Prognostic Value of Neuropsychological Profile in the Progression from Mild Cognitive Impairment to Dementia: A Retrospective Study
Abstract
Mild cognitive impairment (MCI) and dementia, particularly Alzheimer´s disease (AD), encompass a spectrum of cognitive disfunction with severe impact and prevalence in the elderly. To study prognostic factors that can influence the progression of MCI to dementia, we examined the medical records from the dementia outpatient clinic of a tertiary hospital during a 6-year period, and compared the patients initially diagnosed with dementia or MCI, and patients that progressed to dementia to the patients that maintained the diagnosis of MCI. From 782 patients, 20.3% were diagnosed with AD and 12.9% with MCI, 18.4% of which progressed to dementia in a median period of 53 months, with an annual conversion rate of 4.3%. From the neuropsychological test battery applied to these patients, the long-term percent retention index of the Auditory Verbal Learning Test and the memory component of Dementia Rating Scale-2 (DRS-2), showed to predict the progression to all-cause dementia and AD specifically, in a logistic regression model. It also correlates to faster progression to dementia in the survival analysis. Our study emphasizes that MCI is a clear precursor of dementia, particularly in AD and, even in a small cohort analysis, episodic memory dysfunction is a prominent feature in the early stages of the disease and can predict a faster progression to dementia. Furthermore, this was captured by DRS-2, a validated neuropsychological battery for the evaluation of global cognition, that can be applied in general clinical setting.
Keywords
Alzheimer Disease; Dementia; Dementia progression; Mild Cognitive Impairment; Neuropsychological tests.
Introduction
There is an increasingly high global impact associated with dementia, especially Alzheimer´s disease (AD), the 5th cause of death worldwide [1]. In Portugal, approximately 5,91% of people with 60 years or older have dementia, contributing with more than 11,9% of the years lived with incapacity, higher than any form of cancer (2,4%)[2]. In a community based study, Gonçalves-Pereira M. et al. estimated in 2017 that the prevalence rate of dementia in people with 65 and older in Southern Portugal was9.23% [3].
Not with standing, the deposition of amyloid-ß per se, a typical neuro pathological signature of AD, does not predict progression to AD in the short-term (30 months) [4], suggesting the need for a long-lasting phase before the development of clinically evident disease. The symptoms appear typically 10-15 years post the onset of neuropathology, constituting a window of opportunity to intervene on potentially modifiable risk factors [5].
Mild Cognitive Impairment (MCI) is a stage between the expected cognitive decline with normal ageing and the cognitive and functional decline characteristic of dementia. The clinical diagnosis of MCI relies on establishing objective cognitive impairment but with preserved functional independence, which distinguishes this entity from dementia [6]. A recent study showed that 40-53% of individuals with MCI had prodromal AD and 46% were in the high likelihood of developing AD [7].
In that sense, it is relevant to consider what drives the conversion from MCI to dementia. Concerning specific predictors, amyloid deposition and neuronal injury markers offer the most accurate prognosis [7], and the elevation of the tau to amyloid ratio in the CSF precedes the clinical symptoms of MCI and its diagnosis by more than 5 years [8]. However, these measurements are not routinely used in the asymptomatic setting, not constituting suitable candidates for prognostic tests [9]. Furthermore, invasiveness, high cost, and poor availability of these detection methods restrict their widespread use as clinical diagnostic tools. Edmonds et al. showed that subtle cognitive decline measured by NPT can act as a suitable first marker of cognitive decline, allowing earlier identification of adults at risk for progressing to more prominent disease states [10]. Neuropsychological Testing (NPT) allows the identification of cognitive decline, the hallmark of MCI or dementia, and the monitoring of disease course [11]. Severe memory and executive dysfunction are well established risk factors for dementia progression, and they can be evaluated with NPT batteries [12-15].
A clinical diagnosis of MCI, as determined using NPT, with a positive biomarker for AD dramatically increases the risk of short-term progression when compared to MCI with negative biomarkers for AD [16]. This acknowledges the complementarity between NPT and biomarker information.
NPT can be limited to short cognitive screening tools, but the majority of NPT in clinical practice includes an extensive evaluation of multiple cognitive domains. The effects of demographic and cultural characteristics on test performance is best accounted for with the use of normative data. Large regression-based normative data for the Portuguese population are currently available for some neuropsychological tests [17-20].
The purpose of this work was to study the predictive value of NPT in the progression from MCI to dementia in a group of patients referred to the Dementia Outpatient Clinic of a tertiary hospital.
Methods
Subjects and procedures
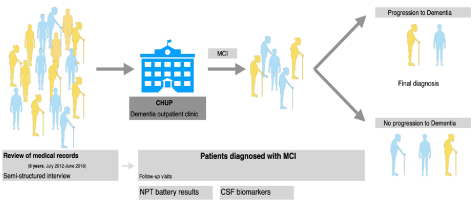
This retrospective study was based on the review of the available medical records from the Dementia Outpatient Clinic of Centro Hospitalar Universitário do Porto (CHUP), conducted by a single neurologist (RT), by means of semi-structured interview. The analysis included the period between July of 2012 and June of 2018 (Figure 1).
For each patient we collected information related to demographic characteristics and diagnostic approach from the clinical e-records. The diagnoses were established according to the current criteria for AD [21], MCI [21], frontotemporal lobar degeneration (FTLD) [22,23], vascular cognitive impairment (VaD) [24] and dementia with Lewy bodies [23].
Cerebrospinal fluid biomarker analysis was ordered by the consulting physician when considered appropriate during the investigation, and the data retrospectively collected. Quantification of amyloid-ß 1-40, amyloid-ß 1-42, Tau and Phosphorilated-tau was done by ELISA assay kits (Innogenetics, Gent, Belgium), according to the manufacturer's instructions. Blood contamination of the CSF was excluded by cytochemical analysis.
Patients were followed with at least annual visits. For the MCI group we calculated the conversion time or the follow-up time (depending on the final diagnosis), based on the time (years) between symptoms' onset, as self-reported by patients or relatives, and the final diagnosis, after completion of the investigation. The available results on NPT were registered for the patients diagnosed with MCI at first visit. The NPT battery included Dementia Rating Scale-2 [25], Auditory Verbal Learning Task [26], Benton visual Retention Test [27], Rey Complex Figure [28], Digit Span [29], Corsi Test [30], Judgment of Line Orientation [31], Trail Making Test [29], Sentence Repetition [32], 9-hole[33], Wisconsin Card Sorting Test [34], Verbal fluency[35], Boston Naming Test [36] and Hospital Anxiety and Depression Scale [37]. These exams were conducted by specialized psychologists from the Neuropsychology Unit of CHUP. Test scores were standardized according to age, sex, and/or education [17-20]. Mini-Mental State Examination conducted by the neurologist in the first visit was also included for analysis.
This study was approved by the Hospital's Local Ethics Committee.
Statistical analysis
For descriptive statistics, qualitative variables were studied using the absolute and relative frequencies. For the quantitative variables, the mean and standard deviation, or median and inter quartile range (p25 -p75) (IQR) were calculated according to the normality of the distribution. Non-parametric tests were used as the distribution of the sample was significantly skewed. Ford emographics, a Mann-Whitney test was used for the quantitative variables and a χ2test for qualitative variables.
For the neuropsychological evaluation analysis, a Mann-Whitney test determined which scores were related to conversion to dementia. A binary logistic regression was carried for the statistically significant tests, with the conversion to dementia as outcome. A Spearman correlation coefficient was calculated to assess for multi collinearity. Hosmer and Leme show tested the fitness of the model (p > 0.05). Receiver operating characteristic (ROC) curves where then obtained, in order to establish measures of discrimination using Youden's Index to determine cut-off points. An area under the curve of > 0.70 was considered acceptable. A cox proportional hazards model was conducted for the NPT with a better correlation on the binary logistic model, using the time since the reported symptom's onset.
Statistical analysis was conducted using IBM Statistical Package for the Social Sciences (SPSS) version 25 [38]. A p value < 0.05 was considered significant.
Results
In this period, 798 patients had an appointment in the dementia outpatient clinic, 16 of whom were excluded due to incomplete data. The final sample consisted of 782, of which 463 where female (59.2%). The delay between the age of first observation and the age of onset of symptoms was greater or equal to 1 year in the majority (81%) of patients. The most common diagnosis was AD with 155 patients (19.8%), followed by VaD (105, 13.4%), MCI (101, 12.9%) and FTLD (64, 8.2%). One hundred and twelve patients (14.3%) were considered to have normal cognitive performance (Table 1). The median age of onset of symptoms was significantly lower (p = .013) for patients with MCI (66, IQR=12) than for patients with AD (72, IQR = 12) or VaD (71, IQR = 9) (p < .001). For AD, 30.2% of patients were early-onset type, with a mean age (standard deviation) of 58 (4.5) years versus 75.3 (5.8) in the late-onset. From the group with the initial diagnosis of MCI (n=101), with a mean age of onset of symptoms of 66 years (SD = 8.6), 13 patients were excluded from the analysis because were considered to have subjective memory complaints (SMC) in follow-up visits. The median MMSE result as 27 (IQR = 3), and 23 (22.8%) of the 88 MCI patients had CSF biomarkers available.
During follow-up of the MCI patients, 16 progressed to dementia. One patient was lost in the follow-up. Ten patients developed AD dementia (62.5%), 1 LBD, 1 VaD, 2 Mixed dementia and 1 semantic dementia. From the patients that progressed to AD, 9 had positive CSF biomarkers (90.0%), which was significantly associated with progression (χ2(1)=6.390, p=0.024). Four patients that remained with the MCI clinical diagnosis had AD biomarkers on the CSF evaluation, with a respective mean follow-up time of 26.25 months - minimum of 18 and a maximum of 34 months. There was no significant association between progression to dementia (AD or other causes) and sex (χ2(1)=0.313, p=0.576) or education (p=0.572) (Table 2). The MMSE at diagnosis was tendentially lower for dementia converters when compared to non-converters, despite not being significantly different (p = 0.081). However, among female patients, those who progressed to dementia had a significantly lower MMSE score (Median of 24.5, IQR=5 p=0.013). We found no correlation between MMSE and age, but there was a significant positive correlation with years of education (Spearman's rho=0.478, p < 0.001).
From the 87 patients diagnosed with MCI, 69 underwent a comprehensive NPT evaluation, of which 11 progressed to dementia (15.9%). NPT values were adjusted for sex, age, and/or education according to the available normative data for the Portuguese population [17-20], except for the WCST tests (of which normative data was unavailable). There were significant differences between converters and stable MCI patients in the following tests (with respective mean rank for progressors): DRS-2 Memory (Mean Rank [MR] 14.45; p =0.001), Digit Span (MR 19.82; p= 0.035), AVLT 30-minute delayed recall (MR 17.36; p = 0.012), AVLT Long-Term Percent Retention (LTPR) index (MR 15.00; p =0.002) and Sentence Repetition: (MR 14.94; p =0.015). We ran logistic regression analysis for each of this statistically significant NPT, adjusted for demographic variables (Table 3). The model containing AVLT LTPR successfully explained 52 % of the variance in dementia progression, correctly predicting 85.7% of cases (χ2(4) =22.184, p < 0.001). A model with the memory component of DRS-2 correctly predicted 89.7% of dementia progression (χ2(4) =21.610, p < 0.001; Nagelkerke R2 =50.1%) and a model with the Sentence Repetition task explained 96% (χ2(4) =19.538, p < 0.001; Nagelkerke R2 =53.0%). All the other models showed inferior predictive properties (Table 3). Digit Span was excluded for having no statistical significance in the respective model (p = 0.617). Prior to consider a logistic regression including all the tests, we assessed for the presence of multi collinearity, similarly to what was done in other studies [39]. Besides the obvious correlation between all the AVLT components, we found significant inter correlation between DSR-2 memory and the other NPT. Given that LTPR depended on the other measures of the AVLT, the further were also dropped at this point. A multi-parameter model with AVLT-LTPR, DRS-2 - memory and SR (χ2(6) =28.024, p < 0.001; Nagelkerke R2 =70.9%) predicted 91.8% of dementia progression.
We applied these same NPT to build models for patients that progressed to AD dementia specifically (n=9), correctly predicting 90.7% of progressors with the AVLT-LTPR (χ2(4) =25.493, p < 0.001; Nagelkerke R2 =63.4%), 94.6% with the DRS-2 memory (χ2(4) =25.291, p<0.001; Nagelkerke R2 =62.0%) and 95.9% with SR (χ2(4) =16.879, p=0.02; Nagelkerke R2 =49.4%). The multi-parameter model for AD dementia progression explained 95.8% of the variance (χ2(6) =30.699, p < 0.001; Nagelkerke R2 =79.6%).
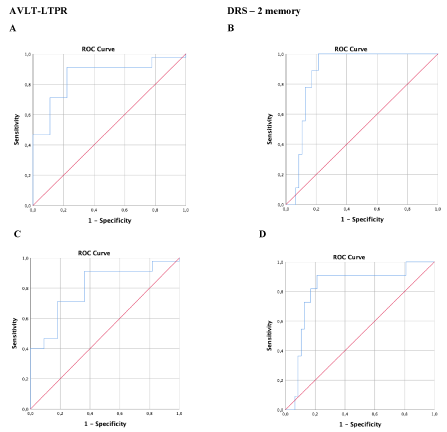
Receiver operating characteristics (ROC) curves were created to obtain discriminative scores (Figure 2). The Area Under the Curve (AUC) for AVLT-LTPR was 0.800 (SE=0.070, p < 0.001, 95% CI = [0.663-0.937]) for all-cause dementia and 0.854 (SE=0.063, p = 0.001, 95% CI = [0.730-0.978]) for AD dementia, both of which acceptable (> 0.70) and suggestive of a highly predictive capacity. According to Youden's index, the approximate cut-off point of -1.03 offers a sensitivity of 81.8% and a specificity of 71.1% in identifying converters to all-cause dementia and 88.9% sensitivity with the same specificity in identifying converters to AD dementia. For DRS-2 memory, the AUC was 0.820 (SE=0.073, p < 0.001, 95% CI = [0.677-0.963]) for all-cause dementia and 0.879 (SE=0.045, p < 0.001, 95% CI = [0.791-0.967]) for AD dementia, suggesting of a highly predictive capacity. According to Youden's index, the approximate cut-off point of -2.7 offers a sensitivity of 88.9% and a specificity of 78.7% for AD dementia, and 81.8% sensitivity with the same specificity in diagnosing all-cause dementia.
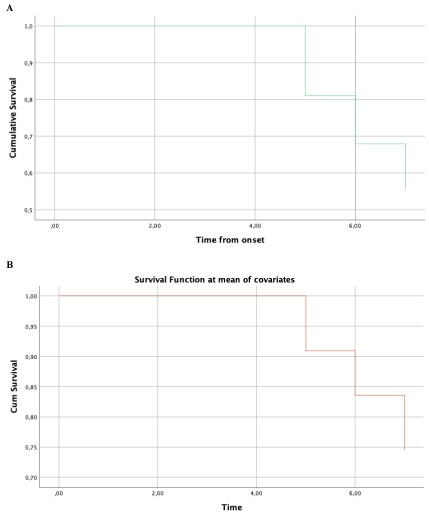
Survival analysis was conducted using Cox's proportional hazard model. Results are presented in Table 4 and Figure 3. Lower LTPR scores significantly correlated to faster progression to dementia (p=0.001), as do lower DRS-2 memory scores (p=0.003).
Of the 87 MCI patients, 27 underwent lumbar puncture for CSF examination, 13 of which showing AD biomarkers. Of this group, 9 progressed to AD dementia and 4 maintained the diagnosis of MCI (p=0.024). The subgroup of MCI patients with positive AD biomarkers in CSF who underwent NPT displayed significantly lower scores in DRS-2 Memory (p < 0.001) and AVLT LOT (p =0.037) when compared to CSF-biomarkers negative MCI cases. A model with DRS-2 memory correctly predicted 90.9% of the eleven patients that had positive AD biomarkers (Table 4).
Discussion
In our study, 19.0% of the patients with MCI progressed to dementia over a median conversion time of 53 months, which constitutes an annual conversion rate of approximately 4.3%, similar to what was obtained in other studies [40-42].The distribution of dementia by types in our study withstands global distribution, with AD and VaD being the most common causes [43,44].
With respect to the progression to dementia, our results do not support significant effects of sex and education. The sample size and the overrepresentation of patients with less education possibly explain the absence of the protective effect of education described in other studies. In our study MMSE score, was not predictive of progression, which is similar to reported in other studies [45, 46]. Additionally, our results are in agreement with previous works in the Portuguese population showing that MMSE is largely influenced by education [17].
It is well established that rapid forgetting over a delay interval is a highly sensitive indicator of early AD [47,48] and predictor of conversion to AD dementia [49,50]. This can be assessed with specific NPT tests such as the AVLT, the most strongly associated with progression in our study, with a high sensitivity to the detection of AD progression.
In our study, the LTPR, a measure dependent on the 30-minute delayed recall of the AVLT [18], showed a sensitivity of approximately 91.0% in predicting the progression to AD dementia, and the survival analysis showed a tendency to faster progression to dementia in patients with abnormal results. Landau et al. reported similar results, with 92% of sensitivity, showing that the predictive value of AVLT was similar to that of neuro imaging or CSF biomarkers for the conversion [51]. In our study, when considering MCI patients with CSF biomarkers of AD, the prognostic utility of the delayed recall of the AVLT was not statistically significant. This is likely due to the small sample with CSF investigation. Additionally, patients with CSF positive AD biomarkers had significantly lower scores on memory performance tests when compared to CSF negative patients, leading to a ceiling effect phenomenon in which NPT loses discriminative power. In agreement to our findings, Bos et al. found that excluding individuals with AD biomarkers in the CSF from normative data for memory tests increased the predictive accuracy for future progression to dementia, particularly for the AVLT delayed recall [52]. Further studies with larger samples of MCI cases with positive AD biomarkers to which the AVLT is applicated may help to understand if there is any correlation to faster progression to AD dementia. Interestingly, other studies found that MCI patients with CSF biomarkers of AD appeared to have significantly lower scores on memory performance tests when compared to CSF negative patients [53-55], encouraging the use of the AVLT to select MCI patients for CSF analysis [53].
We did not consider other dementia types besides AD because only 6 patients progressed to other types of dementia. These findings also suggest that we are currently applying an extensive NPT battery when only a few tests have actual correlation to the progression to dementia, demonstrating a need for refinement on this matter. The Dementia Rating Scale-2 (DRS-2) is a comprehensive and validated neuropsychological battery for the evaluation of global cognition, including attention, initiation/perseveration, visuospatial construction, conceptualization, and memory [56]. Despite showing less power in predicting disease progression when comparing to AVLT, the possibility of using DRS-2 as a cognitive impairment global screening tool can be seen as an advantage in the clinical setting, particularly if wider neuropsychological assessment is not possible. This comes from the fact that DRS-2 is a practical test that can be done by the neurologist during the patient visit, similarly to MMSE or the Montreal Cognitive Assessment (MoCA) [45].
This study is relevant for replicating the previous knowledge of episodic memory impairment as measurable marker of AD dementia in a small sample of a portu guese outpatient dementia clinic. Furthermore, we emphasize the usefulness of operationalizing NPT evaluation in conjunction with CSF biomarker analysis in patient workup. These findings encourage the use of NPT to select suspected AD cases for further CSF biomarker analysis to obtain a more precise diagnosis, which is even more relevant with the advent of therapeutic options for AD [57].
Our study presents a series of limitations that should be accounted for. Despite centered in one single consultant, with a semi-structure clinical interview and similar diagnostic work-up and follow-up, it is a retrospective study. The study underwent in tertiary referral hospital, with an inevitable selection bias associated. Another limitation is the lower proportion of males in the study, a bias present in similar works [58], which can also be a consequence of the selection bias. The number of patients that underwent lumbar puncture for CSF AD biomarkers limited the analysis in this group.
Conclusion
This study addresses possible prognostic factors for the progression from MCI to dementia, that occurred at an annual conversion rate of approximately 4.3%. Age is clearly the most important risk factor for this progression, and no significant differences were found for sex, education or MMSE score. There was an association between episodic memory impairment and progression to dementia, especially AD dementia, reflected in lower AVLT delayed recall test scores. Besides allowing to predict progression, it also correlated with a faster trajectory to dementia onset. Interestingly, the memory component of DRS-2 also displayed a similar correlation, which may act as a practical and non-extensive bedside tool for the clinician. Even in a small sample size, these findings emphasize the importance of neuropsychological evaluation in the identification of cognitive decline in patients at risk for developing dementia and in which additional investigation would be advised, acting as suitable indicators of prognosis.
References
- Geneva. Global Health estimates 2016: Disease burden by cause, age, sex, by country, and by region, 2000-2016. WHO (2018).
- Santana I, Farinha F, Freitas S, et al. (2015) Epidemiologia da demência e da doença de alzheimer em portugal: Estimativas da prevalência e dos encargos financeiros com a medicação. Acta Med Port 28: 182-188.
- Gonçalves Pereira M, Ana Cardoso, Ana Verdelho, et al. (2017) The prevalence of dementia in a portuguese community sample: A 10/66 Dementia Research Group study. BMC Geriatr 17: 261.
- Dubois B, Stephane Epelbaum, Francis Nyasse, et al. (2018) Cognitive and neuroimaging features and brain ß-amyloidosis in individuals at risk of alzheimer's disease (INSIGHT-preAD): A longitudinal observational study. Lancet Neurol 17: 335-346.
- Frankish H, Horton R (2017) Prevention and management of dementia: A priority for public health. The Lancet 390: 2614-2615.
- Petersen R C (2004) Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine 256: 183-194.
- Vos SJB, Frans Verhey, Lutz Frölich, et al. (2015) Prevalence and prognosis of alzheimer's disease at the mild cognitive impairment stage. Brain138: 1327-1338.
- Moghekar A, Shanshan Li, Yi Lu, et al. (2013) CSF biomarker changes precede symptom onset of mild cognitive impairment. Neurology 81: 1753-1758.
- Sorbi S, Hort J, Erkinjuntti T,et al. (2012) EFNS-ENS Guidelines on the diagnosis and management of disorders associated with dementia. Eur J Neurol 19: 1159-1179.
- Edmonds E C, Delano Wood L, Galasko D R, et al. (2015) Subtle cognitive decline and biomarker staging in preclinical alzheimer's disease. J Alzheimer's Dis 47: 231-242.
- Fields J A, Ferman T J, Boeve B F, et al. (2011) Neuropsychological assessment of patients with dementing illness. Nature Reviews Neurology 7: 677-687.
- DeCarli C, D Mungas, D Harvey, et al. (2004) Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology 63: 220-227.
- Greenaway M C, Smith G E, Tangalos E G, et al. (2009) Mayo older Americans normative studies: Factor analysis of an expanded neuropsychological battery. Clin Neuropsychol 23: 7-20.
- Pasquier F (1999) Early diagnosis of dementia: Neuropsychology. J Neurol 246: 6-15.
- Spaan P E J, Raaijmakers J G W and Jonker C (2005) Early assessment of dementia: The contribution of different memory components. Neuropsychology19: 629-640.
- Ingrid S van Maurik, Stephanie J Vos, Isabelle Bos, et al. (2019) Biomarker-based prognosis for people with mild cognitive impairment (ABIDE): A modelling study. Lancet Neurol 18: 1034-1044.
- Isabel Santana, Diana Duro, Raquel Lemos, et al. (2016) Mini-mental state examination: Screening and diagnosis of cognitive decline, using new normative data. Acta Med Port 29: 240-248.
- Sara Cavaco, Alexandra Goncalves, Claudia Pint, et al. (2015) Auditory verbal learning test in a large nonclinical Portuguese population. Appl Neuropsychol Adult 22: 321-331.
- Sara Cavaco, Alexandra Goncalves, Claudia Pint, et al. (2013) Semantic fluency and phonemic fluency: Regression-based norms for the portuguese population. Arch Clinical Neuropsychology 28: 262-271.
- Sara Cavaco, Alexandra Goncalves, Claudia Pint et al. (2013) Trail making test: Regression-based norms for the portuguese population. Arch Clin Neuropsychol 28: 189-198.
- Guy M McKhann, David S Knopman, Howard Chertkow, et al. (2011) The diagnosis of dementia due to alzheimer's disease: Recommendations from the national institute on aging-alzheimer's association workgroups on diagnostic guidelines for alzheimer's disease. Alzheimer's Dement 7: 263-269.
- Takayoshi Shimohata, Ikuko Aiba, Masatoyo Nishizawa (2015) Criteria for the diagnosis of corticobasal degeneration. Brain Nerve 67: 513-523.
- Ian G McKeith, Bradley F Boeve, Dennis W Dickson, et al. (2017) Diagnosis and management of dementia with lewy bodies. Neurology 89: 88-100.
- Perminder Sachdev, Raj Kalaria, John O'Brien, et al. (2014) Diagnostic criteria for vascular cognitive disorders: A VASCOG statement. Alzheimer Dis Assoc Disord 28: 206-218.
- Evelyne Matteau, Nicolas Dupre, Melanie Langlois, et al. (2011) Mattis dementia rating scale 2: Screening for mci and dementia. Am J Alzheimers Dis Other Demen 26: 389-398.
- E Vakil, H Blachstein (1993) Rey auditory-verbal learning test: Structure analysis. J Clin Psychol 49: 883-890.
- Eun Hyun Seo, Dong Young Lee, Il Han Choo, et al. (2007) Performance on the benton visual retention test in an educationally diverse elderly population. J Gerontol B Psychol Sci Soc Sci 62: 191-193.
- Min-Sup Shin, Sun-Young Park, Se-Ran Park, et al. (2006) Clinical and empirical applications of the rey-osterrieth complex figure test. Nat Protoc 1: 892-899.
- Jordi Llinas-Regla, Joan Vilalta-Franch, Secundino Lopez-Pousa, et al. (2017) The trail making test. Assessment 24: 183-196.
- D B Berch, R Krikorian, E M Huha (1998). The corsi block-tapping task: Methodological and theoretical considerations. Brain Cogn 38: 317-338.
- Egas M Caparelli-Daquer, Ricardo Oliveira-Souza, Pedro F Moreira Filho (2009) Judgment of line orientation depends on gender, education, and type of error. Brain Cogn 69: 116-120.
- J A Small, S Kemper, K Lyons (2000) Sentence repetition and processing resources in alzheimer's disease. Brain Lang 75: 232-258.
- Wang Y C, Bohannon R W, Kapellusch J, et al. (2015) Dexterity as measured with the 9-hole peg test (9-HPT) across the age span. J Hand Ther 28: 53-60.
- Faustino B, Oliveira J, Lopes P (2020) Normative scores of the wisconsin card sorting test in a sample of the adult portuguese population. Appl Neuropsychol Adult 1-8.
- Henry J D, Crawford J R, Phillips L H (2004) Verbal fluency performance in dementia of the alzheimer's type: A meta-analysis. Neuropsychologia 42: 1212-1222.
- Messerly J, Marceaux J C (2020) Examination of the reliability and validity of the nab naming test in a diverse clinical sample. Clin Neuropsychol 34: 406-422.
- Snaith R P (2003) The hospital anxiety and depression scale. Health and Quality of Life Outcomes.
- IBM. IBM SPSS Statistics Software for Windows, Version 25. IBM (2017) doi:10.1016/j.jchf.2014.02.009.
- Rushing NC, Sachs Ericsson N and Steffens DC (2014) Neuropsychological indicators of preclinical Alzheimers disease among depressed older adults. Aging Neuropsychology and Cognition 21: 99-128.
- Visser PJ, Kester A, Jolles J, et al. (2006) Ten-year risk of dementia in subjects with mild cognitive impairment. Neurology 67: 1201-1207.
- Ishikawa T and Ikeda M (2007) Mild cognitive impairment in a population-based epidemiological study. Psychogeriatrics 7: 104-108.
- Annerbo S, Wahlund LO and Lökk J (2006)The significance of thyroid-stimulating hormone and homocysteine in the development of Alzheimer's disease in mild cognitive impairment: A 6-year follow-up study. Am J Alzheimers Dis Other Demen 21: 182-188.
- Lobo A, L J Launer, L Fratiglioni, et al. (2000) Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurology 54: S4-9.
- Harvey R J, Skelton Robinson M and Rossor MN (2003) The prevalence and causes of dementia in people under the age of 65 years. J Neurol Neurosurg Psychiatry 74: 1206-1209.
- Nasreddine ZS, Natalie A Phillips, Valérie Bédirian, et al. (2005) The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53: 695-699.
- Luis CA, Keegan AP and Mullan M (2009) Cross validation of the Montreal Cognitive Assessment in community dwelling older adults residing in the Southeastern US. Int J Geriatr Psychiatry 24: 197-201.
- Delis DC, Massman Paul J, Butters Nelson, et al. (1991) Profiles of Demented and Amnesic Patients on the California Verbal Learning Test: Implications for the Assessment of Memory Disorders. Psychol Assess 3: 19-26.
- Butters N, Hughes J, Mohs R, et al. (1991) Detection of Abnormal Memory Decline in Mild Cases of Alzheimer's Disease using Cerad Neuropsychological Measures. Arch Neurol 48: 278-281.
- Dierckx E, S Engelborghs, R De Raedt ,et al. (2009) Verbal cued recall as a predictor of conversion to Alzheimer's disease in Mild Cognitive Impairment. Int J Geriatr Psychiatry 24: 1094-1100.
- Guo Q, Zhao Q, Chen M, et al. (2009) A comparison study of mild cognitive impairment with 3 memory tests among Chinese individuals. Alzheimer Dis Assoc Disord 23: 253-259.
- Landau SM, D Harvey, CM Madison, et al. (2010) Comparing predictors of conversion and decline in mild cognitive impairment. Neurology 75: 230-238.
- Bos I, Stephanie JB Vos, Willemijn J Jansen, et al. (2018) Amyloid-ß, Tau, and Cognition in Cognitively Normal Older Individuals: Examining the Necessity to Adjust for Biomarker Status in Normative Data. Front Aging Neurosci 10: 193.
- Clarens MF, Lucia Crivelli , Ismael Calandri, et al. (2020) Neuropsychological profile of Alzheimer's disease based on amyloid biomarker findings results from a South American cohort. Appl Neuropsychol 30: 1-6.
- Mandecka M, Magdalena Budziszewska, Anna Barczak, et al. (2016) Association between cerebrospinal fluid biomarkers for Alzheimer's disease, APOE genotypes and auditory verbal learning task in subjective cognitive decline, mild cognitive impairment, and Alzheimer's disease. J Alzheimer's Dis 54: 157-168.
- Stricker NH, Emily S Lundt , Sabrina M Albertson, et al. (2020) Diagnostic and Prognostic Accuracy of the Cogstate Brief Battery and Auditory Verbal Learning Test in Preclinical Alzheimer's Disease and Incident Mild Cognitive Impairment: Implications for Defining Subtle Objective Cognitive Impairment. J Alzheimer's Dis 76: 261-274.
- Springate BA, Tremont G, Papandonatos G, et al. (2014) Screening for mild cognitive impairment using the dementia rating scale-2. J Geriatr Psychiatry Neurol 27: 139-144.
- Schneider L (2020) A resurrection of aducanumab for Alzheimer's disease. Lancet Neurol 19: 111-112.
- García Herranz S, Díaz Mardomingo MC and Peraita H (2016) Neuropsychological predictors of conversion to probable Alzheimer disease in elderly with mild cognitive impairment. J Neuropsychol 10: 239-255.
Corresponding Author
João Moura, Hospital Santo António, Largo Professor Abel Salazar, 4099-001 Porto, Portugal.
Copyright
© 2022 Moura J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.