The Mortal Association of Rothmund Thomson Syndrome and H1N1
Introduction
Rothmund Thomson Syndrome (RTS) is a rare autosomal recessive disorder. It was first described in 1868 by a German ophthalmologist named August Rothmund as a syndrome consisting of bilateral juvenile cataract sand rash. In 1923, a British dermatologist named Sydney Thomson used the term "Congenital Poikiloderma" for the association of rash and skeletal anomalies, with out presence of the cataract. In 1957, these two clinical conditions were determined to be the two variants of the same syndrome and were named as RTS [1]. It sex act prevalence is unknown. Three-hundred cases have been reported in the medical literature. It is of the 1200 rare disorders, defined in the National Organization Database [2].
Two sub types were described for RTS. Sub typing is made according to the presence of RECQL4 gene mutation on the eighth chromosome. RECQL4 gene provides instructions form a king one member of a protein family called RECQ helicases. Helicase protein shelps in DNA repair. In RTS type 1, poikiloderma, ectodermal dysplasia, congenital bone defects, and juvenile cataract a represent with out mutation of RECQL4; in RTS type 2, poikiloderma, congenital bone defects, and increased risk of osteo sarcoma are present together with mutation of RECQL4. Two-thirds of patients, known upto date, have been reported to be long to RTS Type 2 [2,3].
Cutaneous rash, the sparsity of hair, eyelashes and eyebrows, short stature, skeletal and teeth anomalies, bilateral juvenile cataracts, and early aging have been reported to be among the clinical characteristics of RTS. In RTS, an increased risk is present for some types of cancer such as bone (osteosarcoma) and skin (squamous cellcarcinoma), when compared to the normal population [1]. Its treatment is non specific. Standard treatment methods are used according to the developing complications. Calcium and vitamin D supplementation are recommended in patients with osteopenia or with the medical history of pathological fracture. For prevention from skin cancer, sun screen creams are used [4]. We aimed to present a 26-year-old H1N1 (+) patient, diagnosed with RTS and having bilateral diffuse pneumonia and to discuss the pulmonary infections in RTS patients with the review of the literature.
Case Presentation
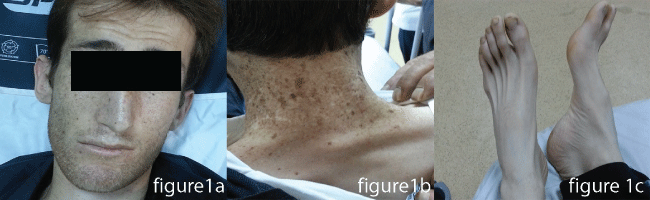
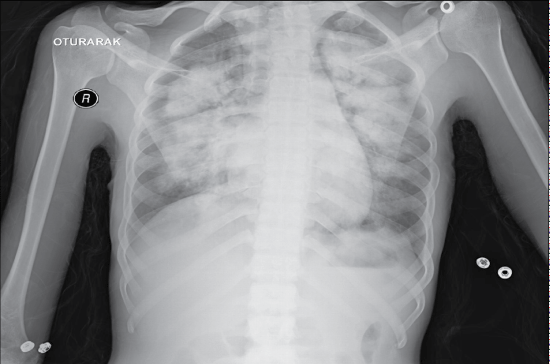
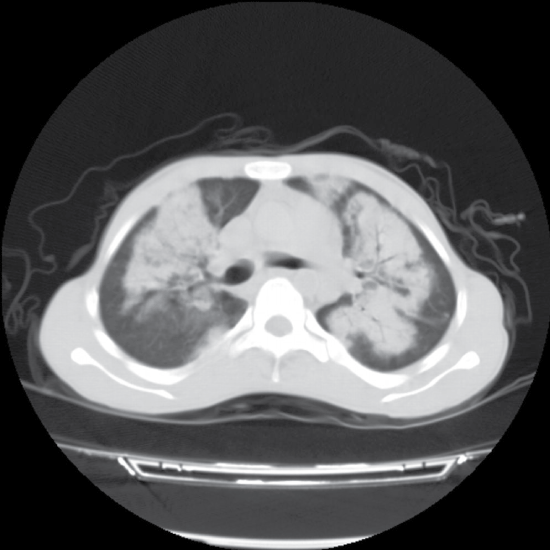
The twenty-six-year-old male patient was admitted to our emergency service with the complaints of fever, cough, and shortness of breath. His vital signs were as follows: Blood pressure 122/67 mmHg, Pulse: 110/min, Body temperature: 38.5 °C, Respiratory rate: 20/min. His SpO2 was measured as 67%. The patient's medical history revealed that he had been previously diagnosed with RTS type-2 by genetic analysis when he was 9-year-old. Because of his recurrent infections on his childhood and bone abnormalities, his parents need for an advance analysis with the physicians who were interesting their child. After this history and advance laboratuary tests he had been taken this diagnose. He had a cachectic and dyspneic appearance. Poikiloderma was present, particularly on his face and neck. He had cataract and sparsity of hair, eyebrows, and eyelashes. He had signs of early aging and symmetrical growth retardation (Figure 1). He had no skeletal anomaly. He had decayed teeth in his mouth, and on auscultation of the breath sounds, he had rales. His laboratory tests revealed normal results. PA chest X-ray was obtained, which revealed bilateral diffuse infiltrations (Figure 2). Chest CT was performed, which showed diffuse infiltrations, together with air bronchograms, bilaterally (Figure 3). Since SpO2 was 80% despite broncho dilator treatment, he was hospitalized in the intensive care unit with the preliminary diagnosis of pneumonia. His laboratory tests for IgA, IgG, and IgM revealed normal levels. He was diagnosed with the H1N1 infection with "Real-time" Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR), and his clinical condition worsen edduring his stay in the intensive care unit. Endo tracheal intubation was performed and the patient died, following 15 days of intensive care follow-up.
Discussion
To our knowledge, our patient has been the first and only patient in the literature with the diagnosis of RTS, who died due to swineflu. Sporadic gastrointestinal, hemorrhagic, and respiratory disorders have been reported but lower respiratory system infections were rarely reported [5] Influenza viruses are RNA viruses belonging to the Orthomyxoviridae family. The virus has the capacity to develop a different genetic variation, due to its molecular characteristics [6]. Swineflu virus was first seen in Mexico. Due to its genetical similarity with the influenza virus seen in pigs, it was named as swineflu. Its clinical features may show great variations, from mild symptoms to severe pneumonia with multifocal infiltrations, acute respiratory distress syndrome, and multiple organ failure. When pulmonary disorders, diabetes mellitus, morbid obesity, auto immunedisorders, administration of immuno suppressive treatments, neurological or cardiovascular disorders, and pregnancy are present, the course of the disease becomes more severe. Deaths due to swineflu have occurred mostly in Mexico; the mortality rate was estimated as 0.4% in Mexico. In other countries, the mortality rates were reported to be quite low in cases of swineflu [7]. In our case with RTS syndrome, swineflu had a mortal prognosis with out the presence of any additional disorder leading to immunodeficiency.
In conducted studies, no risk for recurrent respiratory system infection could be found in RTS patients [8]. Respiratory system disorders are met sporadically in RTS patients [9]. Although a clear relationship has not been identified between infections of there spiratory system and development of immuno deficiency, in some patients, immuno deficiency was reported [5,10-13]. Since he was 26-years-old, there was no history of recurrent infection, and the immuno globulin levels were normal, development of immunodeficiency was not considered in our case. RECQL4 proteins are maintain the structure and integrity of DNA, that helps in DNA repair. It was reported that the disorder in DNA helicases might disrupt the local defense mechanism with out affecting the immune system in general [14]. The negative impact of mutations of this gene, such as chromosomal instability, increased malignancy incidence, and focal immunity disorders, have been shown. The gene defects how sits effect more significantly in systems with rapid cell cycle, such as respiratory tract and skin [3]. We suggest that this situation affected the prognosis directly and led to a mortal clinical course.
References
- Pencovich N, Margalit N, Constantini S (2012) Atypical meningioma as a solitary malignancy in a patient with Rothmund-Thompson syndrome. Surg Neurol Int 3: 148.
- Barisonek KL, Protzman NM, Wobst GM, et al. (2014) Delayed Union of a Jones Fracture in a Patient With Rothmund-Thomson Syndrome: A Case Report and Review of the Literature. J Foot Ankle Surg 55: 291-293.
- Larizza L, Roversi G, Volpi L (2010) Rothmund-Thomson syndrome. Orphanet J Rare Dis 5: 2.
- Wang LL, Plon SE (1999) Rothmund-Thomson Syndrome. Gene Reviews®.
- Snels DG, Bavinck JN, Muller H, et al. (1998) A female patient with the Rothmund-Thomson syndrome associated with an hidrosis and severe infections of the respiratory tract. Dermatology 196: 260-263.
- Gallaher WR (2009) Towards a saneand rational approach to management of Influenza H1N1 2009. Virol J 6: 51.
- Schnitzler SU, Schnitzler P (2009) An update on swine-origin influenza virus A/H1N1: a review. Virus Genes 39: 279-292.
- Wang LL, Levy ML, Lewis RA, et al. (2001) Clinical manifestations in a cohort of 41 Rothmund-Thomson syndrome patients. Am J Med Genet 102: 11-17.
- Polese L, Merigliano S, Mungo B, et al. (2011) Report on a case of Rothmund-Thomson syndrome associated with esophageal stenosis. Dis Esophagus 24: E41-E44.
- Kubota M, Yasunaga M, HashimotoH, et al. (1993) IgG4 deficiency with Rothmund-Thomson syndrome: a case report. Eur J Pediatr 152: 406-408.
- Ito T, Tokura Y, Moriwaki S, et al. (1999) Rothmund-Thomson syndrome with herpes encephalitis. Eur J Dermatol 9: 354-356.
- Broom MA, Wang LL, Otta SK, et al. (2006) Successful umbilical cord blood stem cell transplantation in a patient with Rothmund-Thomson syndrome and combined immuno deficiency. Clin Genet 69: 337-343.
- Porter WM, Hardman CM, Abdalla SH, et al. (1999) Haematological disease in siblings with Rothmund-Thomson syndrome. Clin Exp Dermatol 24: 452-454.
- Reix P, Derelle J, Levrey-Hadden H, et al. (2007) Bronchiectasis in two pediatric patients with Rothmund-Thomson syndrome. Pediatr Int 49: 118-120.
Corresponding Author
Dr. Kocak Abdullah Osman, Medical Faculty, Department of Emergency Medicine, Atatürk University, Turkey, Tel: +90-442-344-7786.
Copyright
© 2017 Osman KA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.