Tinnitus as a Fragment of Consciousness
Abstract
Subjective, chronic tinnitus that is tonal is a human condition that results in the perception of a continuous sound of a particular frequency, in the absence of that sound, by activation of deafferented portions of the auditory cortex. During ongoing behavior, the brain assumes various states as exemplified by the neural activity at the level of the neocortex and hippocampal formation. For the hippocampus, theta oscillations (6-10 Hz) have been associated with behaviors such as walking, running, and swimming, whereas non-theta oscillations have been associated with eating, drinking, and alert immobility. It was found that during walking, running, and swimming one's awareness of tinnitus is reduced; during eating, drinking, and alert immobility, however, one's awareness of tinnitus is enhanced. Tinnitus can be thought of as electrically stimulating one site in a conscious brain which yields the sensation of buzzing. Once the tinnitus is diminished during ongoing behavior other regions of the brain are now accessed by the hippocampus to transmit information (stored as well as sensory) that is expressed as a stream of consciousness and that assists in the execution of motor responses. This idea concurs with the views of William James established over 100 years ago.
Keywords
Theta rhythms, Traveling wave, Hippocampus, Awareness, Behavior, Humans
Introduction
Mammals assume many different states as they transition from waking to sleep. During waking, the brain exhibits low-voltage fast EEG (electroencephalographic) activity at the level of the neocortex; during sleep, spindles and large amplitude EEG activity are exhibited [1-4]. Low-voltage fast activity can also occur during sleep, but this activity is accompanied by skeletomotor atonia plus rapid eye movements. The reduction in muscle tone has been associated with the postsynaptic inhibition of alpha and gamma spinal cord neurons as well as with the presynaptic inhibition of 1a sensory afferents [5,6], and the eye movements are typically disjunctive with looped and torsional trajectories [7-9]. The eye movements during sleep lose their volitional component for the execution of saccades, smooth pursuit, and vergence [10]. Wakefulness therefore is a neocortex anchored to the sensorimotor system and receptive to feedback from the environment as the neocortex exhibits low-voltage fast activity. This activity is pronounced during walking, running, and swimming [1].
Penfield [11] believed that the cadence of consciousness during wakefulness is dependent on an intact hippocampus, which when damaged prevents against the creation of long-term memories [12]. Consciousness as envisaged by James [13] is a linear process or a stream. This is well illustrated when one walks into an office in pursuit of an item and upon forgetting what the item is one must leave the office and retrace the 'stream' to recall the item. Current evidence suggests that the hippocampus of humans and rodents exhibits similar EEG patterns for transferring information about past, present, and future events by way of a theta (6-10 Hz) travelling wave [14,15]. Focal electrical stimulation of neurons in the neocortex evokes fragments of perceptual events rather than a stream of consciousness [16]. Accordingly, the hippocampus may establish a linkage between events as spaced over one's lifetime and as stored in various locations in the central nervous system [17-19].
The hippocampal formation as well as the thalamus and amygdala has been found to be activated in humans with the onset of 480 nm, blue light [20], which is the wavelength of light that best induces waking state [21]. Two patterns of electrical activity in the hippocampus have been correlated with the ongoing behavior of animals [1]. In rats, hippocampal EEG is dominated by theta oscillations during walking, running, swimming, rearing, exploratory head turning, and sniffing. These behaviors have been called Type 1 and they represent volitional movements [22], or arousing conditions [23,24]. In contrast, during eating, drinking, grooming, face washing, and awake-immobility, theta is replaced by 'large amplitude irregular activity'. The behaviors associated with this activity have been called Type 2 and they represent non-volitional acts [22], or non-arousing conditions [23,24].
Tinnitus as related to wakefulness
Some 48 million people in the United States suffer from hearing loss [25], which can culminate in subjective, chronic tinnitus that is tonal [26]. In May of 2016, I woke up to find that there was ringing in my left ear. The ringing can best be described as a high-frequency hissing sound that is continuous. This tinnitus has prevailed unabated for almost one year. The cause is unknown suffice it to say that I have had a history of being on extensive medications during treatment for Burkitt's lymphoma that included the chemo-agent vincristine, which has been associated with inducing tinnitus by damaging the cochlear hair cells [27,28].
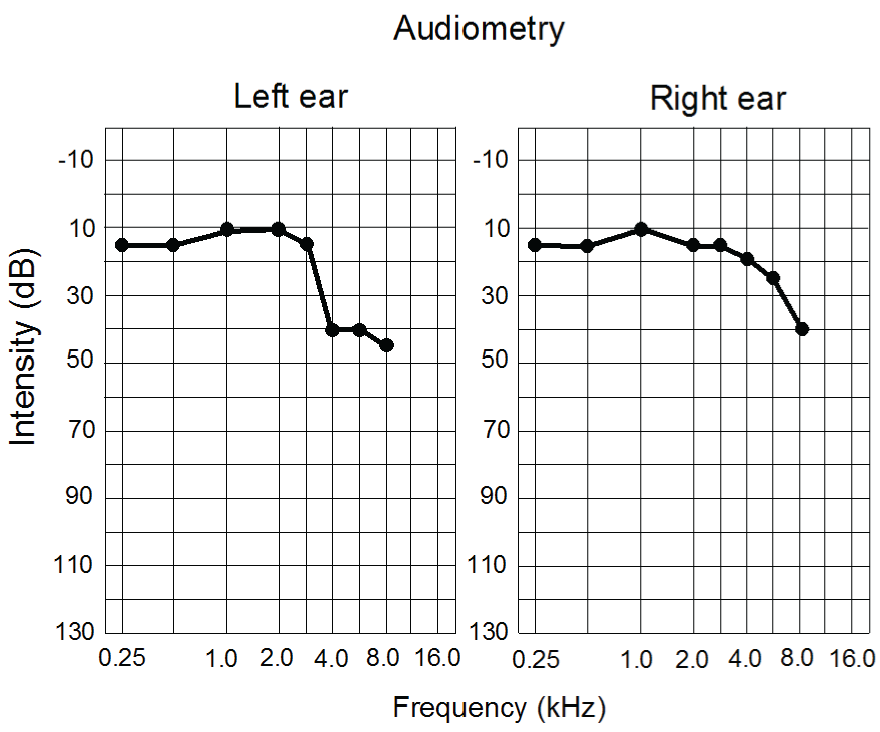
Tinnitus has been described as being similar to phantom-limb pain whereby the auditory cortex contralateral to the affected ear contains a representation of a sound in the absence of any internal or external sound source [26,29,30]. Damaged frequency bands in the cochlea are filled in by plastic changes at the level of the auditory cortex as evidenced by the enhancement of neural activity at the cortical sites coding the damaged bands [27,31,32,33,34]. The frequency bands damaged in my case were between 4 and 6 KHz as established by audiometry (Figure 1). It has been shown that activation of the auditory cortex can ameliorate the symptoms of tinnitus by reconfiguring the plastic changes in the cortex [35,36]. As well, training with appropriate frequency bands delivered through music can reduce the loudness of tinnitus [37].
My awareness of the tinnitus varied as a function of behavioral state and this awareness mapped onto Vanderwolf's [1], categorization of Type 1 and Type 2 behavior. Table 1 summarizes the various behaviors and the subjective levels of tinnitus. What is clear from the table is that during Type 1 behavior it was common for the awareness of tinnitus to be either eliminated or reduced whereas during Type 2 behavior the awareness of tinnitus was pronounced. When viewing a television program non-attentively, however, the tinnitus was potentiated and was no different from that experienced during Type 2 behavior. Indeed the more engaged one was in the execution of a volitional act the more suppressed was the tinnitus. It is well established that during Type 1 behavior the auditory system is suppressed [38,39]. During sleep or dreaming there was never any experience of tinnitus. In the case of dreaming never did I awake from a dream to recall the prevalence of tinnitus. Finally, it was quite apparent that the tinnitus was most evident when engaged in the present rather than when contemplating past or future events.
Overall the intensity of the tinnitus was less robust in the morning hours than late at night. This may have been due to the road traffic being more conspicuous at my home and in my city in the early morning hours as compared to late at night. Road traffic has a peak sound frequency of 1 kHz with a broad distribution ranging from 0.125 to 8 kHz [40], which may have caused some masking of the tinnitus. As well, wind of high velocity (> 11 km/hr) that generated noise [41], matching the sound frequency of the tinnitus (i.e. > 4 KHz), was effective at masking the tinnitus.
Tinnitus can be thought of as the continuous activation of one site within the cerebral cortex [27,33,34], which according to Penfield's electrical stimulation experiments would produce a fragment of perception [16]. A subject is aware that tinnitus is separate from one's perceptual schema much like the perceptual effects of cortex stimulation [11], and tinnitus has characteristics similar to the percept elicited by electrical activation of cortical neurons thereby causing interference of the percept if produced externally [42,43]. This is why tinnitus can be masked using auditory stimuli. Finally, tinnitus can be overridden by behavioral state just as the effect of stimulating the cerebral cortex can be overridden by behavioral state [44].
If the hippocampus is indeed the organ that links the various regions of the brain to have a continuous stream of consciousness [11], then the disengagement of the auditory neurons mediating tinnitus might indicate that the hippocampus is now conveying and sequencing information from other regions of the brain to drive behaviors such as language, music, and locomotion. These behaviors are often accompanied by the need to link the past, present, and future in a continuous stream. Just how hippocampal theta and cortical oscillations correlate with one's awareness of tinnitus is open to exploration in humans as well as animals [45].
In conclusion, the hippocampus may enable the execution of a 'next' command to maintain a stream of consciousness going from the past to the future via its theta travelling wave [14]. One's awareness of tinnitus keeps the wave stationary by locking the hippocampus into a steady state (just a metaphor) causing one to dwell on a single input from the brain, i.e. as induced by the hyperactive cells of the auditory system coding for a single tone thereby bringing the tinnitus into consciousness.
Acknowledgements
I thank Dr. Iamma Radace at the Clinica Pedro Cavalcanti Ltd in Parnamirim, Rio Grande do Norte, Brazil for performing the audiometric test and Drs. Ora Kofman, Nikos K. Logothetis, and Paulo V. Rodrigues for their suggestions on the manuscript. I would like to dedicate this work to my graduate school professor, the late Cornelius Hendrik Vanderwolf.
References
- Vanderwolf CH (1969) Hippocampal electrical activity and voluntary movement in the rat. Electroencephalgr Clin Neurophysiol 26: 407-418.
- Vanderwolf CH (1975) Neocortical and hippocampal activation in relation to behavior: effects of atropine, serine, phenothiazines, and amphetamine. J Comp Physiol Psychol 88: 300-323.
- Vanderwolf CH (1990) An introduction to the electrical activity of the cerebral cortex: relations to behavior and control of subcortical inputs. In: B Kolb, TC Tees, The Cerebral Cortex of the Rat. MIT Press, Cambridge, 151-192.
- Vanderwolf CH, Robinson TE (1981) Reticulo-cortical activity and behavior: a critique of the arousal theory and a new synthesis. Behav Brain Sci 4: 459-476.
- Chase MH, Harper RM (1971) Somatosensory and visceromotor correlates of operantly conditioned 12-14 c/sec sensorimotor cortical activity. Electroencephalogr Clin Neurophysiol 31: 85-92.
- Pompeiano O (1967) The neurophysiological mechanism of postural and motor events during desynchronized sleep. Res Publ Assoc Res Nerv Ment Dis 45: 351-423.
- Fuchs AF, Ron S (1968) An analysis of rapid eye movements of sleep in the monkey. Electroencephalogr Clin Neurophysiol 25: 244-251.
- Jacobs L, Feldman M, Bender MB (1971) Eye movements during sleep. I. The pattern in the normal function. Arch Neurol 25: 151-159.
- Zhou W, King WM (1997) Binocular eye movements not coordinated during REM sleep. Exp Brain Res 117: 153-160.
- Schiller PH, Tehovnik EJ (2015) Vision and the Visual System. Oxford University Press, New York.
- Penfield W (1975) The Mystery of the Mind: A Critical study of Consciousness and the Human Brain. Princeton University Press, New Jersey.
- Scoville WB, Milner B (1957) Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20: 11-21.
- James W (1890) The Principles of Psychology. Harvard University Press, Cambridge, MA.
- Lubenov EV, Siapas AG (2009) Hippocampal theta oscillations are travelling waves. Nature 459: 534-539.
- Zhang H, Jacobs J (2015) Traveling theta waves in the human hippocampus. J Neurosci 35: 12477-12487.
- Penfield W, Rasmussen T (1952) The Cerebral Cortex of Man. Macmillan Press, New York.
- Hebb DO (1949) The Organization of Behavior: A Neuropsychological Theory. John Wiley & Sons, New York.
- Kandel ER, Schwartz JH, Jessell TM, et al. (2013) Principles of Neural Science. (5th edn), McGraw Hill, New York.
- Tehovnik EJ, Chen LL (2015) Brain control and information transfer. Exp Brain Res 233: 3335-3347.
- Vandewalle G, Schmidt C, Albouy G, et al. (2007) Brain responses to violet, blue, and green monochromatic light exposures in humans: prominent role of blue light and the brainstem. PLoS ONE 2: e1247.
- Vandewalle G, Maquet P, Dijk DJ (2009) Light as a modulator of cognitive brain function. Trends Cogn Sci 13: 429-438.
- Van Lier H, Coenen AM, Drinkenburg WH (2003) Behavioral transitions modulate hippocampal electroencephalogram correlates of open field behavior in the rat: support for a sensorimotor function of hippocampal rhythmical synchronous activity. J Neurosci 23: 2459-2465.
- Bradley MM, Miccoli L, Escrig MA, et al. (2008) The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology 45: 602-607.
- Hulse BK, Lubenov EV, Siapas AG (2017) Brain state dependence of hippocampal subthreshold activity in awake mice. Cell Rep 18: 136-147.
- Lin FR, Niparko JK, Ferrucci L (2011) Hearing loss prevalence in the United States. Arch Intern Med 171: 1851-1852.
- Auerbach BD, Rodrigues PV, Salvi RJ (2014) Central gain control in tinnitus and hyperacusis. Front Neurol 5: 206.
- Fetoni AR, Troiani D, Petrosini L, et al. (2015) Cochlear injury and adaptive plasticity of auditory cortex. Front Aging Neurosci 7: 8.
- Han BI, Lee HW, Kim TY, et al. (2009) Tinnitus: characteristics, causes, mechanisms, and treatments. J Clin Neurol 5: 11-19.
- Elgoyhen AB, Langguth B, De Ridder D, et al. (2015) Tinnitus: perspectives from human neuroimaging. Nat Rev Neurosci 16: 632-642.
- Mühlnickel W, Elbert T, Taub E, et al. (1998) Reorganization of auditory cortex in tinnitus. Proc Natl Acad Sci U S A 95: 10340-10343.
- De Ridder D, Elgoyhen AB, Romo R, et al. (2011) Phantom percepts: tinnitus and pain as persisting aversive memory networks. Proc Natl Acad Sci U S A 108: 8075-8080.
- Llinas R, Urbano FJ, Leznik E, et al. (2005) Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci 28: 325-333.
- Rainer K, Andrej K, Silvia H, et al. (1999) Recruitment of the auditory cortex in congenitally deaf cats by long-term cochlear electrostimulation. Science 285: 1729-1733.
- Salvi RJ, Wang J, Ding D (2000) Auditory plasticity and hyperactivity following cochlear damage. Hear Res 147: 261-274.
- De Ridder D, van der Loo E, Vanneste S, et al. (2011) Theta-gamma dysrhythmia and auditory phantom perception. J Neurosurg 114: 912-921.
- Khedr EM, Rothwell JC, Ahmed MA, et al. (2008) Effect of daily repetitive transcranial magnetic stimulation for treatment of tinnitus: comparison of different stimulus frequencies. J Neurol Neurosurg Psychiatry 79: 212-215.
- Okamoto H, Stracke H, Stoll W, et al. (2010) Listening to tailor-made notched music reduces tinnitus loudness and tinnitus-related auditory cortex activity. Proc Natl Acad Sci U S A 107: 1207-1210.
- Brugge JF, Merzenich MM (1973) Responses of neurons in auditory cortex of the macaque monkey to monaural and binaural stimulation. J Neurophysiol 36: 1138-1158.
- Tapia MC, Cohen LG, Starr A (1987) Attenuation of auditory-evoked potentials during voluntary movement in man. Audiology 26: 369-373.
- Sandberg U (2003) The multi-coincidence peak around 1000 Hz in tyre/road noise spectra. Euronoise Naples, 498.
- Zakis JA, Tan CM (2014) Robust wind noise detection. IEEE International Conference on Acoustic Speech Signal Processing, 3683-3687.
- Ojemann GA (1983) Brain organization for language from the perspective of electrical stimulation mapping. Behav Brain Sci 6: 189-206.
- Penfield W (1958) Some mechanisms of consciousness discovered during electrical stimulation of the brain. Proc Natl Acad Sci U S A 44: 51-66.
- Tehovnik EJ, Slocum WM (2004) Behavioural state affects saccades elicited electrically from neocortex. Neurosci Biobehav Rev 28: 13-25.
- Pace E, Luo H, Bobian M, et al. (2016) A conditioned behavioral paradigm for assessing onset and lasting tinnitus in rats. PLoS One 11: e0166346.
Corresponding Author
Edward J Tehovnik, Alameda dos Bosques 880, C59, Parnamirim, RN, Brazil, 59-153-155.
Copyright
© 2017 Tehovnik EJ. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.