Genetic Variation in Biomass Yield and Feedstock Composition and Trait Relationships in Lowland Switchgrass
Abstract
Switchgrass, a leading candidate for lignocellulosic bioenergy feedstock development in the USA, has received widespread researchers' attention in the recent years. For switchgrass to be an economical alternative to fossil fuels, significant biological and technological advancements have to be accomplished primarily in genetic improvement of biomass and ethanol yields. Understanding genetic composition of different populations would help in developing suitable breeding populations and efficient breeding strategies for target trait improvement. The objective of this study was to assess genetic variation among population sources with respect to biomass and ethanol yield and trait relationships. Five population sources were intercrossed to generate half-sib progenies, and half-sib progenies were established in Fall-2009 at Knoxville, TN, and evaluated for key feedstock traits initially (2010 and 2011) under one-cut and later (2012 and 2013) under a two-cut system. Results demonstrated no variation among germplasm source, however, half-sib families were significantly different for both biomass and ethanol yield. Results showed high narrow sense heritability (0.95) for biomass yield suggesting potential benefit of cultivar breeding targeting specific environments. Feedstock composition had low heritability (0.26 to 0.40). Analysis of trait relationship showed tillering ability as the most important trait contributing to biomass yield, explained 66% of variation. Biomass yield plant-1 was the most important predictor of ethanol yield plant-1, explained 99% of variation. Biomass yield was reduced when two-cut system was continued for more than one year, however, several genotypes have shown superior regrowth potential indicating the potential for developing switchgrass cultivars suitable for multiple-cutting management.
Keywords
Switchgrass, Biomass yield, Ethanol yield
Introduction
Switchgrass, a native perennial grass of North American Prairie, is chosen as a model herbaceous species for the development of bioenergy feedstock in the USA [1]. It is a warm-season C4 species with high biomass yield potential on marginal lands with limited fertilizers and water use [2,3]. Switchgrass is an outcrossing species due largely to high level of gametophytic self-incompatibility [4]. This has resulted in natural populations that are highly heterogeneous and each genotype highly heterozygous [5]. Based on adaptation preferences, switchgrass germplasms are broadly classified into lowland and uplands ecotypes that are also associated with 'L' and 'U,' cytotypes as evidenced from variation in chloroplast DNA [6,7]. The lowland ecotypes are found in the wet and hot climates in the south of about 42° latitude, while upland ecotypes are adapted in drier, cooler regions in the north of 34° latitude [8]. The lowland ecotypes such as 'Alamo' and 'Kanlow,' are tall-growing and high-biomass yielding relative to upland cultivars [2,9]. A wide array of ploidy ranging from diploid (2n = 2x = 18) to (2n = 12x = 108) have been reported [10,11]. However, the majority of adapted populations are either tetraploids (i.e., almost all of the lowland and a few upland ecotypes) or octaploids (i.e., most of the upland ecotypes) [10,12]. Research has shown that tetraploid switchgrass follows disomic inheritance [13].
Early switchgrass research was focused on its use as a forage crop, and primary breeding objectives were to improve forage yield and nutrition quality [14]. Significant gain has been made in improving forage yield and in-vitro dry mater digestibility (IVDMD) [15]. With the decision of US Department of Energy to promote switchgrass as a bioenergy feedstock, the cultivar breeding emphasis has shifted toward improvement of lignocellulosic biomass yield and conversion efficiency such as ethanol yield [16]. Energy Independence and Security Act of 2007 (EISA) and the Renewable Fuel Act (RFA) mandates that by the year 2022, 36 billion gallons of renewable fuel are to be produced in the United States with at least 16 billion gallons coming from lignocellulosic sources [16]. With current energy conversion limitations of switchgrass to useable energy, a large amount of biomass is needed to be grown to meet the demands of this initiative. With the large land allocation needed for future research and development to meet such biomass demand, it is imperative to utilize switchgrass for as many purposes as possible to maximize its economic efficiency [5]. Focusing breeding efforts on both high biomass and ethanol yield potential is a possible solution to future land use limitations.
With its continued importance as a warm-season grass to supply hay during summer season, switchgrass cultivars can be bred targeting for potential dual use, as forage as well as bioenergy feedstock. This can be accomplished by implementing a two-harvest system, the first cut would be for forage, while the second is for bioenergy production [5]. Under 2-cut system, the first harvest between pre-boot stage to pre-anthesis for forage use and the second harvest at the end-of-season for use as bioenergy feedstock has been suggested [17]. Earlier results on biomass production under multiple harvest system were not promising, because of low cumulative seasonal biomass yield or marginally higher yields would not be adequate to offset the cost associated with additional harvesting [2,18]. Such results, however, are not surprising given the fact that switchgrass is relatively new in its breeding history and the species has not been bred for multiple-cutting system. We observed a tremendous genetic variation with respect to regrowth after summer-clipping that could be exploited for cultivar improvement targeting both forage and bioenergy feedstock use.
One of the major constraints in switchgrass cultivar improvement is the complex inheritance of traits of primary importance, biomass yield and composition quality. Narrow sense heritability estimates for biomass yield ranged from 0.17 to 0.59 [19-21]. Observed low genetic selection gain in forage digestibility (IVDMD) also indicates poor trait heritability [15]. Breeders often practice indirect selection on secondary trait(s) to improve primary low-heritability traits such as biomass yield. However, for indirect selection to be a success, it requires that the breeding population is genetically diverse in primary trait, there is high genetic correlation between the indirect trait and the primary trait, and the indirect trait has high heritability [8]. In switchgrass, tiller number, plant height, and stem thickness are the key biomass yield related traits [19,22]. In lowland switchgrass grown in the south and southeastern USA, tiller number and plant height have shown moderate to high correlation (r = 0.66 to 0.74) with biomass yield [19,22]. Their low to moderate heritability estimates (0.26-0.47) warrants a cautious approach in using these traits for indirect selection [19].
Genetic improvement of switchgrass with respect to conversion efficiency has also been emphasized [23]. Reduced lignin content is a preferred trait for a fermentation platform due to increased access for hydrolyzing enzyme to the cellulose and hemicellulose substrates. Concerted efforts to reduce recalcitrance and enhance enzymatic digestion via transgenic approaches such as downregulation of key genes in lignin pathway have yielded promising results [24]. However, with the regulatory requirement for the transgenics in cross pollinated species, selection on natural variation would be the most sustainable way to modify feedstock composition and improve conversion efficiency. Significant accomplishment improvement in-vitro dry matter digestibility (IVDMD) and reducing lignin content in forage type switchgrass demonstrated the potential of genetic improvement for conversion efficiency through conventional selection [15,23]. An understanding of the extent of genetic variation in both biomass yield and feedstock composition in various populations would help develop appropriate breeding strategies. The objectives of this study are to assess genetic variation among different lowland switchgrass germplasms for biomass and ethanol yield under single end-season harvesting and two-cut system, and assess potential for developing switchgrass cultivars for dual-use, forage and bioenergy feedstock. The results presented here will be useful for setting breeding priorities and developing future breeding strategies for switchgrass.
Material and Methods
Generation of half-sib families
A polycross nursery comprised of five lowland-switchgrass germplasm sources was established at the University of Tennessee, East Tennessee Research and Education Center (ETREC), Plant Sciences Unit (35° 54' 19.67" N, 83° 57' 14.29" W), Knoxville, TN. The five germplasm sources include, three plant introduction accessions, 'PI 421999', 'PI 422016', and 'PI 607837', acquired from USDA-Germplasm Resource Information Network (USDA-GRIN), and two improved populations, 'Cimarron', and 'NSL-2001-1', obtained from Oklahoma State University (OSU). Plant introduction accessions, PI 421999 and PI422016 were native populations originated from Arkansas and Florida, thus will be hereafter refer to as 'ARK' and 'FL' respectively. PI 607837 (hereafter refer to as 'TX') was developed at the Grassland, Soil, and Water Research Laboratory, Temple, TX from 'Alamo' following two cycles of recurrent restricted phenotypic selection [25]. Of the two OSU developed populations, Cimarron (here after refer to as 'OKS') is a released cultivar derived from Alamo and PMT279; and NSL-2001-1 (hereafter refer to as 'OKN') is an elite breeding population derived from polycrossing several southern and northern lowland genotypes.
Random seed lots of each population source was seeded in the greenhouse in 31 December, 2006 and early germinating 14 plus seedlings of each population were grown for over four and half months, and transplanted to a polycross block on 21 May, 2007. The polycross block was a space-planted plot, 5-rows × 14-plants, plants spaced 1.2 m apart. Each population source was represented by 14 genotypes in the polycross nursery, and plants were placed at random. Open pollinated half-sib (HS) progeny seeds were collected separately from each genotype in Oct. 2008. A total of 70 HS families, i.e., 14 HS progenies per population source (5), were generated.
Field evaluation of half-sib progenies: The HS progeny seed were seeded on April 15, 2009 in the 72-well flats and seedlings were raised in the greenhouse. On June 30, 2009, the seedlings were transplanted in a space-planted field nursery, spaced 1.2 m × 1.2 m, at ETREC Holston Unit in Knoxville, TN (35° 58' 2.31" N, 83° 51' 27.92" W). Families were repeated by 10 pants each. Ten genotypes per HS progeny and 14 HS progeny per germplasm source were evaluated making a total of 700 genotypes in the entire experiment. The entire population was blocked to within the field gradient. No observation was taken during the establishment year, and the plot was mowed at the end of the fall 2009. In the spring of the each post-establishment year the plot was amended with 60 kg ha-1. To control weeds, 2, 4-D at 3.31 L ha-1 was applied post-emergence in establishment each spring during the study period.
Tiller count, plant height (i.e., measured from ground to tip of the panicle), stem thickness (i.e., measured on uppermost fully developed node), and fall biomass yields were recorded for HS progenies at the Holston unit. Tiller counts, plant height, and stem diameter were recorded for each individual plant near maturity during 2010 and 2011. For years 2010 and 2011, single end-season biomass yield was recorded in early November after the first killing-frost using a sickle bar mower set at 15 cm high. In the years 2012 and 2013, the biomass from spring growth was harvested for use in a separate study, which occurred on 30 May to 4 June in 2012 and 10 to 12 June in 2013; and the fall biomass yield (i.e., second cut) was harvested at the end of the fall. For each plant in the HS progeny, a hand-grab sample was taken during harvest, weighed, dried in a batch oven (Wisconsin Oven Corporation, East Troy, WI, USA) for 24-48 hours at 49 ℃, moisture content at harvest estimated, and was used to estimate individual plant's dry matter yield.
The dry biomass samples were then ground using Wiley Laboratory Mill and sieved through a 2-mm screen and analyzed by Near Infrared NIR [26] spectroscopy (FOSS NIR Systems 6500 Feed & Forage Analyzer with Sample Transport Reflectance Only, FOSS Analytical, Hillerod, Denmark). Whole plant samples were analyzed for 2010/2011/2013 growth, while for 2012 growth, stems and leaves were analyzed separately. Acid detergent fiber (ADF = Cellulose + lignin) and neutral detergent fiber (NDF = cellulose + hemicelluloses + lignin) estimates from NIR were used to compute cellulose and hemicellulose contents of biomass samples and expected ethanol content was determined according to the procedure described by Badger [27], assuming 76 and 90% conversion efficiency from cellulose and hemicellulose, respectively. The ethanol content from each plant sample was individually adjusted to total dry matter yield (DM) to obtain the theoretical ethanol yield for corresponding plant.
Data analysis
Most of the HS plants representing FL source suffer severe winter- kill and therefore data representing FL source were excluded from analysis. For the remaining HS progenies, data were analyzed using mixed procedure in SAS 9.3 (SAS institute, Inc. Cary, NC).
Year was used as fixed effects in the model because biomass yields were recorded from the same plant each year. Half-sib progeny and population source were used as fixed effect for mean comparison, and as random effect to estimate component of variation. The least significant difference (LSD) mean separation was used to test for any difference among means due to population sources and due to HS progenies within each population source. The total phenotypic variance was partitioned as follows:
Where is the total phenotypic variation; is the component of variation among germplasm sources; is the component of variation due to half-sib progenies within germplasm source; and is the component of variation due to differences in genotypes within half-sib progenies plus the error variance. In tetraploid species with disomic inheritance, the variation among half-sib progenies is;
Where is additive genetic variation, and is variation due to additive × additive gene interaction. Given a very large denominator, the contribution of to variation among half-sib progenies is expected to be nominal which means total additive genetic variation is equal to 4 times the variation among HS. With this, narrow-sense heritability can be obtained as a ratio of additive genetic variation to total genetic variation, symbolically;
Correlations and simple and multiple regression analysis were performed between biomass yield and its component traits using SAS. Similarly, ethanol yield per plant was regressed on biomass yield and fiber compositions. Plant regrowth potential under 2-harvet system is discussed based on genetic variation among half-sib progenies.
Results and Discussion
Biomass yield
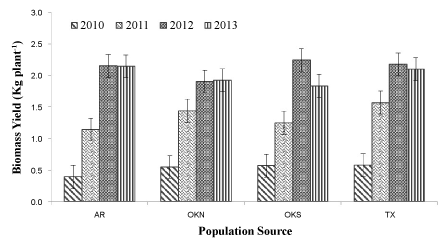
Biomass yields of half-sib progenies derived from four population sources; ARK, OKN, OKS, and TX, were analyzed separately for 2010 to 2011 (yr-2 and yr-3 growth) under 1-cut system and 2012 to 2013 (yr-4 and yr-5 growth) under 2-cut system. Mean annual biomass yield under 1-cut system across years (2010 to 2011) was 1.41 kg plant-1 (Table 1). Biomass yield plant-1 was more than doubled from 0.87 kg in 2010 to 1.97 kg in 2011 attributable primarily to the stand maturity, a common phenomenon observed in perennial grasses where stand may take about 3 years to achieve full production potential. Three population sources, OKN, OKS, and TX were similar in their mean half-sib progeny performances (range: 1.96 to 2.01 kg plant-1), however, their biomass yields were 47% higher when compared with average performance of half-sib progenies derived from ARK source (1.06 kg plant-1). This likely reflects the fact that the former three populations involved 2 or more cycles of selection while ARK represented a native population.
In 2012 and 2013, when biomass was harvested twice each year (i.e., 2-cut system, late-spring-clipping for use as hay, and the end-of-fall clipping for bioenergy use) the population sources were similar in their half-sib progeny performance on both fall and seasonal total biomass production (Table 1). As under 1-cut system, there was significant influence of year. The mean seasonal total biomass yield in 2012 (1.97 kg plant-1) was identical to 2011 biomass yield recorded under 1-cut system. When 2-cut system was continued in 2013, the seasonal biomass yield was dropped to 1.44 kg plant-1 which was 27% less compared to seasonal biomass yield in 2011 (1-cut) as well as in 2012 (2-cut); although biomass yield drop was more drastic for fall harvest than for the spring harvest (Table 1).
Under 2-cut system, average fall-biomass yield was only a third of the full-season biomass yield. Such results were consistent with earlier observations where the first forage cut accounted for around 60-80% of the seasonal total biomass production, but could vary with the amount and the distribution of precipitation.
The low fall biomass yield under 2-cut system could be attributable largely to the nutrient loss due to additional biomass harvest. Under 2-cut system, the first cut removes a large amount of N impacting the fall biomass yield [17,28,29]. Boe and Lee [30] also observed that single cut system used only a 67% of N removed by a two-cut system. Also the timing of the spring-cut may influence the fall biomass yield under 2-cut system. Biomass harvest pre-anthesis has resulted into diminishing stands and biomass yields over time [31]. The spring harvest reported here for 2012 and 2013 occurred at pre-boot stage. With no additional N fertilizer applied following the first-cut, low available soil N could be responsible for reduced fall-biomass yield in 2012 and 2013. The low fall-biomass yield in 2013 compared to 2012 could be partly due to the timing of spring-harvest which occurred 10 days later in 2013 than in 2012 resulting 10 days shorter growth duration for fall 2013 compared to 2012. In addition, lowland switchgrass has not been selected for higher biomass under multiple harvest year-1. Thus a large differences in biomass yield between years 2012 and 2013 within 2-cut system likely indicate the lack of tolerance to multiple harvest system. Sanderson and Moore [32] have reported reduced seasonal biomass yield with increasing harvesting frequencies.
Genetic variation and estimation of heritability
Under 1-cut system, the seasonal biomass yields of half progenies ranged from 0.38 to 2.46 kg plant-1 (Table 2). The components of genetic variation among half-sib progenies remain fairly similar among population sources (Table 3). However, the population sources were different in the relative contribution due to variation among half-sib progenies to total phenotypic variation. Overall analysis of variance revealed that 24% of the total variation was attributable to the variation among half-sib progenies. Among half-sib progeny variation accounted for 9.0, 18.0, 21.0 and 14.3% of the total phenotypic variation in TX, OKS, OKN and ARK sources, respectively (Table 3). A large amount of phenotypic variation was due to plant-to-plant variation within half-sib progenies which also comprised 75% of the additive genetic variation. This portion of genetic variation could only be exploited through among and within family selection procedures [33].
Heritability
Overall estimate of narrow sense heritability () was 0.95. When analyzed separately, the estimates varied widely among germplasm sources, OKN had the highest (0.83) and TX had the lowest heritability (0.36) estimates (Table 3). The heritability estimates obtained from this study were higher compared to those reported from earlier studies [19]. This could reflect the fact that the current study was conducted in a single location, although multiyear data were analyzed. The results suggested the potential benefits of cultivar breeding targeting well-defined ecoregions that could achieve higher genetic gain from selection.
One-cut versus two-cut system
Switchgrass cultivars that are superior in regrowth ability under multiple-harvesting system offer growers an added benefits, particularly by supplying nutritious forage or hay during warm-summer. Results showed that, compared to biomass harvested from a matured stand (2011) under 1-cut system, 2-cut system was less productive (Table 1). These results confirm the earlier observations 18. Although, [34] reported a marginal biomass yield gain (20%) from 2-cut system over 1-cut system, the difference was not large enough to compensate the cost associated with additional harvest.
Since, there has been no attempt to select lowland switchgrass for superior regrowth ability under multiple harvest system its poor performance under multiple harvest system is not surprising. We observed a tremendous diversity among and within HS progenies in regrowth vigor under 2-cut system (data not shown; DNS). When the best 20% HS progenies demonstrating superior regrowth ability from each germplasm source were analyzed, their average yields under 2-cut system was 32 to 88% higher compared to matured-stand-yield recorded for the same HS progenies under 1-cut system (Figure 1). However, it was interesting note that the average 2010 biomass yield of these selected 20% superior genotypes was only 60% of the overall mean biomass yield recorded for the same year (DNS). This could be a concern that superior regrowth under repeated harvest or superior later-year-performance might be associated with poor initial-year vigor. Results also showed that the 2010 biomass yield had low predictive value, explained only 30% of the variation of the later-year's seasonal biomass yield. These results demonstrated that selection based on initial two-year performance could be misleading for the development of feedstock cultivars that are intended for harvesting biomass 10 to 15 years post-establishment. Several genotypes showing vigorous growth after five years of evaluation with latter two years under 2-cut system demonstrate the potential for improving switchgrass with respect to tolerance to multiple-harvest thus ensuring long term biomass yield performance of future cultivars. Further studies are needed to determine whether breeder can simultaneously improve initial year's biomass yield and later year yield performance.
Trait correlation
Analysis using HS progeny mean values across 2010 and 2011 showed biomass yield highly correlated with tillering ability (r = 0.81), moderately correlated with plant height (r = 0.69) and heading days (r = 0.60), but not correlated with tiller diameter (Table 4). Individually, tillering ability, plant height, and heading days explained 66, 46, and 35% of the variation in plant biomass yield (Table 5). Multiple regression analysis showed that tillering ability and plant height together explained 81% (adjusted R-square) of biomass yield variation. Further improvement in prediction is possible by adding days to heading in the model which would explain 83% of the total variation in biomass yield (Table 5). Earlier researchers also reported positive associations of biomass yield with tillering ability and plant height while days to heading seem to be less important [19,22,35]. Lemus, et al., [35] also reported significant negative association of plant height with ash content (-0.80), tissue nitrogen (-0.66) and acid detergent lignin content (-0.52), and lesser of these 3 elements is desirable when switchgrass is grown for use a biofuel feedstock. Although, tillering ability appeared to be the most important trait contributing to biomass production, all of these predictions were made under space-planted conditions, and it is not clear if such relationships would hold true when the crop is planted under sward-planted conditions. When data were analyzed using individual plant data, the adjusted R-squares obtained for tillering ability, plant height, and days to heading, respectively, were 60, 48, and 26% of the R-squares obtained using progeny mean data (DNS). Such discrepancy in results is due to genetic differences among genotypes representing each family as well as due to the environmental variation (noise). This also demonstrates the advantage of using mean data as opposed to single plant data in analysis of associations between biomass yield and its component traits.
Ethanol yield
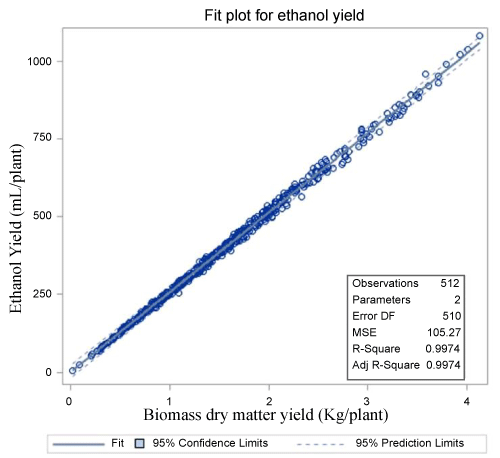
Predicted mean ethanol yield plant-1 under 1-cut system (2010 to 2011) was 364 mL which was reduced to 150 mL for fall biomass harvest under 2-cut system (2012 to 2013) due to the reduced biomass yields (Table 6). There was significant year to year variation in ethanol yields within each harvest system, mean ethanol yield was more than doubled from 2010 to 2011 under 1-cut system while there was a 72% reduction in ethanol yield from 2012 to 2013 under 2-cut system. However, in both cases, the differences in per plant ethanol yields corresponded largely to year to year variation in biomass yield. Low ethanol yield under 2-cut system compared to 1-cut system also reflects the fact that such predictions under 2-cut systems were made only for fall biomass harvest which was about a third of the seasonal biomass yield recorded during the same period. Predicted ethanol yield did not differ among population sources except for ARK under 1-cut system which was only 70% of average ethanol yield estimated for the other three population sources, again the discrepancy is attributable mainly to the differences in biomass yield (Table 6). The biomass yield was the single most important predictor of the ethanol yield plant-1 explaining about 99% of variation (Figure 2). The current study was conducted under space-plant condition, and further studies are needed to confirm if such relationship holds when switchgrass is evaluated under sward.
Germplasm sources differed significantly in ethanol content kg-1 dry biomass, however, the variation was too small (i.e., ranged from 255 to 261 under 1-cut system and 251-258 ml under 2-cut system) indicating potential challenge for its improvement (Table 7). However, there was tremendous variation among pants within HS progenies with overall ethanol content kg-1 dry biomass ranging from 227-290 mL under 1-cut and 213 to 281 mL under 2-cut system (DNS). Population sources were less variable also on key feedstock composition traits. The cellulose and hemicellulose contents, respectively, ranged from 40.5 to 41.5 and 36.6 to 37.1% under 1-cut system and 36.7 to 38.1 and 39.9 to 40.4% under 2-cut system (Table 7). As in the case with ethanol content, there was greater variation among plants within HS progenies with overall range of 36.2 to 45.2 and 29.3 to 43.7% cellulose; and 22.6 to 40.7 and 33.2 to 43.9% hemicellulose, under 1 and 2-cut systems respectively. Compared to season-long grown biomass under 1-cut system, the fall-harvested biomass under 2-cut system was lower in cellulose and lignin and higher in hemicellulose contents (Table 7). This showed that some ethanol yield is likely to be compromised when following 2-cut system due to reduced cellulose content in the feedstock. Mean lignin content, under 1-cut system was 10.1% and did not vary among population sources while it was reduced by half under 2-cut system. Lignin content varied greatly among plants within HS progenies with overall ranged from 5.7 to 13.4% under 1-cut system and 3.2 to 6.8% under 2-cut system.
Further analysis of component of genetic variation under 1-cut system revealed that half-sib progenies were different in ethanol, cellulose, and hemicellulose content kg-1 dry biomass (Table 8). The narrow sense heritability estimates were 0.64, 0.40, and 0.26, respectively for ethanol, cellulose and hemicellulose content kg-1 dry biomass demonstrated some prospect for improvement. Although, overall range of hemicellulose content was similar to cellulose content, its negative association with biomass yield (r = 0.45) suggest a likely trade-off between the two key traits. Half-sib progenies were not different in lignin content and its wide range among plants within HS progenies indicated some prospect, if any, for improvement only through within family selection. Negative association between lignin content and biomass yield could pose some challenge for modification in lignin content via conventional breeding. Transgenic approaches to reduce lignin content in switchgrass have been targeted specifically to reduce expression of syringyl lignin which resulted into improved conversion efficiencies without impacting biomass yield [36]. Future research is needed to investigating natural variation in switchgrass for specific type of lignin.
Understanding of relative contribution of leaf and stem tissues is important to determine plant architecture for bioenergy feedstock production [37]. For this, ethanol, cellulose, hemicellulose, and lignin content were estimated separately for leaf and stem tissues from fall-2012 samples. There was a significant difference between tissue-type in ethanol yield kg-1 biomass (Table 9). As expected stem tissue produced slightly higher ethanol (277 mL) kg-1 biomass compared to leaves (266 mL); however, it was due largely to the differences in hemicellulose content which was higher in the stem (43.6%) compared to leaves (39.2%). The leaf tissue had marginally higher cellulose content (41.7%) than the stem tissue (40.8%) which was in contrast to earlier observations in lowland switchgrass where cellulose was higher in stem tissue than in leaf tissue; while hemicellulose was not different between tissue types [37]. Their observations were from full-season grown matured plants while current results were from fall-grown less matured.
It is clear from above results that there is tremendous potential for biomass yield improvement using both among and within progeny selection procedures. Superior tillering ability and taller plants are likely to contribute to higher biomass yield. Biomass yield, being the single most important predictor for ethanol yield per plant should be the primary focus for cultivar improvement. Very low variation among population sources and their half-sib progenies were less variable with respect to ethanol content and its component traits suggest more complex genetics of these traits, posing challenge in their improvement. Although the current populations of switchgrass are less productive under multiple cutting management tremendous genetic diversity among half-sib families demonstrated potential for improvement in superior regrowth ability under at least 2-cut system. However, poor initial years performance of families selected for superior regrowth after four years post establishment with later two years under 2-cut system could be a concern for breeding. Further study is needed to investigate if simultaneous improvement in initial year and later year performances is possible.
References
- McLaughlin SB, Kszos LA (2005) Development of switchgrass (Panicum virgatum) as a bioenergy feedstock in the United States. Biomass & Bioenerg 28: 515-535.
- Bouton J (2008) Improvement of switchgrass as a bioenergy crop.
- Vogel KP (1996) Energy production from forages (or American agriculture-back to the future). J Soil Water Conserv 51: 137-139.
- Martinez-Reyna JM, mVogel KP (2002) Incompatibility systems in switchgrass. Crop Sci 42: 1800-1805.
- Parrish DJ, Fike JH (2005) The Biology and Agronomy of Switchgrass for Biofuels. Crit Rev Plant Sci 24: 423-459.
- Casler MD, Vogel KP, Taliaferro CM, et al. (2004) Latitudinal adaptation of switchgrass populations. Crop Sci 44: 293-303.
- Missaoui AM, Paterson AH, Bouton JH (2006) Molecular markers for the classification of switchgrass (Panicum virgatum L.) germplasm and to assess genetic diversity in three synthetic switchgrass populations. Genet Resour Crop EV 53: 1291-1302.
- Casler MD (2012) Switchgrass breeding, genetics, and genomics. In: A. Monti, editor Switchgrass. Springer, London, 29-53.
- Wullschleger SD, Davis EB, Borsuk ME, et al. (2010) Biomass Production in Switchgrass across the United States: Database Description and Determinants of Yield. Agron J 102: 1158-1168.
- Hopkins AA, Taliaferro C, Murphy CD, et al. (1996) Chromosome number and nuclear DNA content of several switchgrass populations. Crop Sci 36: 1192-1195.
- Nielsen EL (1947) Plyploidy and winter survival in panicum-virgatum L. Journal of the American Society of Agronomy 39: 822-827.
- Narasimhamoorthy B, Saha MC, Swaller, et al. (2008) Genetic Diversity in switchgrass collections assessed by EST-SSR markers. Bioenerg Res 1: 136-146.
- Okada M, Lanzatella C, Saha MC, et al. (2010) Complete Switchgrass Genetic Maps Reveal Subgenome Collinearity, Preferential Pairing and Multilocus Interactions. Genetics 185: 745-760.
- Vogel KP (2004) Genetic Modification of Switchgrass for Improved Biofuel Production. In: L Sollenberger, Warm-season (C4) grasses, Agronomy Monograph 45. ASA, CSSA, and SSSA, Madison, WI.
- Vogel KP, Mitchell RB, Sarath G, et al. (2013) Switchgrass biomass composition altered by six generations of divergent breeding for digestibility. Crop Sci 53: 853-862.
- Qualls DJ, Jensen KL, Christopher DC, et al. (2012) Analysis of factors affecting willingness to produce switchgrass in the southeastern United States. Biomass and Bioenergy 39: 159-167.
- Vogel KP, Brejda JJ, Walters DT, et al. (2002) Switchgrass biomass production in the midwest USA: Harvest and nitrogen management. Agron J 94: 413-420.
- Sanderson MA, Read JC, Reed RL (1999) Harvest management of switchgrass for biomass feedstock and forage production. Agron J 91: 5-10.
- Bhandari HS, Saha MC, Mascia PN, et al. (2010) Variation among half-sib families and heritability for biomass yield and other traits in lowland switchgrass (Panicum virgatum L.). Crop Sci 50: 2355-2363.
- Newell LC, Eberhart SA (1961) Clone and progeny evaluation in the improvement of switchgrass, Panicum virgatum L. Crop Sci 1: 117-121.
- Talbert LE, Timothy DH, Burns JC, et al. (1983) Estimation of genetic-parameters in switchgrass. Crop Sci 23: 725-728.
- Das MK, Fuentes RG, Taliaferro CM (2003) Genetic variability and trait relationships in switchgrass. Crop Science 44: 443-448.
- Casler MD, Buxton DR, Vogel KP (2002) Genetic modification of lignin concentration affects fitness of perennial herbaceous plants. Theor Appl Genet 104: 127-131.
- Fu C, Mielenz JR, Xiao X, et al. (2011) Genetic manipulation of lignin reduces recalcitrance and improves ethanol production from switchgrass. PNAS 108: 3803-3808.
- Tischler CR, Elbersen HW, Hussey MA, et al. (2001) Registration of TEM-SLC and TEM-SEC switchgrass germplasms. Crop Sci 41: 1654-1655.
- Kiniry JR, Cassida KA, Hussey MA, et al. (2005) Switchgrass simulation by the ALMANAC model at diverse sites in the southern US. Biomass & Bioenerg 29: 419-425.
- Badger PC (2002) Ethanol from cellulose: A general review.
- Fike JH, Parrish DJ, Wolf DD, et al. (2006) Long-term yield potential of switchgrass-for-biofuel systems. Biomass & Bioenerg 30: 198-206.
- Liu XJ, Fike JH, Galbraith JM, et al. (2015) Biosolids Amendment and Harvest Frequency Affect Nitrogen Use Dynamics of Switchgrass Grown for Biofuel Production. Bioenerg Res 8: 560-569.
- Boe A, Lee DK (2007) Genetic variation for biomass production in prairie cordgrass and switchgrass. Crop Sci 47: 929-934.
- Casler MD, Boe AR (2003) Cultivar × environment interactions in switchgrass. Crop Sci 43: 2226-2233.
- Sanderson MA, Moore KJ (1999) Switchgrass morphological development predicted from day of the year or degree day models. Agron J 91: 732-734.
- Casler MD, Brummer EC (2008) Theoretical expected genetic gains for among-and-within-family selection methods in perennial forage crops. Crop Sci 48: 890-902.
- Fike JH, Parrish DJ, Wolf DD, et al. (2006) Switchgrass production for the upper southeastern USA: Influence of cultivar and cutting frequency on biomass yields. Biomass & Bioenerg 30: 207-213.
- Lemus R, Brummer EC, Moore KJ, et al. (2002) Biomass yield and quality of 20 switchgrass populations in southern Iowa, USA. Biomass & Bioenerg 23: 433-442.
- Fu C, Mielenz J, Xleriao X, et al. (2010) Genetic modification of switchgrass for improved biofuel production. In Vitro Cell Dev Biol Anim 46: S26-S27.
- Bhandari HS, Walker DW, Bouton JH, et al. (2014) Effects of ecotypes and morphotypes in feedstock composition of switchgrass (Panicum virgatum L.). GCB Bioenergy 6: 26-34.
Corresponding Author
Eifion Hughes, University of Tennessee, Knoxville, 37996, USA.
Copyright
© 2017 Falasca SL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.