High Altitude Maize (Zea Mays L.) Cultivation and Endemism in the Lake Titicaca Basin
Abstract
Botanists and plant biologists have long asserted maize (Zea mays L.) cannot be cultivated above 3,600 masl. Multidisciplinary evidence has documented an abundance of a particular variety of maize called tunqu by indigenous Aymara speaking populations and cultivated on terraces between 3810 to 4100 masl around the Copacabana Peninsula, in the Lake Titicaca Basin, Bolivia. This is the only known maize variety in the world cultivated above 3600 masl. The Titicaca Basin was transformed by various ancient cultures and civilizations and pre-Columbian landscape modifications such as raised fields and terraces. Such modifications were primarily geared to the cultivation of food crops. Multidisciplinary evidence indicates maize was and continues to be central to indigenous religious rituals and rites in the altiplano. Colonial accounts emphasize this maize was primarily consumed as a fermented intoxicant, maize beer (aqha, or chicha). It was considered sacred and also used for ritual offerings. Such practices and beliefs appear to have ancient origins and have been documented archaeologically with the Yaya Mama religious tradition (ca. 800 BCE). This maize variety has phenotypic characteristics unlike any other known landrace and these characteristics and its adaptation to such altitudes strongly suggest it may be endemic. The Andes are particularly well suited to studying the phenotypic diversity of plants, because the landscape is characterized by discrete ecological zones, which support specific suites of domesticated plants. The Titicaca Basin represents a unique microenvironment where evapotranspiration from the lake reduces the diurnal variation in temperatures enough to permit cultivation of maize. Its cultivation, preparation and consumption among indigenous cultures are analyzed as are its botanical and biological characteristics which distinguish this landrace and support the contention it is endemic.
Keywords
Andes, Maize, Botany, Ethnobotany, Archaeology, Ecology, Plant biology
Introduction
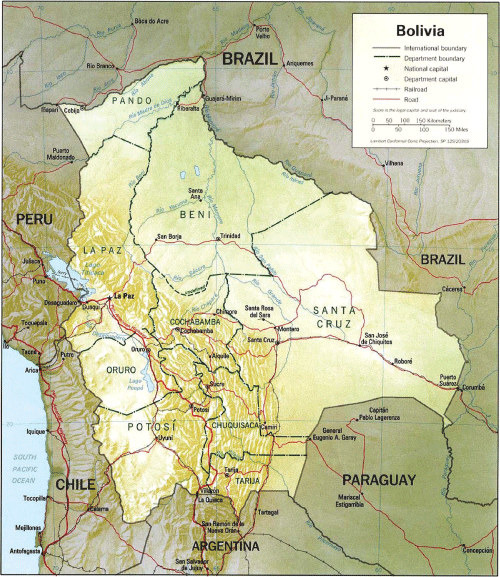
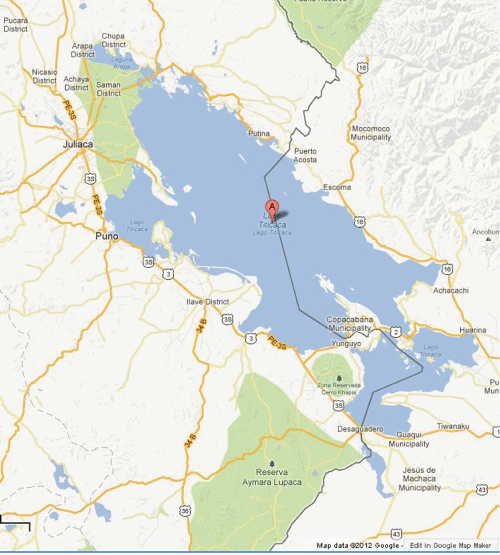
The Andes represent the second highest mountain range in the world and are characterized by complex ecologies and diverse environments, with biota specifically adapted to different regions, sides, and altitudes of the sierra (Figure 1). The Andean cordillera also includes unique microenvironments that affect the diversity and complexity of domesticated and wild plants and animals, and maize (Zea mays L.) is one such plant [1]. Archaeologists, ethnographers, botanists and plant biologists have maintained its cultivation is restricted to altitudes below 3200 masl [2-5]. Nevertheless, large-scale maize cultivation has occurred above 3600 masl in the altiplano of the Lake Titicaca Basin since prehistoric times [6-10]. Indigenous Andean communities (ayullus) along the southwestern part of the basin still herd alpacas and llamas. Since the introduction of foreign domesticated animals, they also raise sheep, cattle, pigs, donkeys, and chickens [9,11]. Native crops are cultivated by indigenous communities on terraces, often without irrigation, along with crops introduced during the Spanish conquest such as, fava beans, barley, and recently, onion (Allium cepa). Primary native food plants in the altiplano include, numerous varieties of quinoa (kañiwa or juyra) and potatoes (ch'ugi), oca (uka or ulluku), native lupin (tarwi), and a particular variety of maize exclusive to this region called tunqu [10]. Native tubers are often freeze dried (ch'unu), and important to indigenous subsistence and their agricultural economy [11,12]. About 60% of the region is engaged in subsistence agriculture [13]. The western portion of the basin adjacent to Lake Titicaca around Juliaca and Puno, Peru is slightly colder than the Bolivian side of the basin, with higher diurnal variation in temperatures [11] (Figure 2).
Archaeological and ethnographic evidence has documented large-scale maize cultivation at c. 3810 to 4200 masl - the highest known cultivation in the world, primarily centered around the Copacabana Peninsula. Previous interdisciplinary research indicated indigenous communities in various regions of the basin cultivate high yields of a maize variety called 'tunqu'. Local campesinos consider this maize as 'sacred' and use it almost exclusively to make maize beer or chicha for ritual feasting, rites, and as offerings [7,9]. Multidisciplinary evidence presented here suggests the tunqu maize may represent an endemic variety, specifically adapted to the microenvironment of certain regions of the Titicaca Basin [1,11].
Evolution and Domestication of Maize
Plant domestication represents a process of evolutionary change involving the genetics and biogeography of plant populations through human and natural selection. The domestication of plant species is generally perceived in the scientific literature as a process of evolutionary change involving the genetics of plants [1,14-18]. Genetic changes are in response to human influence or deliberate conscious selection, or artificial selection (unconscious selection), for certain favorable traits, or to management of plant reproduction and modification of the environment [1,17-22]. Domesticated plants such as maize are essentially cultural artifacts that would not exist in nature without human assistance, unlike teosinte, its wild predecessor and its various subspecies [21,22]. Human adaptation selects for these interrelationships because they provide strong selective advantages for both the plant(s) and human populations dependent upon them. The cultural importance, biogeography, phenotypic variability of early maize varieties in the New World has been the subject of considerable research and publications in the social, biological, and in recent years the molecular sciences.
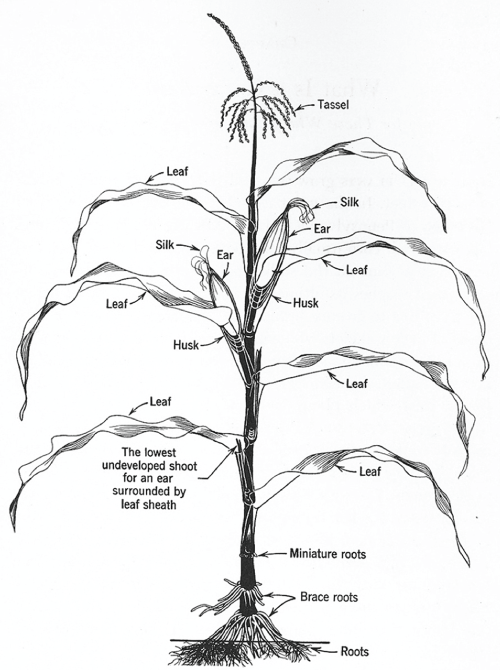
Recent botanical, ethnobotanical, and DNA research have documented the domestication and spread of maize in increasing detail [19,23-26]. Maize or corn (Zea mays L.) is monophyletic. Maize evolved from a single domestication event c. 7000 years ago, a direct descendant of an annual wild grass, teosinte (Zea mays ssp. parviglumis) native to the Balsas River drainage in southern Mexico [25,27]. The genus Zea includes cultivated maize (Z. mays ssp. mays) and the various subspecies of teosintes, classified by plant taxonomists as members of the grass family Poaceae [3,28]. The fruit of the genus Poaceae is a caryopsis or has the appearance of a seed with flowers usually arranged in spikelets with one or more florets further grouped into panicles or spikes (Figure 3). All taxa of Zea have a central spike or terminal branch, a continuation of the central inflorescence axis [28,29]. Teosinte and maize both have male and female flowers on the same branch, and kernels "encased in a hard, leaf-like organ called a glume" is highly branched, with a male inflorescence (tassel) on its central branch and female inflorescences (ears or cobs) on auxiliary branches (Figure 4). What differentiates maize (Zea mays L.) from its wild predecessor teosinte (Zea mays ssp. parviglumis) is that it is totally depend upon humans for its survival, and biogeography [28]. Plant and molecular biologists perceive the domestication process as a gradual and fortuitous accumulation of genetic mutations that create a mutualism and interdependence between human populations, and target plant species and/or populations [1,18,30]. Evolutionary changes due to unconscious selection are initiated by changes in relationships between humans and a particular target species such as maize [21,30]. Selective pressures perceived in terms of a causal relationship usually involve behavioral change toward a maize landrace; selecting certain traits to induce a genetic response, and ultimately morphological and phenotypic modifications [30]. In the case of maize, the ear and kernels provide the most reliable phenotypic traits classification of landraces and their the phylogenetic (historical) relationships [21,31-33]. Maize is highly mutagenic, kernel color and ear morphology are directly affected by wind pollen from maize cultivated in surrounding fields.
Research on maize DNA has proven most effective in establishing its origins [14,25]. Documentation of the maize genome made it possible for botanists and molecular scientists to directly document how domestication and cultivation affect the phylogeny of maize landraces [14,34]. Our current understanding of the bottleneck phenomenon, the phenotypic variation expressed by the different maize landraces through anagenesis and cladogenesis, has allowed research to more clearly understand and further explore why certain maize varieties are maintained while others disappear [14,15,30,35] (Figure 5).
Molecular biologists identified the existence of a Mendelian locus (teosinte glume architecture tga1) that controls the cupule and glume morphology of maize ears or cobs [23,24,36]. These data provide evidence to document the spread of various landraces, as well as the role of human selection to its phenotypic characteristics and morphological traits. This single Mendelian locus (tga1) also controls several aspects of chaff morphology, both macroscopic and microscopic, including the deposition of silica such as phytoliths and epidermal cells [1,7,24]. Moreover, tga1 also controls the induration, orientation, length, and shape of the chaff, and its pleiotropic effects suggest it represents a regulatory locus [23,24]. The diverse mosaic of maize varieties, high genetic diversity among landraces, are related in part to a founder effect, resulting from a population bottleneck followed by conscious selection for specific traits [14,26,30] (Figure 6).
Origins and Biogeography of Maize
Archaeological research on the origins of maize was critical to our understanding of the transition from hunting and gathering to agricultural economies [19,37-39]. Its domestication and spread through different regions of the New World is largely associated with the rise of pre-Columbian agriculture and its perceived importance as a staple to early agricultural economies [15,16,38,40-43]. However, evidence from early cave and rock shelters sites suggest teosinte fruitcases were not exploited for food, but rather for their stalks [17,37-39,41]. Macrobotanical evidence suggests teosinte was initially exploited for its stalk sugar, which was used as a condiment or for fermentation [44]. Several of these rock shelters and caves provided evident of quids or chewed maize stalks in the earliest stratigraphic layers [37-39,41,45]. Archaeological evidence indicates maize stalks were initially chewed to make intoxicants such as pulque, providing insight regarding maize biogeography to various parts of Mesoamerica and throughout the New World [1]. The earliest distinguishing characteristics separating fully domesticated maize from its wild progenitor teosinte at various rock shelters and caves is the glume architecture [19,46]. Thus the tga1 locus controls cob size, row number, kernel shape and size primary phenotypic characteristics that define and differentiate different varieties of maize landraces [14,17,32,46] (Figure 6).
Direct AMS dates on the earliest maize in Latin America are relatively recent c. 5900 to 5450 B.C. [1,47,48]. Most associated 14C dates suggest an initial expansion dated to c. 7000-4500 B.C., and a subsequent expansion into the American SW at around 2000 B.P [1,49,50]. Systematic identification of maize varieties, as well as recent research in the Andes cordillera, provide clear evidence of the incredible mutability of maize and its ability to adapt to a whole host of environmental and climatic conditions [1] (Table 1). Despite its early initial expansion maize does not become a primary economic staple in Mesoamerica and the Andes until several millennia later, c. 1500 to 1000 B.C. [1,41].
The recent multidisciplinary research has transformed previous paradigms regarding its origin, biogeography, phenotypic diversification. Food science is now challenging previous paradigms regarding it economic and cultural significance to complex sociocultural development. Data from stable carbon isotope research on ancient diets, have revised our understanding of its economic and dietary roles to Pre-Columbian Mesoamerica and the Andes, as well as other regions of the Americas [22,51-54].
Endemism, Environment, Species Diversity, and Climate
Endemism refers to species that are unique to a particular ecology, habitat or specific geographic location or region [55,56]. Endemic species are characterized by unique phenotypic characteristics, which distinguish such species from others of the same taxon. Physical, climatic, and biological factors contribute to endemism and endemic species are particularly likely to evolve in geographically and biologically distinct or isolated regions [56] (Figure 7). The greatest incidence of endemism in the Andes occurs in the Huancabamba Depression, northernmost highland Peru and southern highland Ecuador, because these regions represent the lowest part of the cordillera where both plant and animal species can move and spread to both sides of the cordillera [1,57]. The upper Amazon of Peru and Bolivia, and the Lake Titicaca Basin are also characterized by a high incidence of endemism [10,58,59]. The discrete ecological zones of the cordillera are characterized by specific suites of economic plants and animals. Transitional environments and ecologies, or what is categorized as an 'ecotone', also have a high incidence of endemism [1,57].
The pleiotropic effects suggest tga1 represents the regulatory locus which makes the tunqu maize variety in the Copacabana peninsula truly distinct from all other known varieties of maize (Figure 8). This maize variety is endemic because its phenotypic characteristics are specific to the Titicaca Basin, particularly around the Copacabana peninsula, where tunqu has been cultivated since prehistoric times [7,9]. The reduced diurnal variation in temperature caused by evapotranspiration of the lake creates a microenvironment which makes the cultivation of maize as well as other crops possible in such extreme altitudes (Figure 9).
Multidisciplinary research in the Peruvian and Bolivian Amazon have modelled the geographic distribution of numerous endemic plants and species between 1000-1500 masl [59]. However, the concept of endemism and identification of endemic species is largely dependent upon knowledge of the geographical range of a particular species. The Peruvian and Bolivian Amazon Basin is characterized by extreme biodiversity. However, the botanical and biological data with regard to the biogeography of various taxon are not well documented [58,60,61]. However, the phenotypic diversity and biogeography of maize have been extensively documented in the social, as well as botanical and biological sciences [1,14,15,32,34].
The Lake Titicaca basin has been a focus of considerable attention by researchers and its regions and islands are well defined and documented [62-66]. Maize cultivation was initially believed to be restricted to sheltered locations with northern exposure on the shores of the lake or the various islands [67-69], the terraces above Puno or the District of Chucuito overlooking the lake [2,70]. Archaeologists maintained maize had little economic importance in the region, and neglect to discuss the associated altitudes, varieties, or their phenotypic characteristics. Most emphasize its consumption as a vegetable or its ritual or ceremonial importance to the political economy [2,4,70].
Andean maize varieties cultivated above 3200 masl are largely a product of conscious selection of particular traits in combination with adaptation to the extreme environmental conditions [71]. Variation in maize varieties such as tunqu are a result of human selection and adaptation to micro environmental conditions created by the lake. This is particularly apparent on terraces around the Copacabana Peninsula, where tunqu, an endemic variety of maize grows in altitudes over 3800 to 4100 masl [1,7]. Its distinct phenotypic characteristics and restricted geographic distribution has only come to the attention of scholars in recent years, [7,9,10]. This may be related in part to its cultivation and consumption being restricted to indigenous communities in the basin.
The Lake Titicaca Basin straddles the border Peru and Bolivia, and the lake is the largest and highest navigable lake in the world (Figure 1 and Figure 2). The basin is situated in a high altitude plain or altiplano ranging c. 3800 and 4100 masl [9,72-75]. About 90% of the fish species in Lake Titicaca are endemic [76,77]. Overall, the altiplano is a harsh and difficult environment for the cultivation of many domesticated plants [78,79]. However, a significant decrease in diurnal temperature variation around the lake caused by evapotranspiration, creates a microenvironment in which various economic staple including maize been cultivated in these altitudes since ancient times [1,10]. The climate is classified as subtropical highland and cultivation is strongly affected by the semi-arid, and cold dry season cycle. Mean annual temperatures of between -3 °C to 10 °C, with slightly lower diurnal variability in and immediately around the lake in cities such as Copacabana, Bolivia and Puno, Peru (Tables 2 and Table 3). Copacabana and Puno are both located beside the lake with average high temperatures of c. 15.3 °C (59.5 °F), and 18.5 °C (64.8 °F) and average low temperatures of 0.6 °C (33.1 °F) and 1.58 °C (32.6 °F) respectively. Average annual precipitation in these regions of the basin are between 580.7 mm (22.9 inches) and 669 mm (26.4 inches) (Tables 2 and Table 3). The climate around Juliaca, Peru, which is situated in a higher altitude and whose climate is considered to be more characteristic of the basin as whole has average high temperatures of c. 17 °C (62.5 °F), while the average annual precipitation is around 580 mm (22.9 inches) (Table 4). Thus, the basin overall is more arid and characterized by slightly higher diurnal variations in temperature. The subtropical highland climate around the basin is distinct because it is situated in the tropics, it is in one of the highest parts of the cordillera, and consequently has a markedly drier dry season. The thermo-regulation through evapotranspiration, albeit slight and subtle, appears to have dramatic consequences for diurnal temperature variations, climate, as well as the physical qualities of sedimentary deposits [11,80]. Evapotranspiration around the lake water reduces the diurnal temperature variation, by creating a microenvironment more amenable to cultivation [9,79,81]. The agricultural cycle generally spans from early December to April, and cultivation around the lake is totally dependent upon rainfall since the waters of Lake Titicaca are saline [9].
Maize and Andean Culture
The Lake Titicaca Basin essentially represents a built landscape transformed for cultivation over the millennia by various cultures and civilizations extending back to Initial Period [9,63,72]. Initially the archaeological evidence suggested maize was introduced to the Titicaca basin during the Middle Horizon Period by the Tiwanaku civilization. More recent research has identified maize at sites ranging from the Formative to Early Horizon Period [74,75,82]. However, food residues preserved on ancient Yaya Mama pottery, including 247 samples from Ch'isi, Qhot'a, Pata, Kusijata, Cundisa, and Qupakati, produced three AMS dates ranging between 800-460 B.C. associated with Yaya Mama religious tradition on the Copacabana Peninsula [7,9]. Archaeological and ethnographic evidence of agricultural terraces on the peninsula between 3810 to 4200 masl are said to have very ancient origins [7,9]. The basin was subsequently transformed by human engineering and landscape modifications geared to the cultivation of food crops [9]. Yaya Mama culture was egalitarian and non-centralized. The center has a series of temples complexes which played a major role to maintaining ethnic diversity, while at the same time engineer and coordinate widespread regional landscape modifications [6,7,9]. In contrast to the monumentality of Tiwanaku architecture, the Yaya Mama were organized into distinct ayullus or communities, who subsequently cultivated tunqu on terraces around the lake for over three millennia [9,64,65,83].
Maize played a central role to the rise of Andean civilization because of its roles and importance to religious rituals and rites, rather than as a food staple. Ethnographic and ethnohistoric accounts state that it was so commonly consumed as beer (aqha or chicha) as to make a significant contribution to the diet [1,22,84,85]. The term for maize beer is 'chicha' derived from the Nahuatl 'chichiya', which means, "to become sour, bitter", in reference to the fermentation process [86]. In parts of Guatemala, the term "chicha" is still used by some Maya to refer to maize based fermented intoxicants or pulque [87]. The Spaniards spread the term to designate both alcoholic and non-alcoholic beverages made from various plants. The Aymara word for maize, tunqu, appears in the 1612 Aymara dictionary by Bertonio, but until recently, it has been rarely used in the literature. Quechua terms runa simi or sara are more prevalent, and both terms are still used by Aymara and Quechua speakers [1,86]. Tunqu is not mentioned in the 1608 Quichua dictionary of Gonzalez Holguin (1952 [1608]) [88]. Maize beer was central to fostering social alliances and was redistributed by various Andean polities as a form of reciprocity in exchange for corveé labor [4,84]. Inca territorial expansion was based in part upon large-scale maize production associated with widespread terrace construction. Such terrace constructions and maize cultivation largely involved chicha production, and when consumed as food, various landraces played a major role in Andean ethnic identity and regional political economies [4,5,84,85]. One of the earliest Andean locations for such large-scale terrace construction was the Copacabana Peninsula in the Lake Titicaca Basin [6,7,9].
Carl Sauer [1950: 494] [2] stated, "The Spanish annalists give the impression that more of it was drunk and less eaten...". The chronicler Cieza de León (1959 [1553]) [89] was particularly impressed by the drinking powers of the indigenous Andeans and its importance to rituals, rites, and high status elite [90,91]. However, the first conquistador to mention maize cultivation in the Titicaca basin was Martin de Murúa (1987 [1590]:556) [92] who documented maize cultivation at 3650 masl near present day La Paz. The Bolivian altiplano was particularly important to the Spanish Crown and various Colonial governments because to the south around Potosi, Bolivia was Cerro Rico, which has the richest silver ore deposits in the Americas [89,91]. Potosi was also the location of the Spanish colonial mint, and chroniclers state that many thousands of indigenous people died from mercury poisoning working in the Cerro Rico silver mine [86].
Pre-Columbian Andean religions are inherently telluric, naturalistic and spatial. Their origins and creations from previous conditions or states particularly mythological emergence from a wild or natural state into the existing creation cycle [1,84-86]. Ethical order of the sacred landscape was manifest though the cultivation of valley bottomlands, terrace construction, channeling water into agricultural fields, and architectural modification of sacred places (Figure 10). Ritual offerings to huacas continue perpetuate the circulation of time and bring forth regeneration, fertility and fecundity of the earth among indigenous populations (Figure 11). The terrace construction was associated with the civilizing of the natural landscape and its people through the imposition of an ethical order [84-86]. Water is seen to flow through the earth into the underworld and into the sky in the form of the Milky Way [86]. The destinies of many Andean cultures was shaped by the manipulation of water, evident by customs such as ritual washings, ritual beverages, and the manipulation of irrigation technology. However, unlike the elaborate irrigation networks associated with the raised fields at nearby Tiwanaku, there is little evidence of irrigation technology in the Copacabana Peninsula since all of the cultivation associated with the terraces is dependent upon the coming rains [6,9,10].
The cultivation of the tunqu maize in this region of the Andes cordillera was initially brought to the attention of scientists and scholars by George Squier (1973 [1877]:331, 341) [93] who noted that on the Copacabana peninsula and some islands of the lake, the maize stalks were, "scarcely three feet high" and the cobs were "no longer than one's finger" and "closely covered with compact vitreous grains" (Figure 12). The cultivation of the small-sized maize growing on lower terraces on some of the island around the peninsula was confirmed some 20 years later [94]. Although not specified in these later accounts, it appears from the ethnographic and archaeological evidence tunqu maize was consumed both as food source and had important ritual and ceremonial significance [9].
Maize Phenotypic Diversity in the Titicaca Basin
The Andes are particularly well suited to studying the phenotypic diversity of plants, because the discrete ecological zones support specific suites of economic plants. Cultural modifications to the landscape vary in time, but stone-faced terraces particularly on the slopes and hills of the Copacabana Peninsula and those around Puno which also are constructed along the shoreline of Lake Titicaca appear to have an ancient history (Figure 10). The monumental terraces reflect a uniform style and technology of construction [7,9]. In other part of the basin, raised field agriculture was also an adaptive response to the altitude and cold temperatures characterizing the altiplano, as was cultivation on qochas or artificial depressions (suka qullu) and artificially irrigated pastures or bofedales to herd llamas and alpacas [63,74,79,93,95]. Dates for raised field cultivation and these other landscape modifications range from c. A.D. 300 to 1000 [63,64,74].
The stone-faced terraces around Copacabana range in width from one on steep slopes to several meters wide, and in some areas, preserved to a height of three meters [9]. Their distribution is continuous around the peninsula, including the islands of the Sun and Moon and several other islands south of the peninsula, only interrupted by rock outcrops and steep hills (ibid.). Subsistence agriculture is critical to adaptation to such altitudes and most indigenous communities are engaged in cultivation of food crops [13]. In fact, quinoa was originally domesticated c. 3000 B.C. in this region, and this is reflected in the genetic diversity of its varieties [8,96].
Over 52 communities on the Copacabana Peninsula cultivate maize annually, from lake level to as high as 4100 masl, taking advantage of the micro environmental conditions of the lake basin to meet subsistence and surplus needs. Early research described distinct varieties of maize for Peru and Bolivia primarily on the basis of phenotypic characteristics [97], recently such descriptions include genotypic characteristics [8]. Early maize classifications restricted to phenotypic characteristics of the cobs clearly indicated that this variety is endemic and exclusive to this geographical location, its climatic variability, flexibility.
There is a long history of cross pollination of tunqu varieties with other regions of the Andes. The pre-Columbian maize consumed in the basin by the Wari and Tiwanaku cultures was brought in from lower parts of the eastern cordillera and the coast the context of traditional Andean political economies [4]. The Inca cultivated and stored maize at administrative centers in and around Cochabamba, and all along the eastern cordillera that they commonly cross-pollinated with coastal varieties, particularly around Mocquegua and northern coastal Chile [98]. Recent documentation of the maize genome has directly documented the interrelations of various landraces over space and time [14,25,26,33]. Cutler (1946:281-282) [99] illustrates examples of altiplano maize ears from the shores of Lake Titicaca (Bolivia) that closely resemble the small and medium tunqu described here. Significantly, he noted that there was little or no change when planted using irrigation in Cochabamba, implying that the phenotypic characteristics of this variety are a product of both human and natural selection and geographically restricted to the basin, an important characteristic of endemism.
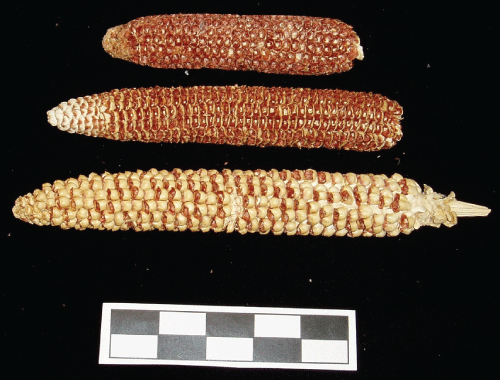
This tunqu maize variety is characterized by small ears and cobs measuring 3.5-5 cm in length (Figure 13). They stand about a half a meter in height (Figure 12). Varieties which grow in the lower terraces closer to the lake generally have larger kernels and cobs and are taller; medium to large cobs range 6-9 cm to 10-13 cm long respectively, and stand between 80 to 165 cm in height (Figure 6). Pointed popcorn varieties called p'asangalla have adapted to the highest elevations and their cobs are between 5-10 cm long (Figure 14). Large cob varieties which grow in the extreme altitudes are generally varieties which were cross pollinated to attain larger kernels and cobs with varieties in lower elevations [1,10]. Weatherwax (1954:62) [29] reported the existence of "pygmy" maize varieties standing about 60 cm high in some areas of this region. However, this may be a product of recent cross pollination with varieties from lower elevations. In the highest elevations a stalk usually produces only one cob, while in the lower elevations they have two or sometimes three cobs per stalk [9]. The cobs have different shapes; those from the highest elevations are rounder, pointed popcorns more slender, basically because they are longer, and usually lack straight rows. Row numbers vary depending if or with what varieties they were cross pollinated from 10 to 14 for the smallest cobs, from 13 to 14 for larger cobs, and the greatest range is with the popcorn varieties which have 5-12 rows. Kernel color is a primary phenotypic characteristic among indigenous communities for differentiating distinct maize varieties, and this is related to their culture, because kernel color and shape has associations to ethnic identity. Indigenous communities even differentiate multicolored kernels from those of a particular color. Pointed popcorn (p'asangalla) varieties are usually a bright yellow or white (Figure 13). According to the chroniclers, kernel color was also important among the Inca as it relates to their uses in rituals, rites, curing, and chicha manufacture [1,85].
Until recently, most investigators have neglected to take into account the subsistence potential of cultivating maize in the basin. The Titicaca Basin is largely believed to lack the potential for economically significant maize cultivation. Overall, the altiplano environment is harsh and difficult for the cultivation of certain plants. However, decreased diurnal temperature variation around the lake caused by evapotranspiration, creates a microenvironment in which maize has been cultivated in these altitudes since Colonial times and extend well into the pre-Columbian past.
Summary and Conclusions
The ecology and environment in the Titicaca Basin is related to the micro-environmental conditions created by the lake. It is the evapotranspiration of the lake that increase the nighttime temperatures just enough to create the conditions for maize cultivation at these extreme altitudes. Both natural and human selection make these endemic landraces distinct in different regions of the Lake Titicaca Basin. The phenotypic characteristics of tunqu maize is distinct, cobs are smaller in length, more bulbous in shape with smaller kernels and fewer row numbers than those cultivated in other regions of the Andes. Whenever maize landraces from the coast or the eastern cordillera are brought to this region and cross-pollinated with maize landraces from they are also phenotypically transformed by adapting to the extreme altitude. Pointed popcorns brought to this region from the Chilean or Peruvian coast or the eastern Andes around Cochabamba all revert in their phenotypic characteristics within one or two growing cycles. In terms of size and cob shape they become smaller and the kernels more rounded, similar to the tunqu landraces. However, they maintain or change their kernel color and shape to varying degrees depending upon what other varieties are cultivated nearby. Adaptation to periodic drought results in tiny cobs, what were once considered by scholars to be "wild" corn (Copacabana 2, 13). These have been identified in northern Chile and southern coastal Peru, indicating altiplano maize from the basin was and continues to be cross pollinated with other regions. It is obvious from the phenotypic characteristics and the fact that exotic landraces respond in similar ways to the microenvironmental conditions around the lake that natural selection is producing varieties, which are truly unique to the species and geographic region and are therefore endemic.
References
- Staller John E (2010) Maize Cobs and Cultures: History of Zea mays L. Berlin Heidelberg: Springer, 140-141.
- Sauer Carl O (1950) Cultivated plants of South and Central America. Handbook of South American Indians. Physical Anthropology, Linguistics and Cultural Geography of South American Indians. Volume 6. In: Julian H Steward, Smithsonian Institution Bureau of American Ethnology Bulletin 143, Washington D.C. U.S. 487-543.
- Harlan JR, de Wet JMJ (1973) On the quality of evidence for the origin and dispersal of cultivated plants. Current Anthropology 14: 51-62.
- Murra John V (1975) Maiz, Tuberculos y Ritos Agricolas. In: Formaciones Económicas y Politicas del Mundo Andino, Instituto de Estudios Peruanos, Lima, 45-57.
- Murra John V (1985) "El Archipielago Vertical" Revisited. In Andean Ecology and Civilization; An Interdisciplinary Perspective on Andean Ecological Complementarity. S Masuda, I Shimada, and C Morris, Tokyo: University of Tokyo Press, 3-13.
- Chávez Karen L (1989) The significance of Chiripa in Lake Titicaca Basin developments. Expedition 30: 17-26.
- Chávez Sergio, Robert G Thompson (2006) Early Maize on the Copacabana Peninsula: Implications for the archaeology of Lake Titicaca Basin. In: Histories of Maize: Multidisciplinary Approaches to the Prehistory, Linguistics, Biogeography, Domestication, and Evolution of Maize. John E Staller, Robert H Tykot, Bruce F Benz, Taylor & Francis Grp, NY: Routledge, 415-428.
- Ortiz Ana Isabel (2012) Los Maíces en la Seguridad Alimentaria de Bolivia. Centro de Investigación y Promoción del Campesinado (CIPCA). La Paz, Bolivia.
- Chávez Sergio (2012) Agricultural Terraces as Monumental Architecture in the Titicaca Basin. In Early New World Monumentality, RL Burger, RM Rosenswig, University Press of Florida, Gainesville, 431-453.
- Staller John E, Sergio Chávez (2014) High Altitude Zea mays L. (tunqu) Cultivation and Endemism in the Lake Titicaca Basin. Paper for the Midwest Conference on Andean & Amazonian Archaeology & Ethnohistory, University of Wisconsin, Milwaukee.
- Vacher Jean J, Emmanuel Brasier de Thuy, Maximo Liberman (1992) Influencia del Lago en la Agricultura Litoral. In El Lago Titicaca; Sintesis del Conocimiento Limnológico Actual 1991, Claude Dejoux y André Iltis, Misión ORSTOM en Bolivia e HISBOL Instituto de Historia Social Boliviana. La Paz: Talleres Graficos hisbol, 517-530.
- Kuczynsky-Godard, Maxime (1944) La Pampa de Ilave y Su Hinterland. Lima: Ministerio de Salud Publica y Asistencia Social, 11-12.
- Carpio Lourdes del, Felipe Yupa Vereau (2014) Diagnóstico Preliminar Para Elaborar Un Seguro Agropecuario En La Región Puno. Lima: La Positiva - Seguros Generales.
- Doebley John F, Brandon S Gaut, Bruce D Smith (2006) The Molecular Genetics of Crop Domestication. Cell 219: 1309-1321.
- Benz Bruce F, Staller John E (2006) The Antiquity, Biogeography, and Culture History of Maize in the Americas. In Histories of Maize: Multidisciplinary Approaches to the Prehistory, Linguistics, Biogeography, Domestication, and Evolution of Maize. John E Staller, Robert H Tykot, Bruce F Benz, Taylor & Francis Grp., NY: Routledge. 647-673.
- Benz Bruce F, Staller John E (2010) The Antiquity, Biogeography, and Culture History of Maize in Mesoamerica. In Histories of Maize in Mesoamerica: Multidisciplinary Approaches. John E Staller, Robert H Tykot, and Bruce F Benz, Taylor & Francis Grp., NY: Routledge, 267-275.
- Benz Bruce F, Cheng L, Leavitt S, et al. (2006) El Riego and Early Maize Agriculture Evolution. In: John E Staller, Robert H Tykot, Bruce F, Benz, Histories of Maize: Multidisciplinary Approaches to the Prehistory, Linguistics, Biogeography, Domestication, and Evolution of Maize. Taylor & Francis Group, Routledge, NY, 73-82.
- Staller John E (2015b) Plant Domestication. In The Archaeology of Food: An Encyclopedia., In: Karen Bescherer Metheny and Mary C. Beaudry, Alta Mira Press. Imprint of Rowland & Littlefield, New York, 407-409.
- Benz Bruce F (2001) Archaeological evidence of teosinte domestication from Guilá Naquitz, Oaxaca. Proceedings of the National Academy of Sciences 98: 2104-2106.
- Benz Bruce F (2006) Maize in the Americas. In Histories of Maize: Multidisciplinary Approaches to the Prehistory, Linguistics, Biogeography, Domestication, and Evolution of Maize. In: John E Staller, Robert H Tykot, Bruce F Benz, Taylor & Francis Grp., NY: Routledge, 9-20.
- Doebley John, Brandon S Gaut, Bruce D Smith (2006) The Molecular Genetics of Crop Domestication. Cell 127: 1309-1321.
- Staller John E (2015a) Maize. In: The Archaeology of Food: An Encyclopedia. Karen Bescherer Metheny and Mary C Beaudry, New York: Alta Mira Press. Imprint of Rowland & Littlefield, 296-298.
- Dorweiler Jane E (1996) Genetic and evolutionary analysis of glume development in maize and teosinte. Ph.D. Dissertation, University of Minnesota. Ann Arbor: University of Michigan Microfilms.
- Dorweiler Jane, John Doebley (1997) Developmental analysis of teosinte chaff architecture1: A key locus in the evolution of maize (Poaceae) Am J Bot 84: 1313-1322.
- Matsuoka Yoshihiro, Yves Vigouroux, Major M Goodman, et al. (2002) A Single Domestication for Maize Shown by Multilocus Microsatellite Genotyping. Proceedings of the National Academy of Sciences 99: 6080-6084.
- Freitas FO, Brendel G, Allaby RG, et al. (2003) DNA from Primitive Maize Landraces and Archaeological Remains: Implications for the Domestication of Maize and its Expansion into South America. Journal of Archaeological Science 30: 901-908.
- Piperno D R, Ranere A J, Moreno J E, et al. (2007) Late Pleistocene and Holocene environmental history of the Iguala Valley, Central Balsas Watershed of Mexico. Proceedings of the National Academy of Sciences USA 104: 11874-11881.
- Doebley John, Hugh Iltis (1980) Taxonomy of Zea (Gramineae): A Subgeneric Classification with Key to Taxa. Amer J Bot 67: 982-993.
- Weatherwax, Paul (1954) Indian Corn in Old America. New York: The Macmillan Company. 150-151
- Smith, Bruce D (2006) Documenting Plants in the Archaeological Record. In: Documenting Domestication: New Genetic and Archaeological Paradigms. Melinda A Zeder, Daniel G Bradley, Eve Emshwiller, Bruce D Smith,. Berkeley: University of California Press, 15-24.
- Doebley, John F (1994) Morphology, Molecules, and Maize. In Corn & Culture in the Prehistoric New World., edited by Sissel Johannessen and Christine A Hastorf, Boulder: Oxford: Westview Press, Inc, 101-112.
- Feuillet, Catherine, Kelly Eversole (2009) Solving the Maze. Science 326: 1071-1072.
- McMullen MD, Kresovich S, Villeda HS, et al. (2009) Genetic Properties of the Maize Nested Association Mapping Population. Science 325: 737-740.
- Buckler ES, Holland JB, Bradbury PJ, et al. (2009) The genetic architecture of maize flowering time. Science 325: 714-718.
- Smith Bruce D (2001) Documenting plant domestication: The consilience of biological and archaeological approaches. Proceedings of the National Academy of Sciences 98: 1324-1326.
- Dorweiler Jane, A Stec, Kermicle J, et al. (1993) Teosinte chaff architecture 1: a genetic locus controlling a key step in maize evolution. Science 262: 233-235.
- Byers, Douglas S (1967) The Prehistory of the Tehuacán Valley, Volume 1, Environment and Subsistence. Austin: University of Texas Press.
- MacNeish, Richard S, (1967) A summary of the subsistence. In: Douglas S. Byers, The Prehistory of the Tehuacán Valley, Volume 1, Environment and Subsistence, Austin: University of Texas Press, 290-309.
- Flannery, Kent V (1986) Food Procurement Area and Preceramic Diet at Guila Naquitz., In Guilá Naquitz: Archaic Foraging and Early Agriculture in Oaxaca. Kent V Flannery, San Diego: Academic Press, 303-315.
- Sauer, Carl O (1952) Agricultural Origins and Dispersals. New York: American Geographic Society.
- MacNeish Richard S (1992) The Origins of Agriculture and Settled Life. Norman: University of Oklahoma Press.
- Smith,C Earle, Jr (1980 ) Ancient Peruvian Highland Maize. In: Guitarrero Cave; Early Man in the Andes. Thomas F Lynch, New York: Academic Press, 121-143.
- Smith, Bruce D (1995) The Emergence of Agriculture. Second Edition, Scientific American Library, New York.
- Smalley John, Michael Blake T (2003) Sweet Beginnings: Stalk Sugar and the Domestication of Maize. Current Anthropology 44: 675-703.
- Smith C Earle Jr (1986) Preceramic plant remains from Guila Naquitz.. In: Guilá Naquitz, Archaic Foraging and Early Agriculture in Oaxaca. Kent V Flannery, San Diego: Academic Press, 265-276.
- Benz Bruce F, Hugh H Iltis (1990) Studies in Archaeological Maize. I. The "Wild" Maize from San Marcos Cave reexamined. American Antiquity 55: 500-511.
- Piperno Dolores R, Kent V Flannery (2001) The earliest archaeological maize (Zea mays L.) from highland Mexico: New accelerator mass spectrometry dates and their implications. Proc Natl Acad Sci U S A 98: 2101-2103.
- Grobman Alexander, Duccio Bonavia, Tom D Dillehay, et al. (2011) Preceramic Maize from Paredones and Huaca Prieta, Peru. Proceedings of the National Academy of Sciences U.S.A 109: 1755-1759.
- Blake Michael (2006) Dating the Initial Spread of Maize. In: Histories of Maize: Multidisciplinary Approaches to the Prehistory, Linguistics, Biogeography, Domestication, and Evolution of Maize. John E Staller, Robert H Tykot, Bruce F Benz, Taylor & Francis Group, Routledge, NY, 55-72.
- Jaenicke-Després Viviane, Bruce D Smith (2006) Ancient DNA and the Interpretation of Archaeological and Genetic Approaches to the Study of Maize Domestication. In: Histories of Maize: Multidisciplinary Approaches to the Prehistory, Linguistics, Biogeography, Domestication, and Evolution of Maize. John E Staller, Robert H Tykot, Bruce F Benz, Taylor & Francis Group, Routledge, NY,83-96.
- White,Christine D, Fred J Longstaffe, Henry Schwartz (2006) Social Directions in the Isotopic Anthropology of Maize in the Maya Region. In: Histories of Maize: Multidisciplinary Approaches to the Prehistory, Linguistics, Biogeography, Domestication, and Evolution of Maize. John E Staller, Robert H Tykot, Bruce F Benz, Taylor & Francis Group, Routledge, NY, 143-160.
- Chisholm Brian S, Michael Blake (2006) Diet in Prehistoric Soconusco. In: Histories of Maize: Multidisciplinary Approaches to the Prehistory, Linguistics, Biogeography, Domestication, and Evolution of Maize. John E Staller, Robert H Tykot, Bruce F Benz, Taylor & Francis Group, Routledge, NY, 153-160.
- Tykot Robert H, Nik J van der Merwe, Richard L Burger (2006) The importance of maize and marine foods to Initial Period/Early Horizon subsistence in highland and coastal Peru. In: Histories of Maize: Multidisciplinary Approaches to the Prehistory, Linguistics, Biogeography, Domestication, and Evolution of Maize. John E Staller, Robert H Tykot, Bruce F Benz, Taylor & Francis Group, Routledge, NY, 187-197.
- Tykot, Robert H, John E Staller (2002) On the importance of early maize agriculture in coastal Ecuador: New data from the Late Valdivia Phase site of La Emerenciana. Current Anthropology 43: 666-677.
- Morrone Juan (1994) On the Identification of Areas of Endemism. Systematic Biology 43: 438-441.
- Kerr Jeremy T (1997) Species Richness, Endemism, and the Choice of Areas of Conservation. Conservation Biology 11: 1094-1100.
- Staller John E (1994) Late Valdivia Occupation in El Oro Province Ecuador: Excavations at the Early Formative Period (3500-1500 B.C.) site of La Emerenciana, Ph.D. dissertation. Department of Anthropology, Southern Methodist University, Dallas, Texas. Ann Arbor: University Microfilms.
- Donoghue M J (2008) A phylogenetic perspective on the distribution of plant diversity. Proc Natl Acad Sci U S A 105: 11549-11555.
- Swenson Jennifer J, Young BE, Beck S, et al. (2012) Plant and animal endemism in the eastern Andean slope: challenges to conservation. BMC Ecol 12: 1.
- Duellman WE (1999) Distribution patterns of amphibians in South America. In Patterns of distribution of amphibians. Duellman WE, Baltimore, MD. Johns Hopkins University Press, 255-328.
- Hughes C, R Eastwood (2006) Island radiation on a continental scale: Exceptional rates of plant diversification after uplift of the Andes. Proceedings of the National Academy of Sciences 103: 10334-10339.
- Browman David L (1985) Cultural primacy of Tiwanaku in the development of later Peruvian states. Diálogo Andino 4: 59-71.
- Kolata, Alan L (1993) The Tiwanaku: Portrait of an Andean Civilization. Blackwell Publishers, Cambridge.
- Stanish, Charles (2003) Ancient Tiwanaku: The Evolution of Complex Society in Southern Peru and Northern Bolivia. University of California Press, Berkeley.
- Stanish Charles, Edmundo de la Vega, Kirk Frye (1993) Domestic Architecture on Lupaqa Area Sites in the Department of Puno. In: Domestic Architecture, Ethnicity, and Complementarity in the South-Central Andes. Mark S Aldenderfer, University of Iowa Press, Iowa City, 83-93.
- Hastorf Christine, William T Whitehead, Maria C Bruno, et al. (2006) The Movements of Maize into the Middle Horizon Tiwanaku, Bolivia. In: Histories of Maize: Multidisciplinary Approaches to the Prehistory, Linguistics, Biogeography, Domestication, and Evolution of Maize. John E Staller, Robert H Tykot, Bruce F Benz, Taylor & Francis Group, Routledge, NY, 429-448.
- Troll Carl (1958) Las Culturas Superiores Andinas y el Media Geografico. Lima: Instituto de Geografia, Facultad de Letras, Universidad Nacional Mayor de San Marcos.
- Bandelier Adolph F (1969) The Islands of Titicaca and Koati. New York: Kraus Reprint Co. 16-18.
- Matos, Jose (1957) La Propiedad de la Isla de Taquile (Lago Titicaca). Revista del Museo Nacional 26: 211-271.
- Tschopik Harry Jr (1951) The Aymara of Chucuito, Peru 1. Magic. Anthropological Papers of the American Museum of Natural History 44. New York. 153-155, 231-285.
- Sevilla, Ricardo (1994) Variation in Modern Andean maize. In Corn & Culture in the Prehistoric New World, Sissel Johannessen, Christine A Hastorf, Boulder: Westview Press.
- Flores Ochoa Jorge A (1979) Pastoralists of the Andes: the Alpaca Herders of Paratia. Institute for the Study of Human Issues, Philadelphia.
- Flores Ochoa, Jorge A (1988) Distorciones en el Uso del Ecosistema de la Puna y los programas de Cooperación Tecnica. In: Alpacas. Jorge , A Flores Ochoa, Llamichos y Paqocheros, Patores de Llamas y , Universidad Nacional San Antonio Abad del Cuzco, 255-271.
- Kolata, Alan L, Oswaldo Rivera, et al. (1996) Rehabilitating Raised-Field Agriculture in the Southern Lake Titicaca Basin of Bolivia: Theory, Practice, and Results. In: Tiwanaku and Its Hinterland; Archaeology and Paleoecology of an Andean Civilization. 1 Agroecology, Smithsonian Institution Press. Alan L Kolata, Washington DC, 203-230.
- Chávez Karen L Mohr, Sergio J Chávez (1997) Research Summary. Willay Newsletter of the Andean Anthropological Research Group. Carbondale Il. 44: 5-7.
- Llames ME, Zagares HE (2009) Lakes and reservoirs of South America. In: Encyclopedia of Inland Waters.Likens GE, Elsevier, UK, 533-543.
- Hales, Jennifer, Paulo Petry (2013) 337 Titicaca. Freshwater Ecoregions of the World (FEOW). The Nature Conservancy.
- Kolata Alan L, Oswaldo Rivera, Juan Carlos Ramirez, et al. (1996) Rehabilitating Raised-Field Agriculture in the Southern Lake Titicaca Basin of Bolivia: Theory, Practice, and Results. In: Tiwanaku and Its Hinterland; Archaeology and Paleoecology of an Andean Civilization. 1 Agroecology. Alan L Kolata, Smithsonian Institution Press, Washington, D.C, 203-230.
- Binford Michael W, Alan L Kolata (1996) The Natural and Human Setting. In: Tiwanaku and Its Hinterland; Archaeology and Paleoecology of an Andean Civilization. Alan L Kolata, Smithsonian Institution Press, Washington, D.C, 23-56.
- Binford, Michael W, Alan L Kolata, et al. (1997) Climatic Variation and Rise and Fall of an Andean Civilization. Quaternary Research 47: 235-248.
- Carney Heath J, Michael W Binford, Alan L Kolata, et al. (1993) Nutrient and Sediment Retention in Andean Raised Field Agriculture. Nature 364: 131-133.
- Browman David L (1989) Chenopod Cultivation, Lacustrine Resources, and Fuel Use at Chiripa, Bolivia. The Missouri Archaeologist 47: 137-172.
- Kolata Alan L (1986) The Agricultural Foundations of the Tiwanaku State: A View from the Heartland, American Antiquity 51: 748-762.
- Staller John E (2006) The Social, Symbolic and Economic Significance of Zea mays L. in the Late Horizon Period. In: Histories of Maize: Multidisciplinary Approaches to the Prehistory, Linguistics, Biogeography, Domestication, and Evolution of Maize. John E Staller, Robert H Tykot, Bruce F Benz, Taylor & Francis Grp, NY: Routledge, 449-467.
- Staller John E (2008) Dimensions of Place: The Significance of Centers to the Development of Andean Civilization: An examination of the Ushnu Concept. In: Pre-Columbian Landscapes of Creation and Origin. John Edward Staller, Springer, New York, 269-313.
- Staller John E, Brian Stross (2013) Lightning in the Andes and Mesoamerica: Pre-Columbian, Colonial, and Contemporary Perspectives. New York and Oxford: Oxford University Press, 77-82.
- Bruman Henry J (2000) Alcohol in Ancient Mexico. Forward by Peter Furst, University of Utah Press. Salt Lake City.
- Gonzalez Holguin, Diego (1952) Vocabulario de 1a Lengua General de Todo e1 Peru Llamada Lengua Qquichua o del Inca. Edición del Instituto de Historia. Lima: Imprenta Santa Maria.
- Cieza de León, Pedro (1998) The discovery and conquest of Peru. Chronicles of the New World Encounter. Alexandra Parma Cook,Noble David Cook. Durham and London: Duke University Press.
- Arriaga Fr Pablo Joseph de (1968) The Extirpation of Idolatry in Peru. Keating C, Lexington.
- Acosta, Jose de (1987) Historia Natural y Moral de las Indias. Edición de Jose Alcina Franch. Madrid: Impresión y encuadernación TEMI.
- Murúa, Martin de (1987) Historia general del Perú, origen y descendencia de los Incas. Madrid: Biblioteca Americana Vetus.
- Squier Ephraim George (1973) Peru: Incidents of Travel and Exploration in the Land of the Incas. New York: AMS Press for Peabody Museum of Archaeology and Ethnology, Cambridge.
- Middendorf Ernst W (1974) Peru; Observaciones y Estudios del Pais y sus Habitantes Durante una Permanencia de 25 Años. Tomo III. Lima: Universidad Nacional Mayor de San Marcos.
- Lynch Thomas F (1983) Camelid Pastoralism and the Emergence of Tiwanaku Civilization in the South-Central Andes. World Archaeology 15: 1-14.
- Christensen SA, Pratt DB, Pratt C, et al. (2007) Assessment of genetic diversity in the USDA and CIP-FAO international nursery collections of quinoa (Chenopodium quinoa Willd.) using microsatellite markers. Plant Genetic Resources 5: 82-95.
- Grobman Alexander, Wilfredo Salhuana, Ricardo Sevilla, et al. (1961) Races of Maize in Peru, Their Origins, Evolution and Classification. National Academy of Sciences - National Research Council, Washington, D.C. 915.
- Rivera Mario (2006) Prehistoric Maize from Northern Chile: An evaluation of the evidence. In: John E Staller, Robert H Tykot, Bruce F Benz, Histories of Maize: Multidisciplinary Approaches to the Prehistory, Linguistics, Biogeography, Domestication, and Evolution of Maize. Taylor & Francis Group, Routledge, NY, 403-413.
- Cutler Hugh C (1946) Maize in South America. Botanical Museum Leaflets, Harvard University 12: 257-291.
- Long Austin, Bruce F Benz, DJ Donahue, et al. (1989) First Direct AMS Dates on Early Maize from Tehuacán, Mexico. Radiocarbon 31: 1035-1040.
- Smith Bruce D (1997a) The initial domestication of Curcurbita pepo in the Americas 10,000 years ago. Science 276: 932-934.
- Smith Bruce D (1997b) Reconsidering the Ocampo Caves and the Era of Incipient Cultivation in Mesoamerica. Latin American Antiquity 8: 342-383.
- Clark John E (1994) The Development of Early Formative Rank Societies in the Soconusco, Chiapas, Mexico.
- Blake Michael, Brian S Chisholm, John E Clark, et al. (1995) Radiocarbon Chronology for the Late Archaic and Formative Periods on the Pacific Coast of Southeastern Mesoamerica. Ancient Mesoamerica 6: 161-183.
- Hard RJ, Roney JR (1998) A massive terraced village complex in chihuahua, mexico, 3000 years before present. Science 279: 1661-1664.
- Pope Kevin O, Mary Pohl, John G Jones, et al. (2001) Origin and Environmental Settings of Ancient Agriculture in the Lowlands of Mesoamerica. Science 292: 1370-1373.
- Matson RG (2003) The Spread of Maize Agriculture into the U.S. Southwest. In Examining the Farming/Language Dispersal Hypothesis. Peter Bellwood and Colin Renfrew, McDonald, Institute for Archaeological Research, Cambridge: University of Cambridge, 341-356.
- Pearsall Deborah M (2003) Plant Food Resources of the Ecuadorian Formative: An overview and Comparison to the Central Andes. In: Archaeology of Formative Ecuador: A Symposium. Dumbarton Oaks, 7 and 8 October 1995, edited by. J. Scott Raymond & R. L. Burger, Washington D.C, 213-257.
- Zarrillo Sonia, Dolores M Pearsall, J Scott Raymond, et al. (2008) Directly dated starch residues document early formative maize (Zea mays L.) in tropical Ecuador. Proceedings of the National Academy of Sciences 105: 5006-5011.
- Rodriquez, María Fernanda, Carlos Alberto Aschero (2007) Archaeological Evidence of Zea mays L. (POACEAE) in the Southern Argentinean Puna (Antofagasto de la Sierra, Catamarca). Journal of Ethnobiology 27: 256-271.
- Rodriquez, María Fernanda, Carlos Alberto Aschero (2007) Archaeological Evidence of Zea mays L. (POACEAE) in the Southern Argentinean Puna (Antofagasto de la Sierra, Catamarca). Journal of Ethnobiology 27: 256-271.
- Creamer, Winifred, Alvaro Ruíz, et al. (2007) Archaeological investigations of Late Archaic sites (3000-1800 B.C.) in the Pativilca Valley, Peru. Fieldiana Anthropology 40: 1-78.
- Haas, Jonathan, Winifred Creamer, Luis Huamán Mesía, et al. (2013) Evidence of Maize (Zea mays) in the Late Archaic Alvaro Ruíz and 2007. Archaeological investigations of Late Archaic sites (3000-1800 B.C.) in the Pativilca Valley, Peru. Proceedings of the National Academy of Sciences 40: 1-78.
- Freitas Fabio O (2001) Estudo genético-evolutivo de amostras modernas e arqueológicas de milho (Zea mays mays, L.) e feijão (Phaseolus vulgaris, L.). Escola Superior de Agricultura Luiz de Queiroz, Brazil.
- Gil Adolfo F (2003) Zea mays on the South American Periphery: Chronology and dietary Importance. Current Anthropology 44: 295-300.
Corresponding Author
Staller John E, Research Associate, Botanical Research Institute of Texas (BRIT), Fort Worth, Texas, USA.
Copyright
© 2016 Staller JE. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.