Infantile Haemangioma: Treatment in a Preterm Infant
Abstract
Introduction
Infantile Haemangioma (IH) is the most frequent benign vascular tumor of infancy, and active treatment is indicated in special situations. Topical timolol, it has been reported to be an effective and safe treatment for superficial IH, with rare adverse effects. There are very few case reports of topical timolol for the treatment of IH in a preterm infant.
Case report
We present a case of a 30-weeks premature infant that developed an ulcer on a IH in scrotum. He was treated with topical timolol 1% cream and in 2 weeks the ulcer was totally reepithelialized, with no local or systemic adverse effects.
Conclusion
In this case, the timolol 1% cream appears to be a safe option, however, prospective studies with serum timolol concentration testing, heart rate and blood pressure monitoring during treatment are needed.
Keywords
Dermatology, Neonatology
Introduction
Infantile Haemangioma (IH) is the most frequent benign vascular tumor of infancy, with an incidence of 23% in preterm infants, 2-3% in neonates and 10% after 1 year. Active treatment is indicated when large size or specific areas are involved, to prevent complications like ulceration, bleeding, infection and to diminish the potential scar. Ulceration is the most common complication and occurs in 16% or IH. There are very few reports concerning treatment of IH in preterm infants, although it's higher incidence at this age. Topical timolol has recently been reported to be an effective and safe treatment for superficial infantile haemangioma.
Case Report
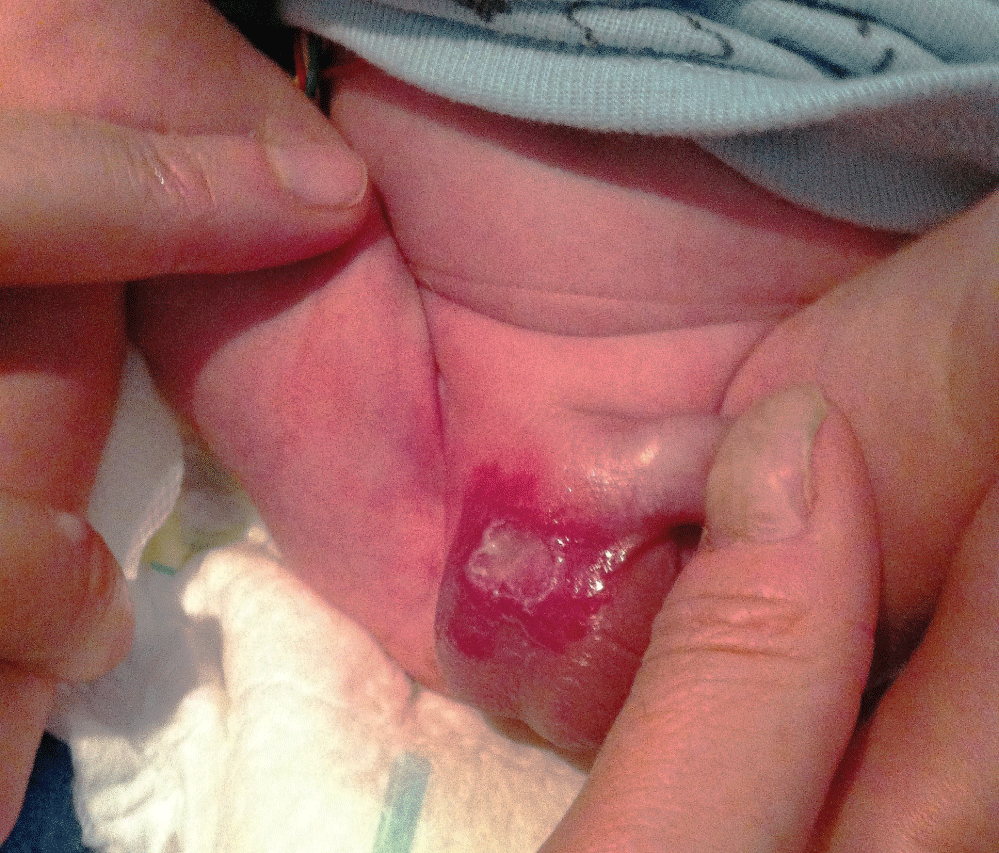
We report a case of a 30-weeks premature infant with a superficial haemangioma in scrotum since birth who developed an ulcer a month later. The patient was hospitalized in neonatal critical care unit for prematurity, fetal transfusion syndrome, low weight for gestational age, apneas and hyaline membrane syndrome. Physical examination revealed a red plaque of 3 cm of diameter on the right part of the scrotum with a central exudative and bleeding erosion of 1.5 cm (Figure 1).
At 37-weeks of post menstrual age (58 days of chronological age) and 1895 g of weight, it was decided to use topical timolol 1% cream, twice a day, to avoid systemic treatment. Vital signs were monitored (heart rate, blood pressure, temperature) during hospitalization. After the first week of treatment the ulcer size was significant smaller. The patient was discharged and the ambulatory treatment continued. 1 week later the ulcer was completely reepithelialized with an atrophic scar at the ulceration site (Figure 2). The patient continued the treatment with favorable evolution until he was 15-months-old, the IH was considerably smaller and the treatment was interrupted. No local or systemic adverse effects have been observed, neither impairment of growth and development of the patient.
Discussion
In 2008 Leaute-Labreze, et al. reported his finding on the effect of systemic propranolol on IH. Since then, propranolol has been the first line treatment; however, it has important side effects like hypotension, bradycardia, bronchospasm, hypoglycemia, hypothermia, among others. Topical timolol maleate a non-selective beta-adrenergic antagonist, widely used in ophthalmology, is 8 times more powerful than propranolol and has been reported as a successful medication for treating superficial IH in the literature. The first report was on 2010 by Guo, et al. who successfully treated a superficial IH of the eyelid. On 2013 Chan, et al. [1] published a randomized controlled trial showing the safety of timolol on small, non ulcerated IH. After that, there have been more reports about timolol efficacy and safety on large superficial IH [2]. Only a few cases of ulcerated IH treated with timolol 0.5% have been described before [3,4]. Frommelt, et al. published a retrospective study of 103 patients who were treated with topical timolol. Only 2 patients had symptomatic adverse effects. These 2 patients were preterm infants with an IH on the eyelid, treated with timolol 0.5% on the upper eyelid and intraocularly. They developed bradycardia, bronchospasm, lethargy and hypothermia. In this case we must consider that the intraocular administration of timolol has an important systemic absorption (80%) and has a rate of 3-4% of adverse effects in children. Systemic absorption after cutaneous application has never been studied and it is likely that transcutaneous absorption will vary greatly [5]. We considered high risk to administer systemic propranolol to a premature infant that had a hyaline membrane syndrome and episodes of apnea.
With the collaboration of the pharmacy unit of our hospital, a timolol 1% cream was elaborated using a solution of 25 mg/ml of timolol and a Beeler base. We used timolol 1%, taking into account that the patient was hospitalized and under monitoring, in the attempt of a faster reephetilization of the ulcer, prevent an imminent infection (diaper area) and a faster and better control of the pain. The first week of treatment, the administration of the timolol cream was under monitoring. There were not local or systemic adverse effects. The ulcer reepithelialized in only 2 weeks. The treatment was continued after the ulcer resolution to stabilize the growth of the IH in the proliferative phase and to avoid another ulcer. We stopped the treatment at 15 months of age, before the disappearance of the IH, because the involution was, at that moment, very slow and the IH was on the regression phase, so eventually it will disappear without the cream. We believe that the highest concentration of timolol and the use of cream as a vehicle could improve the local effect of timolol on the IH, accelerating the reepithelization. In this case, the timolol 1% cream appears to be a safe option, however, prospective studies with serum timolol concentration testing, heart rate and blood pressure monitoring during treatment are needed.
References
- Chan H, McKay C, Adams S, et al. (2013) RCT of timolol maleate gel for superficial infantile hemangiomas in 5- to 24-week-olds. Pediatrics 131: e1739-e1747.
- Neri I, Virdi A, La Placa M, et al. (2014) A perineal infantile haemangioma presenting as early ulcerations. Arch Dis Child Fetal Neonatal Ed 100: 393.
- Beal BT, Chu MB, Siegfried EC (2014) Ulcerated infantile hemangioma: novel treatment with topical brimonidine-timolol. Pediatr Dermatol 31: 754-756.
- Boos MD, Castelo-Soccio L (2016) Experience with topical timolol maleate for the treatment of ulcerated infantile hemangiomas (IH). J Am Acad Dermatol 74: 567-570.
- Frommelt P, Juern A, Siegel D, et al. (2016) Adverse Events in Young and Preterm Infants Receiving Topical Timolol for Infantile Hemangioma. Pediatr Dermatol 33: 405-414.
Corresponding Author
Ximena Rodríguez Vásquez, Department of Dermatology, Hospital Universitario Fundación Alcorcón, Budapest St 1, Alcorcón, P.C. 28922 Madrid, Spain, Tel: +34650813347.
Copyright
© 2017 Rodriguez-Vasquez X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.