack of exercise, and short sleep of < 5h. The number of smokers and workers sleeping for 8-9 h was significantly higher in shift workers than in daytime workers.
Relationships of clinical characteristics with serum 25(OH)D levels
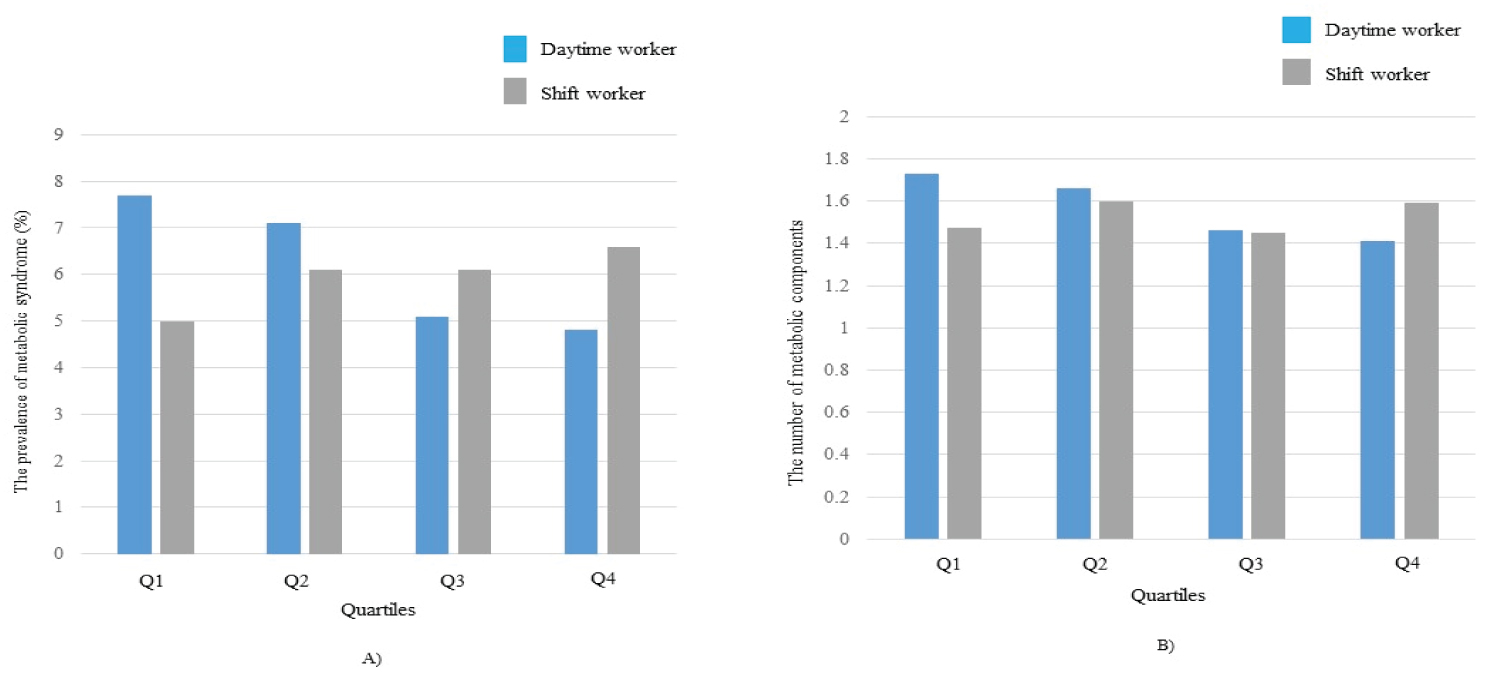
The bivariate correlation analysis in TG revealed negative correlations in both groups (Table 2). Serum 25(OH)D levels in daytime workers showed significant negative correlations with BMI, WC and number of MetS component. Significant correlations were found between serum 25(OH)D and age, WBR, FPG and Cr levels in shift workers. We analyzed the difference in clinical characteristics according to serum 25(OH)D quartiles in two groups. BMI (P = 0.017), WC (P = 0.013), WBR (P = 0.020) and diastolic BP (P = 0.033) showed a significant difference in daytime workers, and TG (P < 0.001), the number of smokers (P = 0.043) and lack of PA (P = 0.034) exhibited an inverse relationship with quartiles (Table 3). The season of data collection showed significant differences among quartiles (P < 0.001). In contrast, age (P = 0.016), FPG (P = 0.047), Cr (P = 0.031) and TG (P = 0.010) showed significant differences in shift workers (Table 4). Higher quartiles were positively related to age (P = 0.016). Lower MetS prevalence and higher MetS components were found in quartiles among daytime workers (Figure 1).
However, the prevalence of MetS was not statistically significant (P = 0.212), whereas the number of MetS components was statistically significant (P = 0.016). Quartiles did not show a significant relationship with the two variables in shift workers (Figure 1).
Associations of MetS with serum 25(OH)D levels
The results of logistic regression analyses between serum 25(OH)D quartiles and MetS prevalence are shown (Table 5). The unadjusted odds ratio (OR) for MetS in each quartile of serum vitamin D in daytime workers did not show a significant association (model 1). Model 2, which was adjusted for age and Cr, revealed a statistical significance in the OR of MetS at the margin in the lowest quartile of serum 25(OH)D (OR = 1.528, 95% confidence interval [CI] = 0.997-2.342, P = 0.052). This did not change after additional adjustments for smoking status and alcohol consumption (model 3). The OR of MetS was significantly increased in the lowest quartile after adjusting for exercise and season (model 4) (OR = 1.580, 95%CI = 1.008-2.476, P = 0.046). This statistical significance remained after adjusting for marital status, education level, and sleep duration (model 5) (OR = 1.599, 95%CI = 1.018-2.511, P = 0.041) and WBR (model6) (OR = 1.580, 95%CI = 1.004-2.488, P = 0.048). By contrast, unadjusted and adjusted OR for MetS in any model in shift workers were not significantly associated.
Associations of individual components of MetS with serum 25(OH)D levels
The association of serum 25(OH)D quartiles with each MetS component was assessed after adjusting for age, Cr, smoking status, alcohol consumption, PA, season, marital status, education level, sleep duration and WBR (Table 6). WC in daytime workers showed significant association with Q1 quartile (OR = 1.915, 95% CI = 1.217-3.013, P = 0.005). The adjusted OR for high TG level of MetS components showed significant association in each quartile of serum 25(OH)D in model 6, and the OR was the highest in the lowest quartile (OR = 2.909, 95% CI = 1.894-4.466, P < 0.001). High BP, high FPG, and low HDL-C levels showed no significant association in each quartile. The adjusted OR for high TG level of MetS components showed significant association in the Q1 quartile (OR = 1.693, 95% CI = 1.029-2.785, P = 0.038) and Q2 quartile (OR = 2.006, 95% CI = 1.273-3.160, P = 0.003) in shift workers in model 6, respectively while the others did not. In addition, the lowest (Q1) and second (Q2) quartiles of Serum 25(OH)D levels showed significant associations with high TG levels regardless of MetS (Table 7).
Discussion
Our study revealed a higher MetS prevalence in daytime workers than in shift workers. The relationship between obesity and vitamin D deficiency was observed in daytime workers, and the association between vitamin D deficiency and MetS and abdominal obesity remained even after adjusting for potential confounding factors. A significant association was found between vitamin D and TG levels in both groups.
Circadian rhythm misalignment occurs due to shifting work, destroying the sleep/wake cycle, and changing meal times, which causes obesity due to excessive appetite and high-calorie food intake due to poor balance of leptin and ghrelin, which are hormones that suppress and stimulate, respectively [18,19]. Additionally, most vitamin D synthesis occurs in the skin, and obesity, less external activity, and less sunlight exposure reduce vitamin D levels [20]. Shift workers may be more vulnerable to vitamin D deficiency than daytime workers, due to less sunlight exposure time, irregular meal times, and lack of vitamin D intake due to instant food. The circadian rhythm misalignment is related to sleep disturbance. Short sleep is associated with weight gain, impaired glucose tolerance, dyslipidemia, high BP, IR, and MetS [21]. This study revealed a higher number of 8-9 h of sleep duration in shift workers than in daytime workers, and the number of daytime workers with < 5h of sleep was significantly higher than that of shift workers. Sleep quality as a crucial determinant of the development of MetS with sleep duration has shown an inverse correlation with MetS; however, this study did not investigate sleep quality [22]. The occurrence of sleep disturbance along with sleep/wake cycle disruption is also related to vitamin D deficiency because vitamin D receptors are expressed in brain areas that regulate the sleep/wake cycle [23].
Shift work has various types, and the impact on the health of shift workers may vary according to the working schedule, the number of nights worked per month or year, and rotation direction [14,24]. Our study included rotating shift workers who do not work only at night, such as night shift workers, but alternatively, work day and night. Therefore, circadian misalignment and lifestyle risks were less in night shift workers. Clockwise rotation (morning-afternoon-night) is a working schedule that changes from day to night, and the opposite is called anticlockwise rotation (afternoon-morning-night) [25]. Clockwise rotation is known to have fewer health hazards than anticlockwise rotation. However, the rotation schedule for the shift pattern was not obtained, and leptin and ghrelin levels were not measured.
Lifestyle factors, such as smoking, alcohol, and lack of exercise, can accelerate the risk of MetS. Smoking increases the risk of hypertriglyceridemia, low HDL-C, and IR and is associated with MetS development [26,27]. Serum vitamin D levels decreased through a specific mechanism by an endocrine disruptor contained in tobacco smoke [28]. Increased ionized calcium caused by smoking inhibits parathyroid hormone (PTH) levels and affects serum vitamin D levels [29]. This study revealed a significantly increased number of smokers in shift workers compared to daytime workers, but it showed an inverse relationship with serum 25(OH)D levels in daytime workers. However, calcium and PTH levels were not measured and the smoking duration and number of smoked cigarettes were not investigated. Alcohol consumption showed a significant increase in daytime workers. Excessive alcohol consumption is associated with hypertension, dyslipidemia, and obesity [30]. Alcohol intake that exceeds 5 g per day was associated with hypertension, hypertriglyceridemia, and low HDL-C [31]. Conversely, those who consumed 0.1-5g of alcohol per day had a significantly lower prevalence of MetS than those who consumed no alcohol. Additionally, proper amount or timing and the type of alcohol consumption rather reduced MetS prevalence [32]. Our study has not obtained the amount of alcohol consumption. Alcohol consumption showed no significant relationship with serum 25(OH)D levels in both groups. Studies have shown positive or negative associations between alcohol consumption and vitamin D levels, which remain controversial [33]. Exercise improves insulin activity and lowers BP by controlling fat and glucose metabolism [34]. Serum 25(OH)D level is related to muscle function and immune response and showed a positive relationship according to the quartiles group of exercise [35], which was consistent with our study. The exercise may have relatively decreased while working indoors for a long time, and the shift worker may have had active time outside of work. A study revealed an increased risk of MetS in daytime workers than in shift workers, with lack of exercise as a risk factor [36].
As age increases, vitamin D levels decreases by reduced outdoor activities and lack of vitamin D synthesis due to skin atrophy, various food intake changes, and decreased renal function [37]. Daytime workers showed a significant increase in the mean age than shift workers. However, there was no significant correlation between age and serum 25(OH)D levels and significant difference in age according to vitamin D level changes in daytime workers. In contrast, positive correlation was found between age and serum 25(OH)D levels in shift workers. These findings may be because of the absence of the elderly population above 50 years. Positive relationships were found in age and Cr according to quartiles of serum 25(OH)D levels in shift workers. Another study also showed that age and 25 (OH)D level were proportional to healthy individuals with normal renal function [38].
Studies revealed a higher MetS prevalence due to relatively reduced healthcare system access in adults with low education levels than in adults with high education levels [39]. Our study revealed a significantly lower educational level in shift workers. The increased MetS prevalence in daytime workers is due to lifestyle and age differences in both groups despite the higher educational level than shift workers. The married group is less exposed to smoking, alcohol, and stress than the divorced or unmarried group, resulting in a lower MetS prevalence [40]. Stress reduction due to mental and emotional support, improvement of cardiovascular disease mortality and morbidity, and better health behavior were reported [41]. MetS prevalence in daytime workers was higher than in shift workers although there were more married people in shift workers than in daytime workers because many of the daytime workers are married but live apart from their spouses due to their working environment. The positive or inverse relationship between income and MetS was reported [42]. However, this factor has no available information.
Similar to our study, some studies revealed no difference between the two groups, or rather, the prevalence of MetS was lower in shift workers. One study for healthcare workers showed no significant difference between shift and daytime workers for the risk of MetS after adjustment of potential confounders [24]. This result may be explained by the clockwise rotation of shift workers or the reverse causation hypothesis that the healthier they are, the more they prefer to work in shifts. Another study revealed a lower risk of MetS for nurses working in shifts than daytime working nurses engaged in management because of increased PA in patient care [43]. Another study revealed a lower prevalence of MetS in shift workers of car-manufacturing companies compared with daytime workers [36]. The shift workers comprised two-shift (n = 1,125) and three-shift workers (n = 182), unlike our study. This result was not clearly explained because of work-related information, including shift rotation, psychological stress, work duration, economic status, and academic career. Additionally, they considered the possible effect of health workers as one of the causes.
We found many potential involved confounders in serum 25 (OH)D levels and vitamin D deficiency as potential risk factors for MetS. Vitamin D is fat-soluble and distributed in fat cells, thus vitamin D decreases as obesity increases [20]. WC is an indicator of abdominal obesity and is related to IR [44]. WC showed significant differences among quartiles of serum 25 (OH)D levels, with significant associations in daytime workers. However, our study did not measure insulin concentration and investigate the association between IR and MetS. Additionally, an inverse relationship between vitamin D levels and BMI was found in daytime workers and without a significant difference with shift workers. Previous studies revealed an inverse relationship between vitamin D levels and obesity indicators, such as BMI and WC [20]. Actually, the second quartile (Q2) showing significant association with MetS is quartiles of serum 25(OH)D levels of approximately below 20 ng/mL, and WC showed significant association in Q1 and Q2, and the criteria for vitamin D deficiency in this study was 20 ng/mL. Therefore, intervention is needed to prevent MetS for workers with vitamin D levels below 20 ng/mL.
Our study revealed that serum 25 (OH)D level plays an important role in lipid metabolism, such as TG. The mechanism is unclear, but increased intestinal absorption of calcium by serum vitamin D reduces intestinal absorption of fatty acids and synthesis and secretion of TG in the liver [45]. Calcium increases bile acid secretions, which reduces cholesterol levels [46]. Low serum 25(OH)D levels increase PTH, and this hormone reduces lipolysis, lipoprotein lipase activity, and peripheral TG removal [47]. Impaired β-cell function and IR by vitamin D deficiency are related to hypertriglyceridemia and decreased HDL-C [48].
Seasonal variation showed significant differences among quartiles of serum 25(OH)D levels in both groups. Vitamin D levels are the lowest in winter and the highest in summer and autumn in blood collection [49,50]. This study revealed a higher number of shift workers who underwent blood sample examinations in the summer and vice versa in autumn. The number of daytime workers was higher than shift workers in winter, which may have lowered serum 25(OH)D levels. However, since there were relatively more workers who received blood collection in the autumn than shift workers, it is not clear whether seasonal variation affected the association between serum 25(OH)D levels and MetS.
This study has several limitations. First, cross-sectional design limits the determination of the causal relationship between variables and MetS. Second, generalizing our study results is difficult due to the small sample size. Third, information, such as alcohol consumption, smoking status, exercise, and sleep duration, depended on the self-rating questionnaire, so they are subject to recall bias. Fourth, data such as sun exposure time, working outdoors, and dietary regimen information were unavailable. Fifth, calcium, PTH, and IR, which could have affected the serum 25(OH)D level, were not measured.
Conclusion
In conclusion, shift workers may have different health effects depending on their working conditions and environment, lifestyle factors, and sociodemographic status. Serum 25(OH)D deficiency is a risk factor for MetS and hypertriglyceridemia in male workers. However, a prospective study with a large population is necessary.
Acknowledgements
This work was supported by the following grant from the Korean Hydro & Nuclear Power Project (A20LF05).
References
- Andreadis EA, Tsourous GI, Tzavara CK, et al. (2007) Metabolic syndrome and incident cardiovascular morbidity and mortality in a Mediterranean hypertensive population. Am J Hypertens 20: 558-564.
- Kotani K, Satoh Asahara N, Nakakuki T, et al. (2015) Association between metabolic syndrome and multiple lesions of intracranial atherothrombotic stroke: A hospital-based study. Cardiovasc Diabetol 14: 108.
- Brown AE, Walker M (2016) Genetics of Insulin Resistance and the Metabolic Syndrome. Curr Cardiol Rep 18: 75.
- Park YW, Zhu S, Palaniappan L, et al. (2003) The metabolic syndrome: Prevalence and associated risk factor findings in the US population from the third national health and nutrition examination survey, 1988-1994. Arch Intern Med 163: 427-436.
- Kim M, Lee S, Shin KS, et al. (2020) The change of metabolic syndrome prevalence and its risk factors in korean adults for decade: Korea national health and nutrition examination survey for 2008-2017. Korean J Fam Pract 10: 44-52.
- Bikle DD (2014) Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol 21: 319-329.
- Jeon SM, Shin EA (2018) Exploring vitamin D metabolism and function in cancer. Exp Mol Med 50: 1-14.
- Park JH, Hong IY, Chung JW, et al. (2018) Vitamin D status in South Korean population: Seven-year trend from the Knhanes. Medicine (Baltimore) 97: e11032.
- Manicourt DH, Devogelaer JP (2008) Urban tropospheric ozone increases the prevalence of vitamin D deficiency among Belgian postmenopausal women with outdoor activities during summer. J Clin Endocrinol Metab 93: 3893-3899.
- Coppeta L, Papa F, Magrini A (2018) Are shiftwork and indoor work related to d3 vitamin deficiency? A systematic review of current evidences. J Environ Public Health 2018: 8468742.
- Costa G (2003) Shift work and occupational medicine: An overview. Occup Med (Lond) 53: 83-88.
- Rivera AS, Akanbi M, O'Dwyer LC, et al. (2020) Shift work and long work hours and their association with chronic health conditions: A systematic review of systematic reviews with meta-analyses. PLoS One 15: e0231037.
- Shan Z, Li Y, Zong G, et al. (2018) Rotating night shift work and adherence to unhealthy lifestyle in predicting risk of type 2 diabetes: Results from two large US cohorts of female nurses. BMJ363: k4641.
- Walsh JS, Bowles S, Evans AL (2017) Vitamin D in obesity. Curr Opin Endocrinol Diabetes Obes 24: 389-394.
- Gallagher JC (2013) Vitamin D and aging. Endocrinol Metab Clin North Am 42: 319-332.
- Sooriyaarachchi P, Jayawardena R, Pavey T, et al. (2022) Shift work and the risk for metabolic syndrome among healthcare workers: A systematic review and meta-analysis. Obes Rev 23: e13489.
- Liu XC, Huang Y, Lo K, et al. (2021) Quotient of waist circumference and body mass index: A valuable indicator for the high-risk phenotype of obesity. Front Endocrinol (Lausanne) 12: 697437.
- Alberti KG, Eckel RH, Grundy SM, et al. (2009) Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International atherosclerosis society; and International association for the study of obesity. Circulation 120: 1640-1645.
- Lee SY, Park HS, Kim DJ, et al. (2007) Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract 75: 72-80.
- Nguyen J, Wright KP Jr (2009) Influence of weeks of circadian misalignment on leptin levels. Nat Sci Sleep 2: 9-18.
- Klok MD, Jakobsdottir S, Drent ML (2007) The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev 8: 21-34.
- Xi B, He D, Zhang M, et al. (2014) Short sleep duration predicts risk of metabolic syndrome: A systematic review and meta-analysis. Sleep Med Rev 18: 293-297.
- Kim YJ, Yeom HE (2020) Interactive impact of sleep duration and sleep quality on the risk of developing metabolic syndrome in korean adults. Healthcare (Basel) 8: 186.
- Romano F, Muscogiuri G, Di Benedetto E, et al. (2020) Vitamin D and sleep regulation: Is there a role for vitamin D? Curr Pharm Des 26: 2492-2496.
- Kumar SE, Antonisamy B, Kirupakaran H, et al. (2021) A cross-sectional study among hospital employees-metabolic syndrome and shift work. Indonesian J Occup Safety Health 10: 258-264.
- Shiffer D, Minonzio M, Dipaola F, et al. (2018) Effects of clockwise and counterclockwise job shift work rotation on sleep and work-life balance on hospital nurses. Int J Environ Res Public Health 15: 2038.
- Lee WY, Jung CH, Park JS, et al. (2005) Effects of smoking, alcohol, exercise, education, and family history on the metabolic syndrome as defined by the ATP III. Diabetes Res Clin Pract 67: 70-77.
- Chiolero A, Faeh D, Paccaud F, et al. (2008) Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr 87: 801-809.
- Diamanti Kandarakis E, Bourguignon JP, Giudice LC, et al. (2009) Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr Rev 30: 293-342.
- Cutillas Marco E, Fuertes Prosper A, Grant WB, et al. (2012) Vitamin D deficiency in South Europe: Effect of smoking and aging. Photodermatol Photoimmunol Photomed 28: 159-161.
- Choi S, Kim K, Lee JK, et al. (2019) Association between change in alcohol consumption and metabolic syndrome: Analysis from the health examinees study. Diabetes Metab J 43: 615-626.
- Kim SK, Hong SH, Chung JH, et al. (2017) Association between alcohol consumption and metabolic syndrome in a community-based cohort of Korean adults. Med Sci Monit 23: 2104-2110.
- Vieira BA, Luft VC, Schmidt MI, et al. (2016) Timing and type of alcohol consumption and the metabolic syndrome - ELSA-Brasil. PLoS One 11: e0163044.
- Tardelli VS, Lago MPPD, Silveira DXD, et al. (2017) Vitamin D and alcohol: A review of the current literature. Psychiatry Res 248: 83-86.
- Cannata F, Vadala G, Russo F, et al. (2020) Beneficial effects of physical activity in diabetic patients. J Funct Morphol Kinesiol 5: 70.
- Wicinski M, Adamkiewicz D, Adamkiewicz M, et al. (2019) Impact of vitamin D on physical efficiency and exercise performance-A review. Nutrients 11: 2826.
- Kawada T, Otsuka T, Inagaki H, et al. (2010) A cross-sectional study on the shift work and metabolic syndrome in Japanese male workers. Aging Male 13: 174-178.
- Nasri H, Ardalan MR (2012) Association of serum vitamin D level with age in individuals with normal renal function. J Nephropharmacol 1: 7-9.
- Moore JX, Chaudhary N, Akinyemiju T (2017) Metabolic syndrome prevalence by race/ethnicity and sex in the united states, National health and nutrition examination survey, 1988-2012. Prev Chronic Dis 14: E24.
- Ben Shlomo Y, Smith GD, Shipley M, et al. (1993) Magnitude and causes of mortality differences between married and unmarried men. J Epidemiol Community Health 47: 200-205.
- Troxel WM, Matthews KA, Gallo LC, et al. (2005) Marital quality and occurrence of the metabolic syndrome in women. Arch Intern Med 165: 1022-1027.
- Mackenbach JP, Stirbu I, Roskam AJ, et al. (2008) European union working group on socioeconomic inequalities in health. Socioeconomic inequalities in health in 22 European countries. N Engl J Med 358: 2468-2481.
- Jung H, Dan H, Pang Y, et al. (2020) Association between dietary habits, shift work, and the metabolic syndrome: The Korea nurses' health study. Int J Environ Res Public Health 17: 7697.
- Tabata S, Yoshimitsu S, Hamachi T, et al. (2009) Waist circumference and insulin resistance: A cross-sectional study of Japanese men. BMC Endocr Disord 9: 1.
- Cho HJ, Kang HC, Choi SA, et al. (2005) The possible role of Ca2+ on the activation of microsomal triglyceride transfer protein in rat hepatocytes. Biol Pharm Bull 28: 1418-1423.
- Jiang W, Miyamoto T, Kakizawa T, et al. (2006) Inhibition of LX Ralpha signaling by vitamin D receptor: Possible role of VDR in bile acid synthesis. Biochem Biophys Res Commun 351: 176-184.
- Querfeld U, Hoffmann MM, Klaus G, et al. (1999) Antagonistic effects of vitamin D and parathyroid hormone on lipoprotein lipase in cultured adipocytes. J Am Soc Nephrol 10: 2158-2164.
- Karnchanasorn R, Ou HY, Chiu KC (2012) Plasma 25-hydroxyvitamin D levels are favorably associated with β-cell function. Pancreas 41: 863-868.
- Klingberg E, Olerod G, Konar J, et al. (2015) Seasonal variations in serum 25-hydroxy vitamin D levels in a Swedish cohort. Endocrine 49: 800-808.
- Itoh H, Mori I, Matsumoto Y, et al. (2011) Vitamin D deficiency and seasonal and inter-day variation in circulating 25-hydroxyvitamin D and parathyroid hormone levels in indoor daytime workers: A longitudinal study. Ind Health 49: 475-481.
Corresponding Author
Seung Jin Choi, Health & Medical Section, Radiation Health Institute, Korea Hydro & Nuclear Power Co., Ltd., Seoul, Korea, 38, Seosomun-ro, Jung-Gu, Seoul, 04505, Republic of Korea, Tel: +82-2-6106-4312; +82-10-3156-9215.
Copyright
© 2023 Sung SH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.