Synthesis and Preliminary Assays of a Novel Molecularly Imprinted Polymer Dedicated to the Extraction of Carbamazepine from River Water
Abstract
Monitoring of trace organic contaminants in aquatic media has become a major concern today. The presence of Carbamazepine (CBZ), anticonvulsant and analgesic used in the treatment of pain associated with trigeminal neuralgia, has been reported in effluents from sewage treatment plants due to its low biodegradability. To monitor its content in waters, a Molecularly Imprinted Polymer (MIP) of CBZ was synthesized to extract this molecule from river waters by Solid Phase Extraction (SPE). This MIP was prepared in dichloromethane with carbamazepine, 2-(trifluoromethyl) acrylic acid and divinyl-benzene as template, functional monomer and crosslinker, respectively. After synthesis, the template was completely removed by sonication. The selectivity between MIP and its related Non Imprinted Polymer (NIP) was greater than 2 and CBZ was completely recovered at the elution step regardless of the volume of sample loaded in the 1-100 mL range. The specificity of the material was evaluated by adding some Tricyclic Antidepressants (TCAs) to water samples containing CBZ. Analysis of these drugs was carried out by Capillary Electrophoresis (CE) after the introduction of a new developed background electrolyte. The results showed that TCAs exchanged strong specific and non-specific interactions with the polymer, which resulted in a decrease in elution yield of CBZ (64%).
Keywords
Molecularly imprinted polymer, Carbamazepine, Tricyclic antidepressants, TFMAA, Solid-phase extraction, Capillary electrophoresis
Introduction
The presence of human pharmaceuticals and their metabolites in the aquatic environment has drawn significant attention during the past decade due to their potential in altering the normal endocrine function and physiological status of wildlife [1,2]. Among these compounds, Carbamazepine (CBZ) (Figure 1) is of increasing concern due to its low biodegradability in treated water, surface water, groundwater and drinking water [3,4]. This drug is used as an anticonvulsant primarily in the treatment of epilepsy to control seizures. Like most other pharmaceuticals, CBZ and its metabolites enter the water cycle through human excretions. Previous investigations have demonstrated that biodegradation of CBZ in wastewater treatment plants was almost impossible [5-8]. Thus, CBZ has been found in some effluents in concentrations up to 16 nM [9]. It has been detected at concentrations of 5 nM in river, stream and groundwater [10,11] and at a maximum concentration of 22 nM in landfill leachate [12]. In drinking water, it was found at a concentration below 1 nM [11].
The analysis of compounds at low concentrations in complex matrices requires a sensitive analytical method and an efficient sample preparation procedure in order to extract and pre-concentrate the analytes at trace levels. In the last decade, Molecularly Imprinted Polymers (MIPs) have been developed to selectively adsorb contaminants from environmental waters [13]. MIPs are cross-linked polymers, which are synthesized in the presence of a template molecule, a monomer and a porogen solvent. Cross-linkers enclose the pre-polymerisation complex formed by the functional monomer and the template. After polymerization, removal of the template leaves cavities with geometrical and functional properties able to specifically recognize the template molecule and its analogues. In this context, several MIPs have been synthesized for CBZ extraction from biological and environmental samples [14-18]. In all cases, CBZ was used as template. The influence of different parameters such as the type of monomer and cross-linker, the porogenic ability of the solvent and also the ratio of starting materials was studied. The most used monomer was Meth Acrylic Acid (MAA), which forms stable complexes either by electrostatic interactions or hydrogen bonding with the Car Bamyl Moiety of CBZ. Two cross-linkers, Ethylene Glycol Di Metha Arylate (EGDMA) and Di Vinyl Benzene (DVB-80), were assessed in order to optimize MIP capacity for complex samples. In all cases, aprotic porogenic solvents (dichloromethane, acetonitrile and toluene mixtures) were used so as not to disrupt the interactions between the monomer and the template.
In this paper, a novel carbamazepine MIP has been synthesized with a more acidic monomer 2-(Trifluoromethyl) Acrylic Acid (TFMAA) than methacrylic acid in order to bind efficiently CBZ in mineral waters. The MIP-SPE protocol has been optimized to ensure good selectivity and high recoveries for CBZ pre-concentration in ultrapure water as well as in mineral and river waters. Cross-reactivity was tested by adding to the sample four Tri Cyclic Antidepressants (TCAs) - Clomipramine (CLO), Imipramine (IMI), Desipramine (DES) and Trimipramine (TRIMI)-whose structures and functions are close to that of CBZ (Figure 1). These basic drugs are easily ionized and, therefore, SPE fractions have been analyzed by Capillary Electrophoresis (CE) [19-21].
Materials and Methods
Chemicals
2,2’-Azobisisobutyronitrile (AIBN, 98%), 18-crown-6, heptakis (2,6-di-O-methyl)-β-cyclodextrin (DM-β-CD, DS = 12.6 per CD), phosphoric acid and sodium hydroxide were purchased from Fluka (Saint-Quentin-Fallavier, France). Acetic acid (AcOH), ammoniac (NH3), 2-(N-cyclohexylamino) ethanesulfonic acid (CHES), 3-(N-cyclohexylamino)-2-hydroxy-1-propanesulfonic acid (CAPSO), Carbamazepine (CBZ), carbamazepine-13C6 (13C-CBZ) 100 µg mL-1 in methanol, Clomipramine hydrochloride (CLO), Desipramine hydrochloride (DES), Imipramine hydrochloride (IMI), Trimipramine maleate salt (TRIMI), Divinylbenzene 80% (DVB-80), Ethylene Diamine Tetraacetic Acid disodium salt (EDTA), formic acid (HCOOH), potassium chloride (KCl), Sodium Dodecyl Sulfate (SDS), sodium dihydrogen phosphate, sodium tetraborate (Na2B4O7.10 H2O), α- and β-cyclodextrin (α-CD and β-CD), and 2-(Trifluoromethyl) Acrylic Acid (TFMAA, 99%) were purchased from Sigma-Aldrich (Saint-Quentin Fallavier, France). 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD, DS = 6.3 per CD) was purchased from Wacker Chemie (Munich, Germany). Trimethyl-β-cyclodextrin (TM-β-CD, DS = 18 per CD) was purchased from Cyclolab (Budapest, Hungary). Acetonitrile (ACN), Chloroform (CHCl3), dichloromethane (CH2Cl2) and methanol (MeOH) (HPLC grade) were purchased from VWR International (Fontenay-sous-Bois, France). Ultrapure water (18 MΩ. cm) was produced from an Elgastat apparatus (Elga, Villeurbanne, France).
Syringes and hydrophilic Polyvinylidene Fluoride (PVDF) Millex-HV Syringe Filters, pore size 0.45 µm, were purchased from Millipore (Molsheim, France). SPE cartridges (3 mL capacity) and polyethylene frits were purchased from Agilent Technologies (Massy, France). A mineral water was purchased from a local supermarket. River water (Oise, France) was filtered through 0.45 µm nylon filter prior any extraction by MIP-SPE.
Instrumentation
For MIP-SPE development, a Merck-Hitachi LaChrom HPLC system L-7100 pump (Merck KGaA, Darmstadt, Germany) was coupled to a Kratos Spectroflow 783 UV spectrophotometric detector (Applied Biosystems, Les Ulis, France). Data were collected and analyzed using EZ-Chrom Elite software (version 2.5). The column was a Hyperprep column (Thermo) (150 mm × 4.6 mm, I.D. 5 µm) with a C18 bonded-silica stationary phase maintained at 25 ℃.
The flow rate was set at 1 mL min-1. Manual injections were conducted using a Rheodyne (Cotati, CA, USA) Model 7125 injection valve fitted with a 20 µL loop. The mobile phase composition was NH3-AcOH (pH 3.5) buffer/ACN/MeOH, (60/35/5, v/v/v). The UV detector was set at 230 nm wavelength.
For trace analysis of mineral and river waters samples spiked with CBZ 1 µg L-1, an Acquity UPLC QSM (Waters, Guyancourt, France) system was used. This instrument was equipped with a quaternary pump Acquity UPLC H-Class Quaternary Solvent manager, a vial auto sampler and a triple quadrupole mass spectrometry with an electrospray ionization in positive mode. The column was a Kinetex® C18 (Phenomenex) (100 mm × 2.1 mm, I.D. 2.6 µm). The injected volume was 10 µL. The mobile phase was composed of MeOH and acidified water (0.1% HCOOH). The solvent gradient profile started with 10% MeOH and reached 75% MeOH within 15 min, then 95% within 0.1 min. From 15.1 to 17 min, 95% MeOH was maintained, and from 17.1 to 21 min the MeOH concentration decreased to 10%. The flow rate was 0.4 mL L-1 and the column oven temperature was set to 40 ℃ throughout the analytical run (21 min). Analyses were performed by tandem MS with electrospray ionization in positive-ion mode using Multiple Reaction Monitoring (MRM). Each sample was spiked with 13C-CBZ 1 µg L-1 before being analyzed. CBZ and 13C-CBZ were respectively detected by the following m/z transitions: 237.1-194.1 and 243.2-200.2.
The CE system was a 1600 Hewlett Packard3DCE (Agilent, Waldbronn, Germany) equipped with a photodiode array detection system. Agilent software 3D-CE Chemstation (rev B.04.02) was used to pilot the CE system and for signal acquisition. Analyses were performed in bare fused-silica capillary (100 cm total length, 91.5 cm effective length, 50 µm i.d.) purchased from Polymicro Technologies (Phoenix, AZ, USA). New capillaries were conditioned by 1 M NaOH for 15 min and water for 5 min. The separation was conducted at a voltage of + 30 kV (current of +4.5 µA) and a temperature of 25 ℃.
All rinse cycles were carried out at 950 m bar. The Background Electrolyte (BGE) in the separation vials was renewed every three runs. Between runs, the capillary was flushed for 2 min with water and 4 min with BGE. The UV detection was set at 214 nm.
The microwave system used to improve template elimination from MIP was a Start SYNTH Microwave Synthesis Labstation (Milestone, Shelton, United States).
Solution preparation
The stock solutions (1000 mg L-1) of CBZ, DES, IMI, CLO and TRIMI were prepared by dissolving 10 mg in 10 mL methanol and stored at 4 ℃ in the dark for one month. Dilutions were made daily to a suitable concentration in water for MIP-SPE and CE or in mobile phase for HPLC. The standard concentrations for CE linearity assessment were 0.1, 0.5, 1, 5 and 10 mg L-1.
The running CE buffers were prepared fresh each day. Their pH was checked with a Meter Lab PHM 201 Portable pH-Meter (Radiometer Analytical, Villeurbanne, France).
Phosphoric acid aqueous solution (pH 2.5) was prepared for the conditioning step of MIP-SPE.
Preparation of MIPs
The template (CBZ, 0.83 mmol), functional monomer (TFMAA, 2.82 mmol), cross-linker (DVB-80, 13.50 mmol) and initiator (2.2’-azobisisobutyronitrile, 0.38 mmol) were dissolved in 3.35 mL of porogenic solvent (CH2Cl2). The reaction mixture was purged for 5 min with nitrogen bubbling and then polymerized at 60 ℃ for 24 hours. The polymer was ground and sieved to 45 µm. MIP and NIP were exposed to six successive extractions with ACN, CHCl3 and MeOH. Each extraction consisted in putting the tube containing the MIP and NIP for 30 min in an ultrasonic bath with 10 mL of each solvent, then for 10 min in the centrifuge (7500 rpm at 20 ℃) in order to separate the washing solution from the polymer. The cycle was repeated until complete elimination of CBZ. A Non-Imprinted Polymer (NIP) was prepared in the same manner but without the template in the pre-polymerization mixture. Then, the dried powder was subjected to decantation in acetone to remove fine particles.
SPE protocol
250 mg of polymer (MIP or NIP) were introduced into a polypropylene SPE cartridge (3 mL capacity) maintained between two polyethylene frits (porosity 10 µm). Thus, the MIP and NIP-SPE cartridges were conditioned by 10 mL MeOH and 5 mL phosphoric acid aqueous solution (pH 2.5). A water sample was spiked with CBZ 5 mg L-1 and loaded onto MIP and NIP. Then, after an aqueous washing step, several organic solvent mixtures were percolated as washing and elution fractions (Table 1).
Performance of MIP
MIP/NIP selectivity determination
The SPE fractions recovered at the loading and aqueous washing steps were directly analyzed by HPLC, while those obtained for organic washing and elution steps were first evaporated to dryness under a gentle nitrogen flow and recovered in 1 mL mobile phase before being analyzed by HPLC. All experiments were performed in duplicate (n = 2) and recoveries of CBZ were determined for each SPE step.
MIP specificity assessment
To assess the specificity of the MIP-CBZ, the described SPE protocol was applied to ultrapure water spiked with a mixture of CBZ, IMI, DES, CLO and TRIMI (5 mg L-1). The sample and MIP/NIP elution fractions were analyzed using HPLC and CE.
Application of MIP-SPE to mineral and river water
The optimized SPE protocol was applied to a mineral water sample in order to assess its abitility to specifically extract CBZ. Thus, different volumes (1-100 mL) of mineral water were loaded on a MIP cartridge while keeping constant the amount of CBZ loaded (5 µg). On this purpose, CBZ concentrations were 5 mg L-1 for 1 mL of mineral water loaded, 0.5 mg L-1 for 10 mL, 0.1 mg L-1 for 50 mL and 0.05 mg L-1 for 100 mL, respectively. Further analyses were performed by HPLC. Moreover, MIP-SPE was also applied to 100 mL of river water spiked with CBZ 1 µg L-1. The extraction was duplicated and elution fractions were spiked with 13C-CBZ 1 µg L-1 before being analyzed by UPLC - tandem MS for quantification by MRM.
Results and Discussion
MIP synthesis
The MIP was prepared from CBZ and TFMAA with a molar ratio of 1/3.34 (n/n), which means an excess of monomer compared to template in order to favor the pre-polymerization template-monomer complex formation. Furthermore, TFMAA was chosen instead of the more commonly used monomer MAA because of its stronger ability to exchange hydrogen bonding. To our knowledge, this is the first time TFMAA was used for preparing a MIP of CBZ.
Articles dealing with MIPs of CBZ report the use of either EGDMA or DVB-80 as cross-linkers; however, as DVB was proved to minimize non-specific polar interactions thanks to it’s a polar character, it was also used as cross-linker [16]. Moreover, we assumed that DVB was able to develop Π-Π stacking with aromatic groups of CBZ during MIP synthesis, which would contribute to the building of specific cavities around each molecule of template. Hence, CH2Cl2 was preferred to toluene or ACN-toluene mixtures [14] as synthesis solvent to avoid any interference with the Π-Π interactions between the template and the cross-linker. Moreover, hydrogen bonds between template and monomer should not be disrupted in an aprotic solvent such as CH2Cl2.
Optimization of MIP-SPE protocol
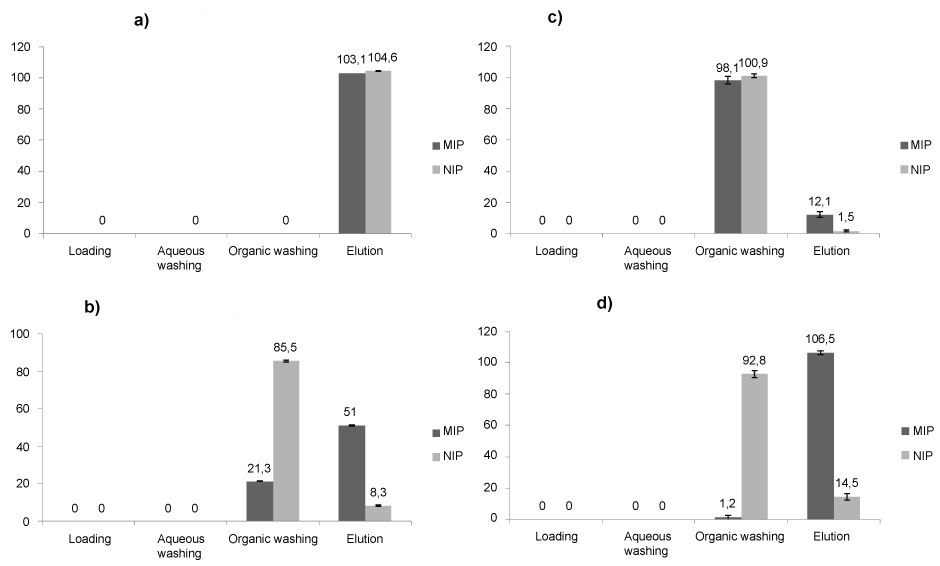
The selectivity of MIP versus NIP and the quantitative recovery of CBZ at the elution step of MIP-SPE were the main focuses for developing the SPE protocol. The selectivity is improved by promoting the Specific Interactions (SI) present inside imprints while reducing the Non-Specific Interactions (NSI) with the polymer material. For this, different washing mixtures were tested (Table 1). Recoveries obtained for MIP and NIP are reported in Figure 2.
Thus, after an acidic (pH 2.5) conditioning step, we observed that CBZ was totally retained by both MIP and NIP at the loading step and, also, at the first washing step (5 mL of ultrapure water). Since no difference appears between MIP and NIP, the retention of CBZ was supposed to be mainly hydrophobic NSI.
Next, the organic washing step was subjected to modifications such as the nature and percentage of solvents in order to reach a good MIP/NIP selectivity. As reported in Figure 2a, pure ACN failed to break NSI since equal amounts of CBZ were recovered in the elution fractions of MIP (103.1%) and NIP (104.6%). As ACN is a polar but aprotic solvent, MeOH was added in order to enhance the competitive effect of the washing solution against NSI hydrogen bond. Thus, 2% MeOH (Figure 2b) improved the selectivity of MIP versus NIP with respective elution recoveries of 51% versus 8.3%, but decreased the extraction rate of CBZ by MIP. Higher percentage of MeOH (20%, Figure 2c) confirmed the high competitor effect of this protic solvent on both NSI and SI since elution recoveries were 1.5% for NIP and 12.5% for MIP.
Therefore, CH2Cl2, previously used as porogenic solvent during the MIP synthesis, was tested as organic washing solvent. As often mentioned in literature, the use of porogenic solvent during the washing step promotes the specific recognition of target analytes by restituting the initial conformation of imprints [18]. This was verified in our case since high elution recoveries and selectivity was achieved (106.5% for MIP versus 14.5% for NIP, Figure 2d).
Then, CH2Cl2-MeOH mixtures were tested at different compositions for the elution step. We observed that percolation of CH2Cl2-MeOH mixture (80/20, v/v) followed by MeOH allowed full CBZ recovery (100-110%). This is certainly due to the protic character of MeOH that was effectively able to break the hydrogen bonds established between the CBZ and the polymer. Moreover, the presence of CH2Cl2 in the first elution fraction ensured an optimum solvation of CBZ.
Thus, after an acidic (pH 2.5) conditioning step, we observed that CBZ was totally retained by both MIP and NIP at the loading step and, also, at the first washing step (5 mL of ultrapure water). Since no difference appears between MIP and NIP, the retention of CBZ was supposed to be mainly hydrophobic NSI.
Application of SPE on mineral and river waters
The MIP-SPE protocol described in the previous section was applied to mineral water and proved its efficiency to extract CBZ with good recoveries. However, a decrease of selectivity was observed since recoveries obtained at the elution step of NIP-SPE were higher than those obtained with ultra-pure water samples.
As divalent metal ions are known to form coordinate bonds with ligands possessing -COOH and -NH groups, we assumed that Ca2+ and Mg2+ may form coordinate bonds with the carboxyl moiety of TFMAA and the amino moiety of CBZ leading to strong NSI which were not removed by aqueous and organic washing steps.
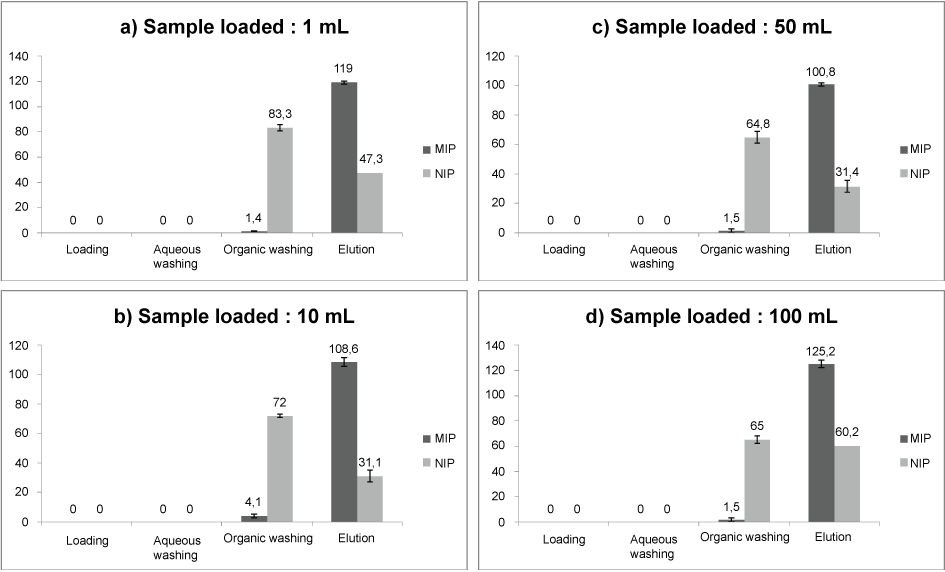
For this reason, the first washing step was done with an EDTA solution (18.6 mg L-1) instead of ultrapure water. Indeed, the addition of EDTA, a strong complexing agent of divalent cations, was expected to prevent the formation of any coordination complex between TFMAA and CBZ and, therefore, to maintain a good selectivity [22]. As expected, elution recoveries equal to 119% for MIP versus 47.3% for NIP were obtained in these conditions as shown in Figure 3a.
With this modified protocol, pre-concentration assays were carried on at several loaded volumes (10, 50 and 100 mL) of mineral water and constant loaded amount of CBZ (5 µg) as described in the experimental section 4.7. The MIP extraction of CBZ was very good with elution recoveries of 100% (Figure 3b, Figure 3c and Figure 3d).
For all experiments, the Imprinting Factor (IF), defined as the MIP/NIP recovery ratio was calculated. IF values were found to be equal to 3.8, 3.9, 4.5 and 2.0 for a volume of mineral water of 1, 10, 50 and 100 mL, respectively. Thus, it is obvious that whatever the loaded volume, the imprinting factor was greater than unity meaning that the developed SPE protocol succeeded in promoting SI regarding to NSI, even in the presence of a mineral matrix.
In order to prove that MIP-SPE protocol was suitable for extraction of CBZ at trace level in a river water, 100 mL of Oise river water, spiked with CBZ 1 µg L-1, was percolated through MIP-SPE cartridge. The MIP-SPE protocol was the same as that previously applied to mineral water. Both elution fractions were gathered, evaporated until dryness under a gentle stream of nitrogen at room temperature and recovered in 1 mL MeOH-H2O (90/10, v/v), spiked with 13C-CBZ 1µg L-1 and analyzed by UPLC - tandem MS. The MIP-SPE was duplicated. The CBZ recoveries obtained were 125 and 124%. These recoveries are very close to that obtained with mineral water (Figure 3d). During this study, it was obvious that residual template (CBZ) remained in MIP even after drastic ultrasonic extractions (section 4.4). Indeed, MIP-SPE performed on blank mineral water provided results indicating that mineral water would contain 0.35 µg L-1 CBZ which is wrong and proves the bleeding of the template from MIP during MIP-SPE protocol. Therefore, in order to avoid any false positive result, more efficient washing steps were developed for MIP preparation. Microwave Assisted Extraction (MAE) was selected according to its reported efficiency [23,24]. The best results (highest amount of CBZ eliminated per mg MIP) was obtained by MAE extractions (5 min heating ramp up to 60 ℃ followed by an isotherm extraction at 60 ℃ for 1 hour) applied to 50 mg of MIP in 20 mL MeOH-HCOOH (90/10, v/v). However, after five successive extraction cycles, the MIP bleeding was still 10 µg L-1 CBZ in elution fraction. As MAE is suspected to affect the MIP physical and chemical integrity, the elimination of template was finished off by simple percolation of MeOH-HCOOH (90/10, v/v) through MIP-SPE cartridges.
Test of cross reactivity
After demonstrating the ability of this MIP to recognize the target molecule (CBZ), the specificity of MIP was then evaluated. Four TCAs were selected as potential interfering compounds, because their chemical structures are to some extent similar to that of CBZ. These basic drugs (pKa~10) are easily ionized and can be analyzed by Capillary Electrophoresis (CE) [19-21].
A novel CE separation method was developed to monitor MIP-SPE fractions by using a BGE composed of CAPSO (50 mM), KCl (0.143 mM) and methanol (60%). Its pH, measured before adding MeOH, was equal to 9.5. This BGE was chosen taking into account the interesting study of Madej, et al. [19]. By adding MeOH and KCl to BGE, the electro-osmotic mobility was reduced and the resolution was improved. In fact, MeOH lowers ionization of silanol groups and the presence of KCl also strengthens the decrease effect of the charge density on the inner wall of the capillary due to the adsorption of K+ onto SiO- sites by electrostatic interactions. Unfortunately, CLO and TRIMI still migrated as a single peak. Wu, et al. analyzed seven TCAs (desipramine, imipramine, clomipramine, doxepin, nortriptyline, amitriptyline and protriptyline) by CE/UV [20] and observed that the separation was possible after adding a native complexing cyclodextrin. For this purpose, several CDs were tested (α-CD, β-CD, HP-β-CD, sulfated CD, dimethyl-β-CD, trimethyl-β-CD) as complexing agents of BGE. The best result was obtained with 2,6-di-O-methyl-β-CD at 1 mM where the resolution between CLO and TRIMI was almost satisfactory (Rs ~1). However, a significant improvement (Rs ~1.4) was obtained by adding 0.2 mM SDS to the BGE with an increase of peak efficiencies (600,000 theoretical plates).
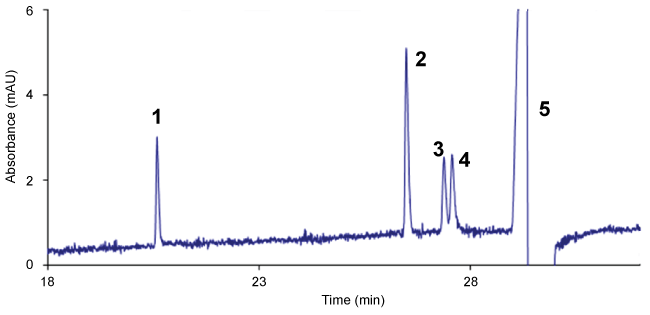
Using an electrokinetic injection (30 kV, 10 s), the Limit of Quantification (LOQ) for CLO and TRIMI has been greatly improved (30 µg L-1). This high sensitivity is certainly due to an online pre-concentration step FASI (Field Amplified Sample Stacking) since the conductivity of the samples is much lower than that of the separation buffer (50 mM ionic strength) [25]. The analysis time was approximately 26 min (Figure 4). The analyses were repeatable with a relative standard deviation of about 1.1% (n = 6). Linearity was validated for each of the four compounds with determination coefficient (r2) higher than 0.993.
From these results one can conclude that the separation of the four TCAs was possible by CE with an optimized BGE containing 50 mM CAPSO, 0.2 mM SDS, 60% MeOH, 0.143 mM KCl and 1 mM 2,6-di-O-methyl-β-CD at a pH of 9.5.
The previously developed SPE protocol was used as following: conditioning with MeOH (5 mL) and acidified water pH 2.5 (10 mL), loading with ultrapure water (1 mL) spiked with CBZ and ATCs (5 mg L-1), aqueous washing with 5 mL of ultrapure water, organic washing with CH2Cl2 (2 mL) and finally elution with CH2Cl2/MeOH (80/20, v/v) and MeOH (5 mL). The elution yield of CBZ decreased by loading CBZ and TCA spiked water (64%) compared with CBZ spiked water (106.5%), as shown in Figure 2d. The TCAs certainly have a competitive effect with respect to interactions between CBZ and MIP. However, extraction was still quantitative for CBZ with a satisfactory selectivity (IP = 2.2). The CE method developed for separating IMI, DES, TRIMI and CLO was applied to determine the amount of TCAs recovered at each step of the SPE protocol. Calculated recoveries showed that TCAs were totally retained by MIP as well as by NIP until the elution step due probably to strong non specific interactions.
Conclusion
This work describes the synthesis of a new molecularly imprinted polymer for extraction of antiepileptic drug CBZ from river waters. This material was prepared from CBZ as template and TFMAA as acidic functional monomer, both of them interacting via hydrogen bonding. The cross-linker (DVB) was selected to interact by Π-Π interactions with CBZ. The MIP-SPE protocol was optimized by using CH2Cl2 as organic washing solvent and enabled the selective and total extraction of CBZ from pure and river waters with a good yield (106%). However, the presence of interfering molecules of Tricyclic Antidepressants (TCAs) added to waters samples induced a decrease of CBZ extraction yield (64%) and the retention of TCAs on the MIP. SPE fractions were analyzed by CE/UV with the help of a novel background electrolyte composed of 50 mM CAPSO, 0.2 mM SDS, 60% MeOH, 0.143 mM KCl and 1 mM 2,6-di-O-methyl-β-CD (pH 9.5).
Acknowledgements
We gratefully acknowledge the Region Centre (France) for its financial support (research project CAPTENVIRO, APR 2012).
References
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. (2009) Endocrine-Disrupting Chemicals: an Endocrine Society Scientific Statement. Endocr Rev 30: 293-342.
- Roy P, Pereira BM (2005) A treatise on hazards of endocrine disruptors and tool to evaluate them. Indian J Exp Biol 43: 975-992.
- Schuman E (2008) Thesis Environmental Technology, Sub-Department of Environmental Technology Wageningen University.
- Andreozzi R, Marotta R, Pinto G, et al. (2002) Carbamazepine in water: persistence in the environment, ozonation treatment and preliminary assessment on algal toxicity. Water Res 36: 2869-2877.
- Heberer T (2002) Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data. Toxicol Lett 131: 5-17.
- Tiehm A, Schmidt N, Stieber M, et al. (2011) Biodegradation of Pharmaceutical Compounds and their Occurrence in the Jordan Valley. Water Resources Management 25: 1195-1203.
- Keen OS, Baik S, Linden KG, et al. (2012) Enhanced Biodegradation of Carbamazepine after UV/H2O2 Advanced Oxidation. Environ Sci Technol 46: 6222-6227.
- Dong MM, Trenholm R, Rosario-Ortiz FL (2015) Photochemical Degradation of Atenolol, Carbamazepine, Meprobamate, Phenytoin and Primidone in Wastewater Effluents. J Hazard Mater 282: 216-223.
- Bahlmann A, Weller MG, Panne U, et al. (2009) Monitoring carbamazepine in surface and wastewaters by an immunoassay based on a monoclonal antibody. Anal Bioanal Chem 395: 1809-1820.
- Ternes TA (1998) Occurrence of drugs in German sewage treatment plants and rivers. Water Research 32: 3245-3247.
- Heberer T (2002) Occurrence and distribution of organic contaminants in the aquatic system in Berlin Toxicol. Lett 131: 5-17.
- Metzger JW (2004) Drugs in Municipal Landfills and Landfill Leachates. In: Springer-Verlag Berlin Heidelberg, Pharmaceuticals in the Environment, 133-137.
- Speltini A, Scalabrini A, Maraschi F, et al. (2017) Newest applications of molecularly imprinted polymers for extraction of contaminants from environmental and food matrices: A review. Analytica Chimica Acta 974: 1-26.
- Beltran A, Marce RM, Cormack PAG, et al. (2009) Synthesis by precipitation polymerisation of molecularly imprinted polymer microspheres for the selective extraction of carbamazepine and oxcarbazepine from human urine. J Chromatogr A 1216: 2248-2253.
- Zhang YL, Zhang J, Dai CM, et al. (2013) Sorption of carbamazepine from water by magnetic molecularly imprinted polymers based on chitosan-Fe3O4. Carbohyd Polym 97: 809-816.
- Beltran A, Caro E, Marce RM, et al. (2007) Synthesis and application of a carbamazepine-imprinted polymer for solid-phase extraction from urine and wastewater. Ana Chim Acta 597: 6-11.
- Dai CM, Geissen SU, Zhang YL, et al. (2010) Performance evaluation and application of molecularly imprinted polymer for separation of carbamazepine in aqueous solution. J Hazard Mater 184: 156-163.
- Spivak D, Gilmore MA, Shea KJ (1997) Evaluation of binding and origins of specificity of 9-Ethyladenine imprinted polymers. J Am Chem Soc 119: 4388-4393.
- Madej K, Wozniakiewicz M, Karabinowska K (2012) Capillary electrophoresis screening method for six tricyclic antidepressants in human serum. Acta Pol Pharm 69: 1023-1029.
- Wu SM, Wu HL, Ko WK, et al. (2000) Determination of amitriptyline and nortriptyline in spiked plasma by capillary zone electrophoresis with β-cyclodextrin. Analytica Chimica Acta 413: 125-129.
- Cantú MD, Hillebrand S, Queiroz MEC, et al. (2004) Validation of non-aqueous capillary electrophoresis for simultaneous determination of four tricyclic antidepressants in pharmaceutical formulations and plasma samples. J Chromatogr B Analyt Technol Biomed Life Sci 799: 127-132.
- Zhu QZ, Degelmann P, Niessner R, et al. (2002) Selective trace analysis of sulfonylurea herbicides in water and soil samples based on solid-phase extraction using a molecularly imprinted polymer. Environ Sci Technol 36: 5411-5420.
- Lorenzo RA, Carro AM, Alvarez-Lorenzo C, et al. (2011) To remove or not to remove? The challenge of extracting the template to make the cavities available in molecularly imprinted polymers (MIPs). Int J Mol Sci 12: 4327-4347.
- Ellwanger A, Berggren C, Bayoudh S, et al. (2001) Evaluation of methods aimed at complete removal of template from molecularly imprinted polymers. Analyst 126: 784-792.
- Claude B, Nehme R, Morin P (2011) Analysis of urinary neurotransmitters by capillary electrophoresis: sensitivity enhancement using field-amplified sample injection and molecular imprinted polymer solid phase extraction. Anal Chim Acta 699: 242-248.
Corresponding Author
Reine Nehmé, Université d’Orléans - CNRS, ICOA, UMR 7311, BP 6759, F-45067, Orléans, France.
Copyright
© 2017 Claude B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.