Antimicrobial Medical Textile - An Important Part of the Complex Infection Control Measures in the Burn Units
Keywords
Burns, Infection control, Antibacterial textile, Nosocomial infection, Coating ZnO
Introduction
Burn wound infections are one of the most important and potentially serious complications that occur in the acute period following injury. Infection and sepsis are among the most significant causative factors in burn related mortality and morbidity. The improved techniques for burn patient management and effective hospital infection control measures increase survival rate for severely burned patients. The burned patient is at high risk for Nosocomial Infections (NCI) and is susceptible to colonization from organisms in the environment and have propensity to disperse organisms into surrounding environment [1]. The colonized patients are a main reservoir for the pathogen strain. Invasive interventions including intravenous and urinary catheterization, and intubation pose a further risk of nosocomial infections [2-4]. The primary modes of cross-contamination of pathogens between burns patients are believed to be direct and indirect contact either from the hospital environment and equipment, or via staff. Other important sources include contaminated equipment such as hospital textile which poses unique risk for cross-contamination in burn care environment [2,5,6]. The healthcare worker's uniforms are a potential reservoir of infection and their contamination can be directly attributed to patients [7]. Although the person-to-person transmission route is the most likely, the role of the environment should not be ignored, and hospital linen may contribute to the spread of nosocomial infections. Routine nursing activities such as dressing and bed changes increase bacterial dispersal from burns patients, potentially contaminating healthcare workers. In this way, they could become vectors for transmission of nosocomial infection between patients [8-10]. The focus of medical care has to be the infection prevention [11,12]. Strict infection control practices and appropriate empirical antimicrobial therapy are essential to help reducing the incidence of infections due to antibiotic resistant microorganisms [13]. Emerging bacterial resistance to multiple drugs is an increasing problem in burn wound management. The prevention and control of infectious diseases among immunocompromised burned patients present a specialized problem, as the environment in burn units can become contaminated with resistant organisms [1,3,14,15].
Hospital textiles include bed sheets, blankets, towels, personal clothing, patient apparel, uniforms, and gowns. Textiles are a common material in healthcare facilities; therefore, it is important that they do not pose as a vehicle for the transfer of pathogens to patients or hospital workers. Contaminated textiles and fabrics often contain high numbers of microorganisms from body substances, including blood, skin, stool, urine, vomitus, and other body tissues and fluids and provide an excellent environment for the growth of microorganisms because of their large surface area and the ability to retain moisture [5,16-19]. The important future focus is the use of particles with antimicrobial activity for textile modification in order to enhance antimicrobial properties of medical textiles without adding antimicrobial agents into textiles which can have possible harmful or toxic effects [20-22]. Different strategies for antimicrobial finishing for textiles have been studied as a way to control microbe's growth and cross contamination. Application of chemicals in textiles has been used to impart antimicrobial activity to textile materials for medical environments [23-25]. There is a growing awareness of the use of antibacterial fabrics in the form of medical clothes, protective garments, and bed spreads to minimize the chance of the nosocomial infections [26,27]. In the recent years metal oxides nanoparticles coated onto cotton fibers represent important composites that are increasingly being developed for use in novel health-related applications. These coated fabrics do not possess skin irritation and sensitization properties. The ability of the impregnated textile on the reduction of microbial growth or elimination of microorganisms is useful in wound dressings, medical staff uniforms, bed sheets and others [24,26-29]. The aim of new technologies such as antimicrobial textiles is to support the efforts of burn care team to limit bioburden and prevent transmission of infection among burn patients. Antibacterial textile has to be part of the strict infection control protocol [4,5,30].
Although it is well known that contaminated textiles in healthcare facilities can be a source of substantial numbers of pathogenic microorganisms, reports of healthcare associated diseases linked to contaminated fabrics are a few. In the present pilot prospective study, we assess, analyze and discuss the efficacy and safety of the antibacterial and antifungal medical textiles (bed sheets, covers, pillows and patient's gowns), produced on the bases of sonochemical process [31]. The improvement of the original sonochemical process for the synthesis and simultaneous deposition of metal oxide NPs on fabrics is making the synthetic route safer and more cost efficient using water instead of ethanol:water solution. Previously, the metal oxide NPs were prepared by the basic hydrolysis of the corresponding metal acetates in 9:1 ethanol:water (v:v). The introduction of ignitable ethanol in an industrial plant not only requires special protective equipment, but is also costlier than using water as the only solvent. When water replaced the ethanol:water solution, the amount of oxides on the textile was smaller, however, the particle size was also reduced and as a result the antibacterial activity was not hampered [31].
The aim of the present study is to examine the antimicrobial efficiency, comfort and safety of the antibacterial and antifungal (ZnO) coated by sonochemical process medical textile in comparison to conventional, non-antimicrobial linen in a clinical trial in a Burn department. The patients were dressed, and lied on antibacterial (ZnO) coated fabrics - NP-coated bed sheets, patient gowns, pillow cover, and bed covers.
Materials and Methods
A pilot prospective study was conducted in a Burn department in UMHATEM "N.I. Pirogov", Sofia, Bulgaria, between May and August 2013. The protocol adhered to the provisions of the Declaration of Helsinki, and was approved by the institutional review board committee. Thirty-seven patients were enrolled in the study immediately after hospitalization with burns and different soft tissue lesions spread on 5 to 30% of Total Body Surface Area (TBSA). The study group were dressed, and lied on antibacterial ZnO coated fabrics and their propensity to bacterial infection was monitored and compared to control patient group using uncoated conventional textiles. The conventional textile used in the hospital has no antibacterial treatment and contains 65% cotton and 35% polyester. It stands washing at 90 ℃ and dry sterilization at 135 ℃. The material endures at least 200 washes without any linear deformation and able to absorb blood and body fluids. The patients were divided into two groups: Studied (21 using coated textiles) and controlled (16 using standard type of hospital textiles). A set of sonotextile was applied to each patient and it was changed every 12 hours (7 am & pm). The characteristics of the washing are as follows: Duration of washing-60 min; temperature 60 ℃; The ECOS laundry detergent kept a neutral pH level. The following parameters are observed and analyzed for a seven day period - demographic, biochemical, antibacterial effect as well as evaluation for side effects. The number and type of adverse experiences like itch, skin erythema and rush were reported as none, mild, moderate and severe. Microbiological samples were collected for both groups from the throat, nose, armpit, perineum and also collected from the hospital textile on the 1st, 4th and 7th day. Quantitative and qualitative analyses of all isolated pathogens were performed. The body site's samples were taken using cotton-tipped sterile swabs and transported to the microbiological laboratory within 30 minutes or in Steward's transport medium. Cultures were done using standard procedures on blood agar, on the selective medium Endo agar and on Sabouraud agar/Chromagar Candia (BD - Bekton Dickinson, USA). After overnight incubation at 35-37 ℃ for 18-24 h, the cultures were inspected for microbial growth. The identification of the isolated strains was done using conventional procedures or the automated system Vitek-2 Compact, Bio-Merieux (France). Contact plates - Contact E, Merck (Merck EAD Sofia, Bulgaria), were used to take samples from the textile, put into incubator for 18-24 h at 35-37 ℃ and then examined for microbial growth. The microbial growth was both qualitatively assessed for the presence of pathogens and quantitatively analyzed for determination of the total microbial number of all growing microorganisms. The following breakpoints were used: no microbial growth; 1 CFU/cm2 - very weak growth; 3.5 CFU/cm2 - weak growth; 17 CFU/cm2 - moderate growth; 58 CFU/cm2 - heavy growth; above 140 CFU/cm2 - very heavy growth. The target pathogens included: Staphylococcus aureus, beta-hemolytic streptococci of various serological groups, Enterococcus spp., Enterobacteriaceae, Pseudomonas aeruginosa, Acinetobacter baumannii, other Non-Fermentative Gram-negative bacteria and Fungi [2,5,32,33].
Data were entered and processed with statistical package SPSS 13.0. The statistic significant level for rejecting the null hypothesis was chosen as p < 0.05. The following statistical methods were applied: Descriptive analysis; Variation analysis; Graphic analysis; alternative analysis; exact test of Fisher; Nonparametric test of Shapiro-Wilk for determining the type of distribution; Student's t-test for two independent samples; Student's t-test for two related samples; U test of Mann-Whitney for two independent samples; Mauchly's test of sphericity; Repeated measures ANOVA (Analysis of Variance), Nonparametric test of Friedman; Wilcoxon signed rank test for two related samples.
Results and Discussion
The age of the included patients varies from 23 to 86 years. The two groups (study group with coated textile and control group with conventional textile) are statistically equal in terms of gender (р = 1.000, exact test of Fisher). There is no statistical significant difference between two groups by weight, height and Body Mass Index as could be seen on Table 1.
Blood biochemistry and haematology assays were conducted to identify any indications of adverse effects arising from absorption of the ZnO nanoparticles. Serum levels of Zn were not evaluated due to its proved lack of toxicity and widely spread use including children [34,35]. The levels of platelets, MCV and eosinophils were dynamically studied for the observed period. The values of these parameters are very important for toxicity evaluation of the examined textile. There were statistic significant changes during the study in the next hematological characteristics: Thrombocytes (among study group), MCV (among controls and study group), bilirubin (among controls), ALAT (among study group), GGTP (among study group) and eosinophils (among controls and study group). The changes of renal and liver function were strictly followed: Aspartate Aminotransferase (ASAT), Alanine Aminotransferase (ALAT), Gamma-Glutamyl Transferase (GGT), serum albumin, creatinine, urea, creatinine clearance and bilirubin. Both groups do not show typical changes of liver and kidney toxicity based on extrapolated data of the observed parameters [21,22,36,37]. Many haematological and biochemical parameters lay outside the normal range for all participants during the study period, and the majority of parameters return to normal range by the end of the study. The registered deviations are connected with the burn disease and surgical interventions but not as a toxicity manifestation [1,36].
The appearance of itch, skin erythema and rush with varied intensity were registered during the study period. As can be seen at Table 2 there were not statistic significant changes during the study and there was not statistic significant difference between study group and control group during the study.
Depending on the extent and nature of the burn injury the susceptibility to infection increases. The type and amount of the microbial burden colonizing the wound influence the risk of morbidity and mortality. The leading nosocomial pathogens that colonize and infect burn wounds are Acinetobacter baumannii, Staphylococcus aureus, Pseudomonas aeruginosa and Klebsiella pneumoniae [38]. Their resistance to various antimicrobial agents increases. The predominant burn wounds flora changes during hospitalization from Gram-positive bacteria such as Staphylococci, Streptococci, Enterococci to Gram-negative bacteria like Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa [39-41].
Staphylococcus aureus, both Methicillin Susceptible (MSSA) and Resistant Strains (MRSA) frequently colonizes the nostrils, throat and other skin sites of human population. Staphylococcus aureus remains the principal etiological agent of burn wound infection and, along with Acinetobacter baumannii - a common cause of early burn wound infection in many burn centers [42,43]. Over recent decades and with the liberal use of broad-spectrum antibiotics, Methicillin-Resistant S. aureus (MRSA) has become the predominant pathogen in the Intensive Care Unit (ICU) [44-46]. In order to successfully prevent and control the MRSA and MSSA infections in burn patients it is necessary to reveal their primary reservoirs and models of transmission. There are two main models of transmission of S. aureus: Endogenous - from one to another body site of the same patient and exogenous - from one to another patient or from hospital environment to the patients [47,48]. A study from the Netherlands found that two-thirds of patients whose burn wounds were colonized with methicillin-susceptible S. aureus had acquired the organism exogenously [49]. During the hospital stay of each patient, his linen usually and very fast becomes colonized with his own normal, resident microflora as well as with nosocomial pathogens.
In Sofia burns unit, the most common bacterial species in the last 5 years is S. aureus - 23.7% of all isolated pathogens and MSSA prevail. The rates of MRSA constantly fall and now is 7.6%. Gram (+) flora as a whole has a tendency to decrease and now it is 37.9% and Gram (-) flora predominates - 62.1%.
The main nosocomial pathogens isolated in our study from various body sites and the hospital textile were S. aureus and A. baumannii (Table 3).
All the S. aureus isolates were MSSA and all the A. baumannii isolates were Multidrug-Resistant - MDR (resistant to all the beta-lactam antibiotics including carbapenems, aminoglycosides, fluoro-quinolones and trimetoprim/sulfametoxazole, susceptible only to tigecyclin and colistin).
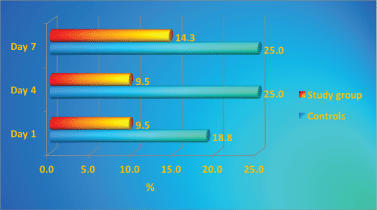
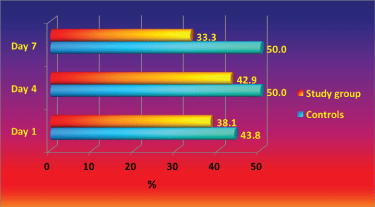
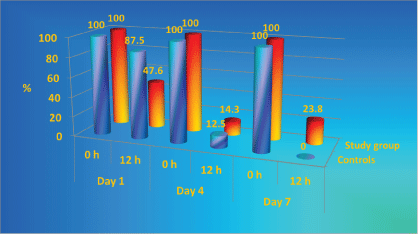
The throat and nose colonization with S. aureus and its dynamics during the study is presented in Figure 1 and Figure 2. Throughout the study period higher percentage of controls were colonized with S. aureus in comparison with the patients with antibacterial textile. The study group throat contamination remained at stable levels, while in the control group increased from day 0 to day 4 and 7. Nasal colonization in controls increased from day 1 to day 7, while in the study group the levels of colonization decreased.
During the study period, the percentage of the textile colonized with S. aureus in controls is twice as higher as it is in the study group (Figure 3) [50-52].
Acinetobacter baumannii is a Gram-negative bacillus that is aerobic, pleomorphic and non-motile. Acinetobacter baumannii is an opportunistic bacterial pathogen and one of the leading pathogens responsible for hospital-acquired infections. A. baumannii has become a great concern to scientists and clinicians and the focus of significant attention, because of its ability to rapidly develop extensive antibiotic resistance spectrum [53-55]. This has led to the emergence of Multidrug-Resistant A. baumannii Strains (MDRAB) and Pandrug-Resistant A. baumannii Strains (PDRAB). This pathogen has become one of the most common colonizing pathogens in immunocompromised burn patients with a high incidence among them, particularly those who have a prolonged hospital stay. A. baumannii survives in the hospital environment for a prolonged period, even on dry surfaces and the hands of health care personnel, and the carriage of MDRAB in human hosts may also be prolonged, which can facilitate transmission and outbreaks of this opportunistic bacterial pathogen [56-58]. Commonly associated with aquatic environments, it has been shown to spread in healthcare facilities colonizing the skin as well as being isolated in high numbers from the respiratory and oropharyngeal secretions of infected individuals.
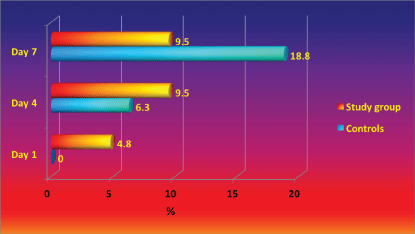
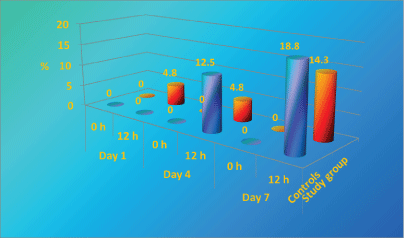
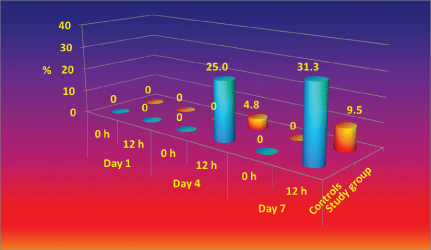
A. baumannii is the second leading pathogen causing for nosocomial infection in our Burns Department and it's rate raised to about 18% of all isolated pathogens in the last 3 years. A. baumannii nosocomial strains, isolated in the department are resistant to most of the antibiotcs. As it is presented in Figure 4, perineum colonization of the controls with A. baumannii increase progressively from day 1 to day 7 from 0% to 18.8%. In the beginning the number of colonized patients in study group is significantly higher than controls, however after day 4 the study group remain stable [43,59,60].
Colonization of control's bed linen with Acinetobacter baumannii increased significantly from Day 1 to Day 7 (from 0% to 18.8%), while in study group remained comparatively stable with small increase in the end of the study period (Figure 5). It is important to underline that A. baumannii is one of the most difficult to manage nosocomial pathogens, not affected by lots of the known antiseptics and antibiotics [46].
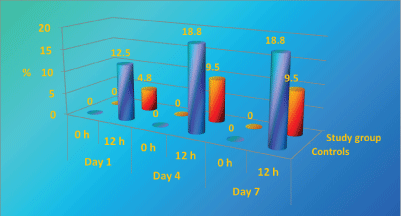
Before its first use hospital linen is sterile. After the patients have contact with the linen its colonization begins initially with their own microflora and usually after about 4 days with nosocomial flora [13,15]. In our study, as show in Figure 6, at 12 h on day 1, the colonization in study group is significantly lower than controls (p < 0.01) and on day 7 higher rate of study group remains without microbial growth ("sterile") than controls with statistic significant difference p < 0.05, i.e. There is no growth in 23.8% of the samples in the study group and there is no growth in 0% in the controls (all samples in the control group are contaminated at 12 h on day 7).
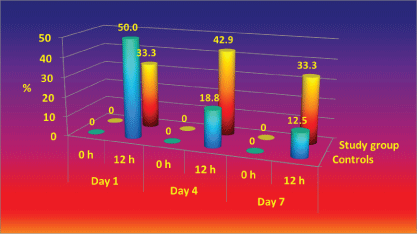
The results of the quantitative assays demonstrated that when or as colonization of the linen has occurred, the coated textiles (the study group) had higher percentage of the weak microbial growth (1-3.5 CFU/cm2 ) in comparison with standard textiles at 12 hours on day 7 (Figure 7).
In the controls linen, the percentage of the assays with moderate growth (17 CFU/cm2 ) is statistic significantly higher than in the study group (Figure 8).
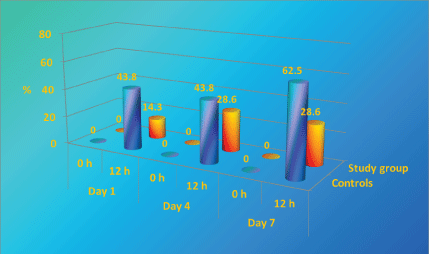
In the controls, there was a statistically significant increase in the cases with heavy growth (58 CFU/cm2) at days 4 and 7 while in the study group such cases were rare (Figure 9).
As it can be seen in controls (Figure 10) there is an increase in share of tests with heavy growth. On day 7 the increase is statistically significant and covers almost 1/3 of all tests. Patients in study group register a much weaker (10%) and later (only on day 7) increase of tests with heavy growth.
The cases with very heavy growth (above 140 CFU/cm2) were quite rare for both groups. Quantitative and qualitative analyses of all isolated pathogens in both groups confirms the antimicrobial effect of the impregnated with new sonotechnology textile against one of the most common pathogens for nosocomial infection in Burns Departments - S. aureus and A. baummanii. The ZnO coated textiles possess noticeable antibacterial effect, as they maintained lower contamination level and better microbiological characteristics both on the patients and the bed linen in comparison with conventional linen even regard multidrug-resistant A. baumannii. The high percentage of study group patients without microbial growth ("sterile") during the study (23.8%) demonstrates this effect. According to the results the new ZnO coated textile does not show toxicity. Side effects as itching, erythema and rush were observed in both groups without significant difference. We did not discover any publications in literature, analysing results of clinical tests of antibacterial hospital bed linen. In this aspect, in our opinion, this is the first such publication. More researches are necessary in order to prove the necessity of use of antibacterial hospital bed linen in clinical practice as part of measures for infection control in Burns Departments and ICU. A comprehensive concept is required to control the spread and transmission of all multi-drug resistant microorganisms on a burn unit [26,56].
Conclusion
The negative financial effect of nosocomial infections is due to the high cost of their treatment, uncertain treatment success, prolonged hospital stay, long term disability, and increased antimicrobial resistance. The frequency of multiple antimicrobial resistances is increasing throughout. The programs controlling the spread of NCI among the patients and the medical staff have proven their effect over the occurrence and the price of the treatment. The reduction of the microbial contamination of the hospital environment is a challenge to burn team. The complete eradication of the infection in burned patients is impossible. The infection control program where a new antimicrobial textile is used in burn patients could reduce infection and mortality rates in burn centers. The ZnO coated textile possesses antibacterial effect, as it managed to maintain lower contamination level and better microbiological characteristics both in the patients and bed linen in comparison with the conventional linen. The results of this study clearly demonstrate that the use of the antibacterial textile can be an option in the measures taken in burn centers to reduce an important source of nosocomial pathogens. One of the most important advantages compared with conventional linen is its ability to sustain a comparatively stable and low growth level of MDR-Acinetobacter baumannii, one of the nosocomial pathogens which are extremely difficult to eradicate.
Acknowledgements
This research was carried out as part of the activities of the SONO Consortium, Contract No. NMP-2008-1.2-1-228730. SONO, is an IP Project of the 7th EC Program.
References
- Coban YK (2012) Infection control in severely burned patients. World J Crit Care Med 1: 94-101.
- Bashe SE, Maclean M, Gettinby G, et al. (2013) Quantifying bacterial transfer from patients to staff during burns dressing and bed changes: implications for infection control. Burns 39: 220-228.
- Posluszny JA, Conrad P, Halerz M, et al. (2011) Surgical burn wound infections and their clinical implications. J Burn Care Res 32: 324-333.
- Rafla K, Tredget EE (2009) Infection control in the burn unit. Burns 37: 5-15.
- Fijan S, Turk SS (2012) Hospital textiles, are they a possible vehicle for healthcare-associated infections? Int J Environ Res Public Health 9: 3330-3343.
- Kramer A, Schwebke I, Kampf G (2006) How long do nosocomial pathogens persist in inanimate surfaces? A systematic review. BMC Infect Dis 6: 130.
- Wilson JA, Loveday HP, Hoffman PN, et al. (2007) Uniform: an evidence review of the microbiological significance of uniforms and uniform policy in the prevention and control of healthcare-associated infections. Report to the Department of Health (England). J Hosp Infect 66: 301-307.
- Dahmardehei M, Alinejad F, Ansari F, et al. (2016) Effect of sticky mat usage in control of nosocomial infection in Motahary Burn Hospital. Iran J Microbiol 8: 210-213.
- Hota B (2004) Contamination, disinfection, and cross-colonization: Are hospital surfaces reservoirs for nosocomial infection? Clin Infect Dis 39: 1182-1189.
- Ling ML, Apisarnthanarak A, Thu le TA, et al. (2015) APSIC Guidelines for environmental cleaning and decontamination. Antimicrob Resist Infect Control 4: 58.
- Carling PC, Von Beheren S, Kim P, et al. (2008) Intensive care unit environmental cleaning: An evaluation in sixteen hospitals using a novel assessment tool. J Hosp Infect 68: 39-44.
- DuBose JJ, Inaba K, Shiflett A, et al. (2008) Measurable outcomes of quality improvement in the trauma intensive care unit: the impact of a daily quality rounding checklist. J Trauma 64: 22-27.
- Baier C, Ipaktchi R, Ebadi E, et al. (2017) A multimodal infection control concept in a burn intensive care unit - lessons learnt from a meticillin-resistant Staphylococcus aureus outbreak. J Hosp Infect.
- Altoparlak U, Erol S, Akcay MN, et al. (2004) The time-related changes of antimicrobial resistance patterns and predominant bacterial profiles of burn wounds and body flora of burned patients. Burns 30: 660-664.
- Bache SE, Maclean M, Gettinby G, et al. (2015) Airborne bacterial dispersal during and after dressing and bed changes on burns patients. Burns 41: 39-48.
- Coladonato J, Smith A, Watson N, et al. (2012) Prospective, nonrandomized controlled trials to compare the effect of a silk-like fabric to standard hospital linens on the rate of hospital-acquired pressure ulcers. Ostomy Wound Manage 58: 14-31.
- Creamer E, Humphreys H (2008) The contribution of beds to healthcare-associated infection: the importance of adequate decontamination. J Hosp Infect 69: 8-23.
- Kniehl E, Becker A, Forster DH (2005) Bed, bath and beyond: pitfalls in prompt eradication of methicillin-resistant Staphylococcus aureus carrier status in healthcare workers. J Hosp Infect 59: 180-187.
- Sasahara T, Hayashi S, Morisawa Y, et al. (2011) Bacillus cereus bacteremia outbreak due to contaminated hospital linens. Eur J Clin Microbiol Infect Dis 30: 219-226.
- Kurtz EJ, Ylverton CB, Camacho FT, et al. (2008) Use of a silklike bedding fabric in patients with atopic dermatitis. Pediatr Dermatol 25: 439-443.
- Bondarenko O, Juganson K, Ivask A, et al. (2013) Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review. Arch Toxicol 87: 1181-1200.
- Brandt O, Mildner M, Egger AE, et al. (2012) Nanoscalic silver possesses broad-spectrum antimicrobial activities and exhibits fewer toxicological side effects than silver sulfadiazine. Nanomedicine 8: 478-488.
- Grigore ME, Biscu ER, Holban AM, et al. (2016) Methods of Synthesis, Properties and Biomedical Applications of CuO Nanoparticles. Pharmaceuticals (Basel) 9.
- Stankic S, Suman S, Haque F, et al. (2016) Pure and multi metal oxide nanoparticles: synthesis, antibacterial and cytotoxic properties. J Nanobiotechnology 14: 73.
- Tang ZX, Lv BF (2014) MgO nanoparticles, as antibacterial agent: Preparation and activity. Brazilian J Chem Eng 31: 591-601.
- Lazary A, Weinberg I, Vatine JJ, et al. (2014) Reduction of healthcare-associated infections in a long-term care brain injury ward by replacing regular linens with biocidal copper oxide impregnated linens. Int J Infect Dis 24: 23-29.
- Borkow G, Gabbay J (2008) Biocidal textiles can help fight nosocomial infections. Med Hypotheses 70: 990-994.
- Hanh TT, Van Phu D, Thu NT, et al. (2014) Gamma irradiation of cotton fabrics in AgNO3 solution for preparation of antibacterial fabrics. Carbohydr Polym 101: 1243-1248.
- Zaidi S, Misba L, Khan AU (2017) Nano-therapeutics: A Revolution in Infection control in post antibiotic era. Nanomedicine 13: 2281-2301.
- Maria Magdalane C, Kaviyarasu K, Judith VJ, et al. (2017) Facile synthesis of heterostructured cerium oxide/yttrium oxide nanocomposite in UV light induced photocatalytic degradation and catalytic reduction: Synergistic effect of antimicrobial studies. J Photochem Photobiol B 173: 23-34.
- Perelshtein I, Lipovsky A, Perkas N, et al. (2015) Making the hospital a safer place by sonochemical coating of all its textiles with antibacterial nanoparticles. Ultrason Sonochem 25: 82-88.
- Halstead FD, Lee KC, Kwei J, et al. (2017) A systematic review of quantitative burn wound microbiology in the management of burns patients. Burns.
- Jakimiak B, Röhm-Rodowald E, Staniszewska M, et al. (2006) Microbiological assessment of efficiency of antibacterial modified textiles. Rocz Panstw Zakl Hig 57: 177-184.
- Bonafini C, Marzotto M, Bellavite P (2017) In vitro effects of Zinc in soluble and homeopathic formulations on macrophages and astrocytes. Homeopathy 106: 103-113.
- Schilling K, Bradford B, Castelli D, et al. (2010) Human safety review of "nano" titanium dioxide and zinc oxide. Photochem Photobiol Sci 9: 495-509.
- Vlachou E, Chipp E, Shale E, et al. (2007) The safety of nanocrystalline silver dressings on burns: a study of systemic silver absorption. Burns 33: 979-985.
- Yang Z, Liu ZW, Allaker RP, et al. (2010) A review of nanoparticle functionality and toxicity on the central nervous system. Journal of the Royal Society Interface 7: S411-S422.
- Gottesman T, Yossepowitch O, Lerner E, et al. (2014) The accuracy of Gram stain of respiratory specimens in excluding Staphylococcus aureus in ventilator-associated pneumonia. J Crit Care 29: 739-742.
- Huang SS, Septimus E, Kleinman K, et al. (2013) Targeted versus universal decolonization to prevent ICU infection. N Engl J Med 368: 2255-2265.
- Milstone AM, Goldner BW, Ross T, et al. (2011) Methicillin-resistant Staphylococcus aureus colonization and risk of subsequent infection in critically ill children: importance of preventing nosocomial methicillin-resistant Staphylococcus aureus transmission. Clin Infect Dis 53: 853-859.
- Sydnor ER, Perl TM (2011) Hospital epidemiology and infection control in acute-care settings. Clin Microbiol Rev 24: 141-173.
- Kang S, Lee J, Kim M (2017) The association between Staphylococcus aureus nasal colonization and symptomatic infection in children in Korea where ST72 is the major genotype: A prospective observational study. Medicine (Baltimore) 96: e7838.
- Ren G, Zhou M, Ding N, et al. (2016) Analysis on distribution features and drug resistance of clinically isolated Acinetobacter baumannii. Exp Ther Med 12: 1715-1718.
- Bloemendaal AL, Fluit AC, Jansen WM, et al. (2009) Acquisition and cross-transmission of Staphylococcus aureus in European intensive care units. Infect Control Hosp Epidemiol 30: 117-124.
- Sui W, Wang J, Wang H, et al. (2013) Comparing the transmission potential of Methicillin-resistant Staphylococcus aureus and multidrug-resistant Acinetobacter baumannii among inpatients using target environmental monitoring. Am J Infect Control 41: 411-415.
- Wang J, Wang M, Huang Y, et al. (2011) Colonization pressure adjusted by degree of environmental contamination: a better indicator for predicting methicillin-resistant Staphylococcus aureus acquisition. Am J Infect Control 39: 763-769.
- DeLeo FR, Otto M, Kreiswirth BN, et al. (2010) Community-associated methicillin-resistant Staphylococcus aureus. Lancet 375: 1557-1568.
- Williams VR, Callery S, Vearncombe M, et al. (2009) The role of colonization pressure in nosocomial transmission of methicillin-resistant Staphylococcus aureus. Am J Infect Control 37: 106-110.
- Kooistra-Smid M, Van Dijk S, Beerthuizen G, et al. (2004) Molecular epidemiology of Staphylococcus aureus colonization in a burn center. Burns 30: 27-33.
- Deeny SR, Cooper BS, Cookson B, et al. (2013) Targeted versus universal screening and decolonization to reduce healthcare-associated meticillin-resistant Staphylococcus aureus infection. J Hosp Infect 85: 33-44.
- Higashino T, Kamiya K (2011) Survival of MRSA attached to materials used in medical facilities at different humidity levels. Med Biol 155: 456-461.
- Oie S, Suenaga S, Sawa A, et al. (2007) Association between isolation sites of methicillin-resistant Staphylococcus aureus (MRSA) in patients with MRSA-positive body sites and MRSA contamination in their surrounding environmental surfaces. Jpn J Infect Dis 60: 367-369.
- Ho PL, Ho AY, Chow KH, et al. (2010) Surveillance for multidrug-resistant Acinetobacter baumannii: a lesson on definitions. Int J Antimicrob Agents 36: 469-471.
- Mezzatesta ML, D'Andrea MM, Migliavacca R, et al. (2012) Epidemiological characterization and distribution of carbapenem-resistant Acinetobacter baumannii clinical isolates in Italy. Clin Microbiol Infect 18: 160-166.
- Peng F, Mi Z, Huang Y, et al. (2014) Characterization, sequencing and comparative genomic analysis of vB-_AbaM-IME-AB2, a novel lytic bacteriophage that infects multidrug-resistant Acinetobacter baumannii clinical isolates. BMC Microbiol 14: 181.
- Khan AH, Baig KF, Mehboob R (2017) Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pacific Journal of Tropical Biomedicine 7: 478-482.
- Nwadike VU, Ojide CK, Kalu EI (2014) Multidrug resistant acinetobacter infection and their antimicrobial susceptibility pattern in a nigerian tertiary hospital ICU. Afr J Infect Dis 8: 14-18.
- Souli M, Galaini I, Giamarellou H (2008) Emergence of extensively drug-resistant and pandrug-resistant Gram-negative bacilli in Europe. Euro Surveill 13: 1-10.
- Siemers F, Fanghänel S, Bergmann P, et al. (2014) Management of a 4MRGN Acinetobacter baumanii outbreak in a burn unit. Handchir Mikrochir Plast Chir 46: 214-223.
- Zanetti G, Blanc DS, Federli I, et al. (2007) Importation of Acinetobacter baumannii into a burn unit: a recurrent outbreak of infection associated with widespread environmental contamination. Infect Control Hosp Epidemiol 28: 723-725.
Corresponding Author
M Аrgirova, M.D., PhD, Department of Burn and Plastic Surgery Clinic, MHATEM "N.I. Pirogov", Sofia, Bulgaria.
Copyright
© 2017 Аrgirova M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.